Differences in Anthocyanin Accumulation Patterns and Related Gene Expression in Two Varieties of Red Pear
Abstract
1. Introduction
2. Results
2.1. Phenotype of ‘Xinli 7′ and ‘Xinqihong’ Red Pear Varieties
2.2. Anthocyanin Accumulation in ‘Xinli 7′ and ‘Xinqihong’
2.3. Comparative Transcriptome Analysis
2.4. Functional Analysis of DEGs
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Materials
5.2. Measurement of Anthocyanin, Chlorophyll, and Carotenoid Content in Peel
5.3. Determination of Fruit Color
5.4. RNA Extraction and First-Strand cDNA Synthesis
5.5. Quantitative Real-Time PCR (qRT-PCR) Analysis
5.6. Library Construction and Transcriptome Sequencing
5.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Honda, C.; Kotoda, N.; Wada, M.; Kondo, S.; Kobayashi, S.; Soejima, J.; Zhang, Z.L.; Tsuda, T.; Moriguchi, T. Anthocyanin biosynthetic genes are coordinately expressed during red coloration in apple skin. Plant Physiol. Biochem. 2002, 40, 955–962. [Google Scholar] [CrossRef]
- Vaknin, H.; Bar-Akiva, A.; Ovadia, R.; Nissim-Levi, A.; Forer, I.; Weiss, D.; Oren-Shamir, M. Active anthocyanin degradation in Brunfelsia calycina (yesterday–today–tomorrow) flowers. Planta 2005, 222, 19–26. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Giusti, M.M. Anthocyanins: Natural colorants with health-promoting properties. Annu. Rev. Food Sci. Technol. 2010, 163, 163–187. [Google Scholar] [CrossRef]
- Yang, Y.N.; Yao, G.F.; Zheng, D.; Zhang, S.L.; Wang, C.; Zhang, M.Y.; Wu, J. Expression differences of anthocyanin biosynthesis genes reveal regulation patterns for red pear coloration. Plant Cell Rep. 2014, 34, 189–198. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Zhang, D.; Huang, C.H.; Qian, M.J.; Zheng, X.Y.; Teng, Y.W.; Su, J.; Shu, Q. Isolation of anthocyanin biosynthetic genes in red Chinese sand pear (Pyrus pyrifolia Nakai) and their expression as affected by organ/tissue, cultivar, bagging and fruit side. Sci. Hortic. 2012, 136, 29–37. [Google Scholar] [CrossRef]
