Systematic Analysis of Combined Antioxidant and Membrane-Stabilizing Properties of Several Lamiaceae Family Kazakhstani Plants for Potential Production of Tea Beverages
Abstract
1. Introduction
2. Materials and Methods
2.1. Laboratory Animals
2.2. Preparation of Plant Extracts
2.3. Estimation of Total Phenolic and Flavonoid Content
2.4. Estimation of Lipid Peroxidation in Liver Microsomes
2.5. Isolation of Rat Erythrocytes
2.6. Estimation of Osmotic Resistance of Erythrocytes
2.7. Statistical Data Analysis
3. Results
3.1. Properties of Plant Extracts
3.2. Influence of Herbal Extracts of Family Lamiaceae
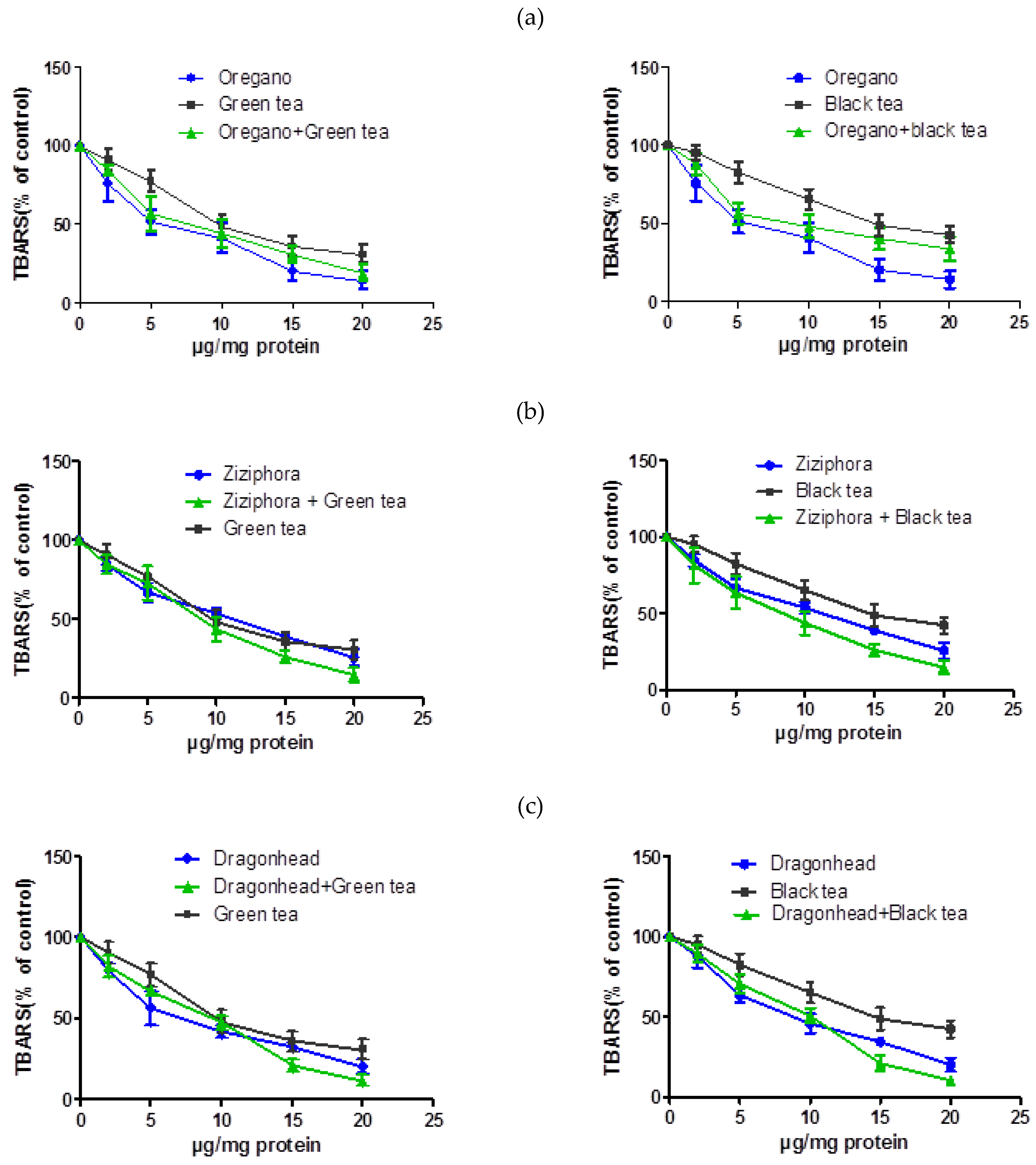
3.3. Antioxidant Properties and Membrane-Stabilizing Properties of Combination Extracts of Plants and Tea
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, T.-Y.; Li, Q.; Bi, K.-S. Bioactive flavonoids in medicinal plants: Structure, activity and biological fate. Asian J. Pharm. Sci. 2018, 13, 12–23. [Google Scholar] [CrossRef]
- Akzhigitova, Z.; Dyusebaeva, M.A.; Tokay, T.; Ydyrys, A.; Lijiang, X.; Jenis, J. Phytochemical Study of Bergenia crassifolia. Chem. Nat. Compd. 2020, 56, 1–3. [Google Scholar] [CrossRef]
- Kozłowska, A.; Szostak-Węgierek, D. Flavonoids—Food Sources, Health Benefits, and Mechanisms Involved. In Bioactive Molecules in Food; Series in Phytochemistry; Springer: Cham, Switzerland, 2017; pp. 1–27. [Google Scholar] [CrossRef]
- Goldstein, B.D. Environmental risks and public health. Ann. N. Y. Acad. Sci. 2006, 933, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Traka, M.H.; Mithen, R.F. Plant Science and Human Nutrition: Challenges in Assessing Health-Promoting Properties of Phytochemicals. Plant Cell 2011, 23, 2483–2497. [Google Scholar] [CrossRef] [PubMed]
- Ydyrys, A.; Mukhitdinov, N.; Ametov, A.; Tynybekov, B.; Akhmetova, A.; Abidkulova, K. The states of coenpopulations of endemic, relict and rare species of plant Limonium michelsonii and their protection. World Appl. Sci. J. 2013, 26, 934–940. [Google Scholar] [CrossRef]
- Zbikowska, H.M.; Szejk, M.; Saluk, J.; Pawlaczyk-Graja, I.; Gancarz, R.; Olejnik, A.K. Polyphenolic–polysaccharide conjugates from plants of Rosaceae/Asteraceae family as potential radioprotectors. Int. J. Biol. Macromol. 2016, 86, 329–337. [Google Scholar] [CrossRef] [PubMed]
- Watson, R.; Preedy, V.; Zibadi, S. (Eds.) Polyphenols in Human Health and Disease, 2nd ed.; Academic Press: Cambridge, MA, USA, 2018. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine Flavonoids in Health and Disease Prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Chen, J.; Mangelinckx, S.; Adams, A.; Wang, Z.-T.; Li, W.-L.; De Kimpe, N. Natural Flavonoids as Potential Herbal Medication for the Treatment of Diabetes Mellitus and its Complications. Nat. Prod. Commun. 2015, 10, 187–200. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.; Venkataraman, K.; Hollingsworth, A.; Piche, M.; Tai, T.C. Polyphenols: Benefits to the Cardiovascular System in Health and in Aging. Nutrients 2013, 5, 3779–3827. [Google Scholar] [CrossRef]
- Sahnoun, Z.; Jamoussi, K.; Zeghal, K.M. Free radicals and antioxidants: Physiology, human pathology and therapeutic aspects (part II). Therapie 1998, 53, 315–339. [Google Scholar] [PubMed]
- Rahal, A.; Kumar, A.; Singh, V.; Yadav, B.; Tiwari, R.; Chakraborty, S.; Dhama, K. Oxidative Stress, Prooxidants, and Antioxidants: The Interplay. BioMed Res. Int. 2014, 2014, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Barrera, G. Oxidative Stress and Lipid Peroxidation Products in Cancer Progression and Therapy. Int. Sch. Res. Not. 2012, 2012, 1–21. [Google Scholar] [CrossRef]
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS Sources in Physiological and Pathological Conditions. Oxidative Med. Cell. Longev. 2016, 2016, 1–44. [Google Scholar] [CrossRef]
- Khan, N. Tea and Health: Studies in Humans. Curr. Pharm. Des. 2013, 19, 6141–6147. [Google Scholar] [CrossRef] [PubMed]
- Ajazuddin, A.A.; Qureshi, A.; Kumari, L.; Vaishnav, P.; Sharma, M.; Saraf, S.; Saraf, S. Role of herbal bioactives as a potential bioavailability enhancer for Active Pharmaceutical Ingredients. Fitoterapia 2014, 97, 1–14. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.C.; Drummond, G.B.; McLachlan, E.M.; Kilkenny, C.; Wainwright, C.L. Guidelines for reporting experiments involving animals: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1573–1576. [Google Scholar] [CrossRef]
- Ohkawa, H.; Ohishi, N.; Yagi, K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979, 95, 351–358. [Google Scholar] [CrossRef]
- Zhamanbaeva, G.T.; Murzakhmetova, M.K.; Tuleukhanov, S.T.; Danilenko, M.P. Antitumor Activity of Ethanol Extract from Hippophae Rhamnoides L. Leaves towards Human Acute Myeloid Leukemia Cells In Vitro. Bull. Exp. Biol. Med. 2014, 158, 252–255. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M.; Lester, P. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Murzakhmetova, M.; Moldakarimov, S.; Tancheva, L.; Abarova, S.; Serkedjieva, J. Antioxidant and prooxidant properties of a polyphenol-rich extract fromGeranium sanguineum L.in vitro andin vivo. Phytother. Res. 2008, 22, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Heimler, D.; Vignolini, P.; Dini, M.G.; Romani, A. Rapid Tests to Assess the Antioxidant Activity ofPhaseolus vulgarisL. Dry Beans. J. Agric. Food Chem. 2005, 53, 3053–3056. [Google Scholar] [CrossRef]