- Feng, S.; Wang, Y.; Yang, S.; Xu, Y.; Chen, X. Anthocyanin biosynthesis in pears is regulated by a R2R3-MYB transcription factor PyMYB10. Planta 2010, 232, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Thomson, G.E.; Turpin, S.; Goodwin, I. A review of preharvest anthocyanin development in full red and blush cultivars of European pear. N. Z. J. Crop Hort. 2018, 46, 81–100. [Google Scholar] [CrossRef]
- Rowan, D.D.; Cao, M.; Lin-Wang, K.; Cooney, J.M.; Jensen, D.J.; Austin, P.T.; Hunt, M.B.; Norling, C.; Hellens, R.P.; Schaffer, R.J. Environmental regulation of leaf colour in red 35S:PAP1 Arabidopsis thaliana. New Phytol. 2009, 182, 102–115. [Google Scholar] [CrossRef]
- Cavallini, E.; Matus, J.T.; Finezzo, L.; Zenoni, S.; Loyola, R.; Guzzo, F.; Schlechter, R.; Ageorges, A.; Arce-Johnson, P.; Tornielli, G.B. The phenylpropanoid pathway is controlled at different branches by a set of R2R3-MYB C2 repressors in grapevine. Plant Physiol. 2015, 167, 1448–1470. [Google Scholar] [CrossRef]
- Xu, W.; Dubos, C.; Lepiniec, L.C. Transcriptional control of flavonoid biosynthesis by MYB-bHLH-WDR complexes. Trends Plant Sci. 2015, 20, 1760185. [Google Scholar] [CrossRef]
- Li, Y.Y.; Mao, K.; Zhao, C.; Zhao, X.Y.; Zhang, H.L.; Shu, H.R.; Hao, Y.J. MdCOP1 ubiquitin E3 ligases interact with MdMYB1 to regulate light-induced anthocyanin biosynthesis and red fruit coloration in apple. Plant Physiol. 2012, 160, 1011–1022. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Schrader, A.; Kokkelink, L.; Falke, C.; Hoecker, U. Light and the E3 ubiquitin ligase COP1/SPA control the protein stability of the MYB transcription factors PAP1 and PAP2 involved in anthocyanin accumulation in Arabidopsis. Plant J. 2013, 74, 638–651. [Google Scholar] [CrossRef] [PubMed]
- Allan, A.C.; Hellens, R.P.; Laing, W.A. MYB transcription factors that colour our fruit. Trends Plant Sci. 2008, 13, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Laura, J. New insights into the regulation of anthocyanin biosynthesis in fruits. Trends Plant Sci. 2013, 18, 477–483. [Google Scholar]
- Honda, C.; Moriya, S. Anthocyanin biosynthesis in apple fruit. Hortic. J. 2018, 87, 305–314. [Google Scholar] [CrossRef]