- Cordell, G.A. Sustainable Medicines and Global Health Care. Planta Med. 2011, 77, 1129–1138. [Google Scholar] [CrossRef] [PubMed]
- Krausz, A.; Gunn, H.; Friedman, A. The basic science of natural ingredients. J. Drugs Derm. 2014, 13, 937–943. [Google Scholar]
- Hoffman, J.B.; Hennig, B. Protective influence of healthful nutrition on mechanisms of environmental pollutant toxicity and disease risks. Ann. N. Y. Acad. Sci. 2017, 1398, 99–107. [Google Scholar] [CrossRef]
- Alok, S.; Jain, S.K.; Verma, A.; Kumar, M.; Mahor, A.; Sabharwal, M. Herbal antioxidant in clinical practice: A review. Asian Pac. J. Trop. Biomed. 2014, 4, 78–84. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Antioxidant potential of green and black tea determined using the ferric reducing power (FRAP) assay. Int. J. Food Sci. Nutr. 2000, 51, 181–188. [Google Scholar] [CrossRef]
- Luczaj, W.; Skrzydlewska, E. Antioxidative properties of black tea. Prev. Med. 2005, 40, 910–918. [Google Scholar] [CrossRef] [PubMed]
- McKay, D.L.; Blumberg, J.B. The Role of Tea in Human Health: An Update. J. Am. Coll. Nutr. 2002, 21, 1–13. [Google Scholar] [CrossRef]
- Lorenz, M. Cellular targets for the beneficial actions of tea polyphenols. Am. J. Clin. Nutr. 2013, 98 (Suppl. 6), 1642S–1650S. [Google Scholar] [CrossRef] [PubMed]
- Tenore, G.C.; Daglia, M.; Ciampaglia, R.; Novellino, E. Exploring the Nutraceutical Potential of Polyphenols from Black, Green and White Tea Infusions—An Overview. Curr. Pharm. Biotechnol. 2015, 16, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Deka, A.; Vita, J.A. Tea and cardiovascular disease. Pharmacol. Res. 2011, 64, 136–145. [Google Scholar] [CrossRef]
- Yuan, J.-M. Cancer prevention by green tea: Evidence from epidemiologic studies. Am. J. Clin. Nutr. 2013, 98 (Suppl. 6), 1676S–1681S. [Google Scholar] [CrossRef] [PubMed]
- Martins, N.; Barros, L.; Santos-Buelga, C.; Henriques, M.; Silva, S.; Ferreira, I.C. Decoction, infusion and hydroalcoholic extract of Origanum vulgare L.: Different performances regarding bioactivity and phenolic compounds. Food Chem. 2014, 158, 73–80. [Google Scholar] [CrossRef]
- Saija, A.; Scalese, M.; Lanza, M.; Marzullo, D.; Bonina, F.; Castelli, F. Flavonoids as antioxidant agents: Importance of their interaction with biomembranes. Free Radic. Biol. Med. 1995, 19, 481–486. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines (Basel) 2018, 5, 93. [Google Scholar] [CrossRef]
- De Oliveira, N.K.S.; Almeida, M.R.S.; Pontes, F.M.M.; Barcelos, M.P.; Silva, C.H.T.D.P.D.; Rosa, J.M.C.; Cruz, R.A.S.; Hage-Melim, L.I.D.S. Antioxidant Effect of Flavonoids Present in Euterpe oleracea Martius and Neurodegenerative Diseases: A Literature Review. Cent. Nerv. Syst. Agents Med. Chem. 2019, 19, 75–99. [Google Scholar] [CrossRef] [PubMed]
- Šmejkal, K.; Malaník, M.; Zhaparkulova, K.; Sakipova, Z.; Ibragimova, L.; Ibadullaeva, G.; Žemlička, M. Kazakh Ziziphora Species as Sources of Bioactive Substances. Molecules 2016, 21, 826. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Y. Studies on the Flavonoids of Dracocephalum integrifolium Bge. J. Integr. Plant Biol. 1980, 22, 266–269. [Google Scholar]
- Bodalska, A.; Kowalczyk, A.; Włodarczyk, M.; Fecka, I. Analysis of Polyphenolic Composition of a Herbal Medicinal Product—Peppermint Tincture. Molecules 2019, 25, 69. [Google Scholar] [CrossRef]
- Duletic-Lauševic, S.; Alimpic, A.; Pavlovic, D.; Marin, P.D.; Lakušic, D. Salvia Officinalis of different origins antioxidant activity, phenolic and flavonoid content of extracts. Agro Food Ind. Hi Tech. 2016, 27, 52–55. [Google Scholar]
- Marchioni, I.; Najar, B.; Ruffoni, B.; Copetta, A.; Pistelli, L.; Pistelli, L. Bioactive Compounds and Aroma Profile of Some Lamiaceae Edible Flowers. Plants 2020, 9, 691. [Google Scholar] [CrossRef]
- Aralbaeva, A.N.; Mamataeva, A.T.; Zhaparkulova, N.I.; Utegalieva, R.S.; Khanin, M.; Danilenko, M.; Murzakhmetova, M.K. A composition of medicinal plants with an enhanced ability to suppress microsomal lipid peroxidation and a protective activity against carbon tetrachloride-induced hepatotoxicity. Biomed. Pharmacother. 2017, 96, 1283–1291. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, A.; Mira, M.L.; Azevedo, M.S.; Manso, C. Mechanisms of hemolysis induced by copper. Free Radic. Res. Commun. 1988, 4, 291–298. [Google Scholar] [CrossRef]
- Gallucci, M.T.; Lubrano, R.; Meloni, C.; Morosetti, M.; Manca di Villahermosa, S.; Scoppi, P.; Palombo, G.; Castello, M.A.; Casciani, C.U. (1999) Red blood cell membrane lipid peroxidation and resistance to erythropoietin therapy in hemodialysis patients. Clin. Nephrol. 1999, 52, 239–245. [Google Scholar] [PubMed]
- Sivonová, M.; Waczulíková, I.; Kilanczyk, E.; Hrnciarová, M.; Bryszewska, M.; Klajnert, B.; Duracková, Z. The effect of Pycnogenol on the erythrocyte membrane fluidity. Gen. Physiol. Biophys. 2004, 23, 39–51. [Google Scholar] [PubMed]
- Pan, M.-H.; Lai, C.-S.; Wang, H.; Lo, C.-Y.; Ho, C.-T.; Li, S. Black tea in chemo-prevention of cancer and other human diseases. Food Sci. Hum. Wellness 2013, 2, 12–21. [Google Scholar] [CrossRef]