- Xu, Y.; Li, S.E.; Huan, C.; Jiang, T.; Brecht, J.K. Effects of 1methylcyclopropene treatment on quality and anthocyanin biosynthesis in plum (Prunus salicina cv. Taoxingli) fruit during storage at a non-chilling temperature. Postharvest Biol. Tec. 2020, 169, 111291. [Google Scholar]
- Shan, X.Y.; Zhang, Y.S.; Peng, W.; Wang, Z.L.; Xie, D.X. Molecular mechanism for jasmonate-induction of anthocyanin accumulation in Arabidopsis. J. Exp. Bot. 2009, 60, 3849–3860. [Google Scholar] [CrossRef] [PubMed]
- An, X.H.; Tian, Y.; Chen, K.Q.; Liu, X.J.; Liu, D.D.; Xie, X.B.; Cheng, C.G.; Cong, P.H.; Hao, Y.J. MdMYB9 and MdMYB11 are involved in the regulation of the JA-induced biosynthesis of anthocyanin and proanthocyanidin in apples. Plant Cell Physiol. 2015, 56, 650–662. [Google Scholar] [CrossRef]
- Ji, X.H.; Wang, Y.T.; Zhang, R.; Wu, S.J.; An, M.M.; Li, M.; Wang, C.Z.; Chen, X.L.; Zhang, Y.; Chen, X. Effect of auxin, cytokinin and nitrogen on anthocyanin biosynthesis in callus cultures of red-fleshed apple (Malus sieversii f. niedzwetzkyana). PCTOC 2015, 120, 325–337. [Google Scholar] [CrossRef]
- Shin, D.H.; Choi, M.G.; Lee, H.K.; Cho, M.; Park, Y.L. Calcium dependent sucrose uptake links sugar signaling to anthocyanin biosynthesis in Arabidopsis. Biochem. Biophys. Res. Commun. 2013, 430, 634–639. [Google Scholar] [CrossRef]
- Zhi, H.; Liu, Q.; Xu, J.; Dong, Y.; Liu, M.; Zong, W. Ultrasound enhances calcium absorption of jujube fruit by regulating the cellular calcium distribution and metabolism of cell wall polysaccharides. J. Sci. Food Agric. 2017, 97, 5202–5210. [Google Scholar] [CrossRef]
- Feng, S.Q.; Sun, S.S.; Chen, X.L.; Wu, S.J.; Wang, D.Y.; Chen, X.S. PyMYB10 and PyMYB10.1 interact with bHLH to enhance anthocyanin accumulation in pears. PLoS ONE 2015, 10, e0142112. [Google Scholar] [CrossRef]
- Li, P.M.; Zhang, Y.Z.; Einhorn, T.C.; Cheng, L.L. Comparison of phenolic metabolism and primary metabolism between green ‘Anjou’ pear and its bud mutation, red ‘Anjou’. Physiol. Plant. 2013, 150, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Dussie, M.C.; Sugart, D.; Azarenko, A.N.; Righetti, T.L. Colorimetric characterization of red pear cultivars. Fruit Var. J. 1997, 51, 39–43. [Google Scholar]
- Steyn, W.J.; Holcroft, D.M.; Wand, S.J.E.; Jacobs, G. Anthocyanin degradation in detached pome fruit with reference to preharvest red color loss and pigmentation patterns of blushed and fully red pears. J. Am. Soc. Hortic. Sci. 2004, 129, 13–19. [Google Scholar] [CrossRef]
- Gonzalez, A.; Zhao, M.; Leavitt, J.M.; Lloyd, A.M. Regulation of the anthocyanin biosynthetic pathway by the TTG1/bHLH/MYB transcriptional complex in Arabidopsis seedlings. Plant J. 2008, 53, 814–827. [Google Scholar] [CrossRef]
- Zhang, X.; Allan, A.C.; Yi, Q.; Chen, L.; Li, K.; Shu, Q.; Su, J. Differential gene expression analysis of Yunnan red pear, Pyrus pyrifolia, during fruit skin coloration. Plant Mol. Biol. Rep. 2011, 29, 305–314. [Google Scholar] [CrossRef]
- Chagné, D.; Wang, K.L.; Espley, R.V.; Volz, R.K.; How, N.M.; Rouse, S.; Brendolise, C.; Carlisle, C.M.; Kumar, S.; Silva, N.D.; et al. An ancient duplication of apple MYB transcription factors is responsible for novel red fruit-flesh phenotypes. Plant Physiol. 2013, 161, 225–239. [Google Scholar] [CrossRef]
- Lin-Wang, K.; Bolitho, K.; Grafton, K.; Kortstee, A.; Karunairetnam, S.; Mcghie, T.K.; Espely, R.V.; Hellens, R.P.; Allan, A.C. An R2R3 MYB transcription factor associated with regulation of the anthocyanin biosynthetic pathway in Rosaceae. BMC Plant Biol. 2010, 10, 50. [Google Scholar] [CrossRef]
- Shin, J.; Park, E.; Choi, G. PIF3 regulates anthocyanin biosynthesis in an HY5-dependent manner with both factors directly binding anthocyanin biosynthetic gene promoters in Arabidopsis. Plant J. 2007, 49, 981–994. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Y.; Wang, J.; Li, P.; Zhao, C.; Chen, Y.; Bi, Y. Phytochrome-interacting factors PIF4 and PIF5 negatively regulate anthocyanin biosynthesis under red light in Arabidopsis seedlings. Plant Sci. 2015, 238, 64–72. [Google Scholar] [CrossRef]
- Tao, R.; Bai, S.; Ni, J.; Yang, Q.; Zhao, Y.; Teng, Y. The blue light signal transduction pathway is involved in anthocyanin accumulation in ‘Red Zaosu’ pear. Planta 2018, 248, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Bai, S.; Zhao, Y.; Qian, M.; Tao, R.; Yin, L.; Gao, L.; Teng, Y. Ethylene response factors Pp4ERF24 and Pp12ERF96 regulate blue light-induced anthocyanin biosynthesis in ‘Red Zaosu’ pear fruits by interacting with MYB114. Plant Mol. Biol. 2019, 99, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Liu, L.; Yuan, C.; Guan, J. Molecular characterization of ethylene-regulated anthocyanin biosynthesis in plums during fruit ripening. Plant Mol. Biol. Rep. 2016, 34, 777–785. [Google Scholar] [CrossRef]
- An, J.P.; Wang, X.F.; Li, Y.Y.; Song, L.Q. EIN3-LIKE1, MYB1, and ETHYLENE RESPONSE FACTOR3 act in a regulatory loop that synergistically modulates ethylene biosynthesis and anthocyanin accumulation. Plant Physiol. 2018, 178, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, H.; Wang, N.; Jiang, S.; Fang, H.; Zhang, Z.; Yang, G.; Wang, Y.; Su, M.; Xu, L.; et al. The ethylene response factor MdERF1B regulates anthocyanin and proanthocyanidin biosynthesis in apple. Plant Mol. Biol. 2018, 98, 205–218. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, Y.; Tao, R.; Yin, L.; Gao, L.; Ke, S.; Qian, M.; Li, J.; Shen, J. Ethylene mediates the branching of the jasmonate-induced flavonoid biosynthesis pathway by suppressing anthocyanin biosynthesis in red Chinese pear fruits. Plant Biotechnol. J. 2020, 18, 1223–1240. [Google Scholar] [CrossRef]
- Mori, T.; Sakurai, M.; Seki, M.; Furusaki, S. Use of auxin and cytokinin to regulate anthocyanin production and composition in suspension cultures of strawberry cell. J. Sci. Food Agric. 1994, 65, 271–276. [Google Scholar] [CrossRef]
- Moro, L.; Hassimotto, N.M.A.; Purgatto, E. Postharvest auxin and methyl jasmonate effect on anthocyanin biosynthesis in red raspberry (Rubus idaeus L.). J. Plant Growth Regul. 2017, 36, 773–782. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, N.; Xu, H.; Jiang, S.; Fang, H.; Su, M.; Zhang, Z.; Zhang, T.; Chen, X. Auxin regulates anthocyanin biosynthesis through the Aux/IAA-ARF signaling pathway in apple. Hortic. Res. 2018, 5, 1–11. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Liu, R.; Hao, H.; Bi, Y. Gibberellins negatively regulate low temperature-induced anthocyanin accumulation in a HY5/HYH-dependent manner. Plant Signal. Behav. 2011, 6, 632–634. [Google Scholar] [CrossRef]
- Zhai, R.; Wang, Z.; Yang, C.; Wang, K.; Espley, R.; Liu, J.; Li, X.; Wu, Z.; Li, P.; Guan, Q.; et al. PbGA2ox8 induces vascular-related anthocyanin accumulation and contributes to red stripe formation on pear fruit. Hortic. Res. 2019, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Karamanoli, K.; Lazaridou, A.; Matsi, T.; Molassiotis, A. Metabolomic and physicochemical approach unravel dynamic regulation of calcium in sweet cherry fruit physiology. Plant Physiol. Biochem. 2017, 116, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xin, G.; Zhang, B.; Yang, J.Y. Optimization of extraction technique of anthocyanin from red peel of ‘Nanguo’ pear. Food Sci. 2009, 30, 97–100. [Google Scholar]
- Wu, J.; Zhao, G.; Yang, Y.N.; Le, W.Q.; Khan, M.A.; Zhang, S.L.; Gu, C.; Huang, W.J. Identification of differentially expressed genes related to coloration in red/green mutant pear (Pyrus communis L.). Tree Genet. Genom. 2013, 9, 75–83. [Google Scholar] [CrossRef]
- Huang, C.H.; Yu, B.; Teng, Y.W.; Su, J.; Shu, Q.; Cheng, Z.Q.; Zeng, L.Q. Effects of fruit bagging on coloring and related physiology, and qualities of red Chinese sand pears during fruit maturation. Sci. Hortic. 2009, 121, 149–158. [Google Scholar] [CrossRef]

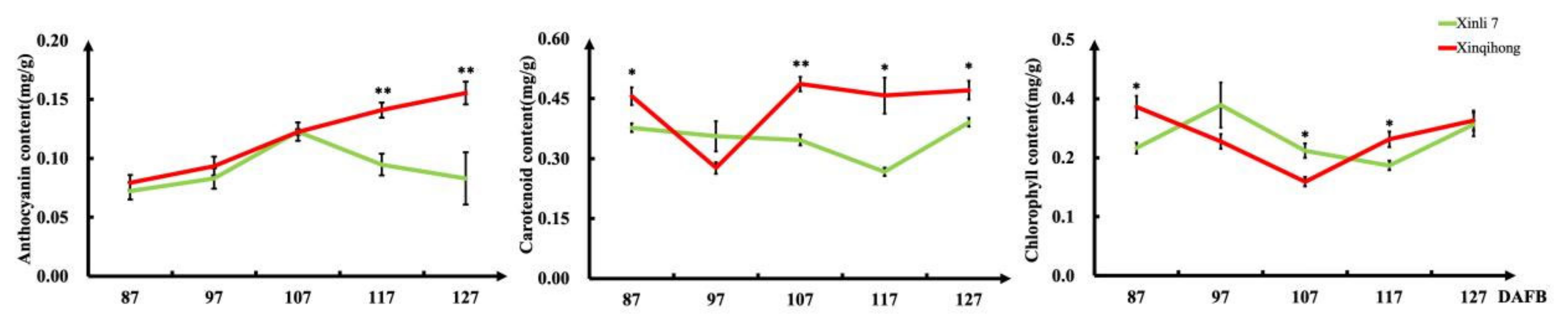
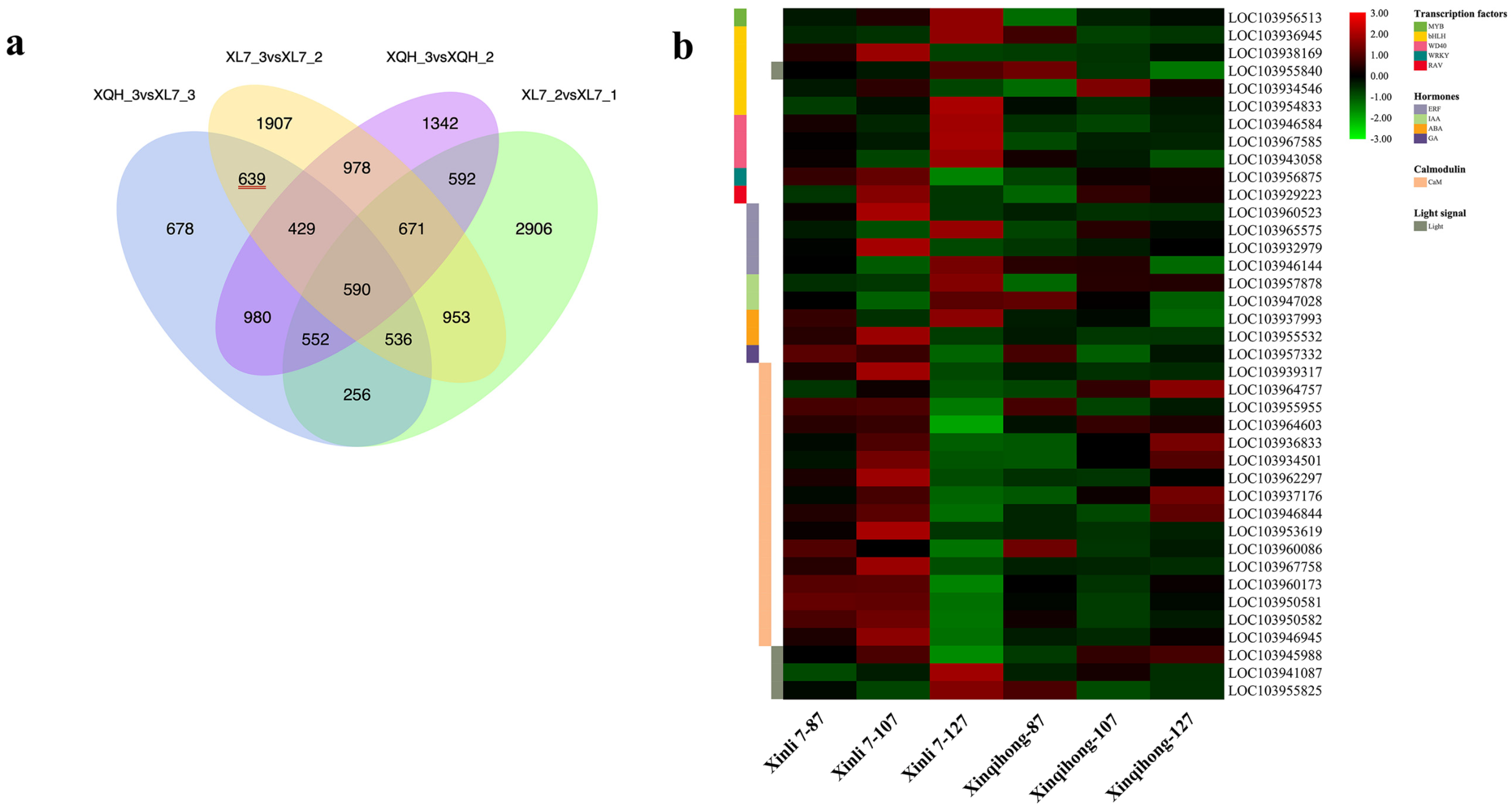
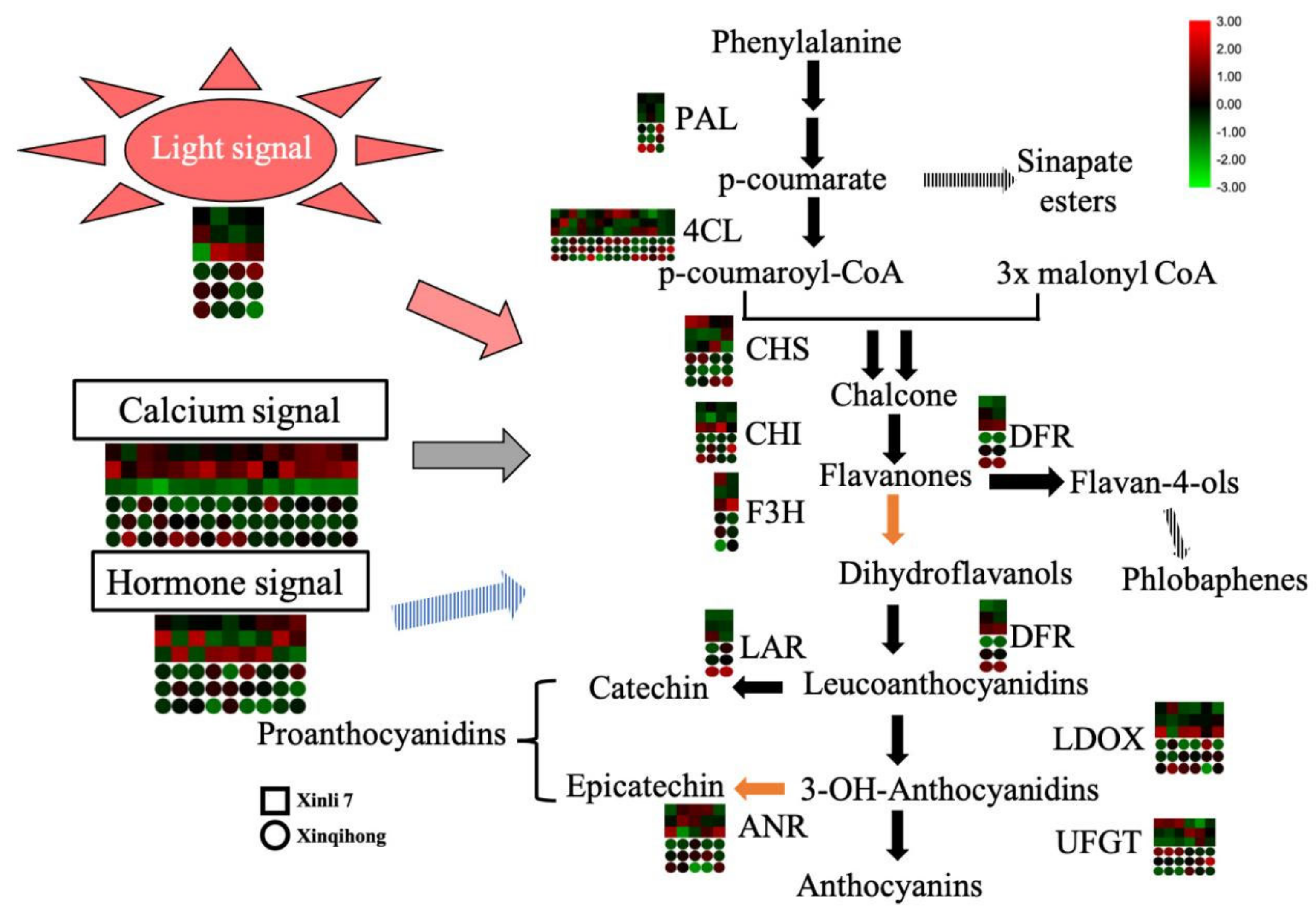
| Trait | Variety | |
|---|---|---|
| Xinqihong | Xinli 7 | |
| Fruit weight, g | 244 ± 13.34 a | 209 ± 4.86 b |
| Fruit shape | Spindle | Spindle |
| Fruit size, cm | 11.32 × 9.99 | 10.5 × 8.7 |
| Shape index | 1.13 ± 0.07 | 1.21 ± 0.07 |
| Color | More red | Less red |
| Soluble solids, % | 12.4 ± 0.43 a | 11.2 ± 0.28 b |
| Titratable acid, % | 0.04 ± 0.01 | 0.06 ± 0.02 |
| Vitamin C, mg/100 g | 1.07 ± 0.04 b | 1.36 ± 0.04 a |
| Fruit hardness, kg/cm2 | 5.69 ± 0.28 a | 4.32 ± 0.44 b |
| Taste | Crispy | Crispy |
| Parameter | Variety | Days after Full Bloom | ||||
|---|---|---|---|---|---|---|
| 87 | 97 | 107 | 117 | 127 | ||
| L* | Xinqihong | 44.85 ± 1.21 c | 42.83 ± 1.38 c | 43.01 ± 2.89 c | 42.57 ± 0.80 c | 42.15 ± 3.32 c |
| Xinli 7 | 50.07 ± 2.62 bcd | 48.02 ± 4.42 cd | 47.42 ± 3.44 cd | 46.23 ± 1.95 d | 51.20 ± 4.02 bc | |
| a* | Xinqihong | 8.84 ± 1.85 d | 13.28 ± 1.52 c | 15.13 ± 2.59 bc | 17.29 ± 1.99 b | 21.38 ± 3.24 a |
| Xinli 7 | 4.77 ± 1.02 b | 11.74 ± 2.39 a | 13.92 ± 5.41 a | 12.47 ± 1.57 a | 11.39 ± 3.48 a | |
| b* | Xinqihong | 24.88 ± 2.05 b | 24.20 ± 1.47 b | 24.11 ± 3.55 b | 22.99 ± 1.39 b | 24.58 ± 3.89 b |
| Xinli 7 | 30.86 ± 1.84 a | 28.55 ± 3.80 ab | 28.80 ± 2.74 ab | 26.20 ± 1.51 b | 31.19 ± 3.34 a | |
| C* | Xinqihong | 26.49 ± 1.56 c | 27.64 ± 1.31 bc | 28.68 ± 1.91 bc | 28.82 ± 1.43 bc | 32.81 ± 2.49 a |
| Xinli 7 | 31.24 ± 1.82 ab | 31.04 ± 2.58 ab | 32.39 ± 2.12 a | 29.06 ± 0.93 b | 33.41 ± 2.53 a | |
| h* | Xinqihong | 70.29 ± 4.95 b | 61.23 ± 3.48 c | 57.58 ± 7.98 cd | 53.11 ± 3.95 de | 48.82 ± 7.43 e |
| Xinli 7 | 81.19 ± 1.95 b | 67.22 ± 7.02 c | 64.29 ± 10.12 c | 64.49 ± 3.91 c | 69.65 ± 6.89 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Deng, Z.; Sun, H.; Song, J.; Li, D.; Zhang, S.; Wang, R. Differences in Anthocyanin Accumulation Patterns and Related Gene Expression in Two Varieties of Red Pear. Plants 2021, 10, 626. https://doi.org/10.3390/plants10040626
Liu J, Deng Z, Sun H, Song J, Li D, Zhang S, Wang R. Differences in Anthocyanin Accumulation Patterns and Related Gene Expression in Two Varieties of Red Pear. Plants. 2021; 10(4):626. https://doi.org/10.3390/plants10040626
Chicago/Turabian StyleLiu, Jianlong, Zhiwei Deng, Hongwei Sun, Jiankun Song, Dingli Li, Shaoling Zhang, and Ran Wang. 2021. "Differences in Anthocyanin Accumulation Patterns and Related Gene Expression in Two Varieties of Red Pear" Plants 10, no. 4: 626. https://doi.org/10.3390/plants10040626
APA StyleLiu, J., Deng, Z., Sun, H., Song, J., Li, D., Zhang, S., & Wang, R. (2021). Differences in Anthocyanin Accumulation Patterns and Related Gene Expression in Two Varieties of Red Pear. Plants, 10(4), 626. https://doi.org/10.3390/plants10040626