| Species | Total Polyphenols (μg GAE /mg) | Total Flavonoids (μg RE/mg) | Lipid Peroxidation IC50 (µg/mg protein) | Membrane-Stabilizing Properties IC50 (µg/mL of RBC) |
|---|---|---|---|---|
| Origanum vulgare * | 374.5 ± 15.2 | 325.2 ± 23.3 | 5.5 ± 0.9 | 75.1 ± 8.1 |
| Ziziphora bungeana ** | 401.5 ± 25.6 | 336.3 ± 42.1 | 11.5 ± 2.5 | 194 ± 11.6 |
| Dracocephalum integrifolium * | 299.4 ± 13.2 | 157.5 ± 15.3 | 7.1 ± 1.5 | 73.5 ± 6.8 |
| Mentha piperita *** | 137.5 ± 10.2 | 82.3 ± 7.6 | 5.8 ± 0.8 | >200 |
| Leonurus turkestanicus *** | 305.2 ± 25.3 | 285.1 ± 10.2 | - | >200 |
| Thymus serpyllum * | 264.8 ± 9.6 | 142.3 ± 15.2 | 3.3 ± 0.7 | 194 ± 6.5 |
| Salvia officinalis * | 251.5 ± 16.8 | 118.2 ± 8.7 | 9.2 ± 2.8 | 75.8 ± 4.8 |
| Tea bush—Camellia sinensis (green tea) | 168.7 ± 8.5 | 28.7 ± 5.8 | 9.7 ± 3.1 | 114.3 ± 9.5 |
| Tea bush—Camellia sinensis (black tea) | 115.3 ± 8.9 | 18.8 ± 305 | 14.8 ± 4.5 | >200 |
| Species | Extract Concentration (μg Dry Substance/mL ES) | ||||
|---|---|---|---|---|---|
| 0 | 25 | 50 | 100 | 200 | |
| Origanum vulgare * | 100 | 82.9 ± 3.4 | 65.7 ± 3.0 | 40.9 ± 4.8 | 33.4 ± 2.0 |
| Ziziphora bungeana ** | 100 | 94.5 ± 4.1 | 79.8 ± 3.5 | 66.8 ± 3.6 | 57.3 ± 6.3 |
| Dracocephalum integrifolium * | 100 | 95.2 ± 2.1 | 89.4 ± 4.5 | 71.2 ± 4.9 | 67.3 ± 4.9 |
| Mentha piperita *** | 100 | 99.5 ± 2.2 | 97.9 ± 6.2 | 89.2 ± 4.5 | 65.4 ± 3.2 |
| Leonurus turkestanicus *** | 100 | 105.6 ± 6.8 | 101.3 ± 5.6 | 96.0 ± 5.7 | 84.9 ± 6.9 |
| Thymus serpyllum * | 100 | 74.4 ± 7.6 | 58.6 ± 3.9 | 57.0 ± 5.7 | 50.1 ± 2.9 |
| Salvia officinalis * | 100 | 64.3 ± 3.5 | 61.7 ± 4.9 | 42.4 ± 8.4 | 34.6 ± 2.3 |
| Green tea * | 100 | 68.8 ± 5.6 | 60.2 ± 6.2 | 52.1 ± 2.3 | 45.4 ± 2.7 |
| Black tea * | 100 | 87.5 ± 6.5 | 78.7 ± 6.8 | 64.1 ± 3.4 | 53.9 ± 5.5 |
| Species by Common Name | Extract Concentration (μg Dry Substance/mg Protein) | |||||
|---|---|---|---|---|---|---|
| 0 | 2 | 5 | 10 | 15 | 20 | |
| Oregano ** | 100 | 77.3 ± 9.6 | 44.5 ± 4.5 | 39.0 ± 7.2 | 20.3 ± 6.5 | 12.4 ± 2.9 |
| Ziziphora bungeana *** | 100 | 84.8 ± 3.9 | 66.9 ± 5.9 | 53.9 ± 3.5 | 39.1 ± 1.6 | 25.8 ± 5.3 |
| Dragonhead *** | 100 | 91.5 ± 3.2 | 65.1 ± 4.9 | 49.4 ± 2.5 | 34.5 ± 2.9 | 20.3 ± 4.3 |
| Peppermint ** | 100 | 78.7 ± 7.3 | 62.2 ± 3.6 | 40.3 ± 4.9 | 24.4 ± 3.2 | 16.1 ± 3.9 |
| Motherwort | 100 | 124.3 ± 3.3 | 117.0 ± 4.8 | 112.3 ± 7.6 | 110.1 ± 6.4 | 106.3 ± 11.3 |
| Thyme * | 100 | 68.4 ± 3.1 | 26.6 ± 3.7 | 21.6 ± 4.4 | 8.8 ± 2.9 | 4.0 ± 1.3 |
| Sage ** | 100 | 84.6 ± 4.4 | 52.2 ± 5.6 | 29.3 ± 6.2 | 14.1 ± 3.7 | 8.5 ± 2.5 |
| Green tea ** | 100 | 90.8 ± 6.7 | 77.3 ± 7.0 | 48.2 ± 7.6 | 35.9 ± 5.9 | 30.9 ± 6.0 |
| Black tea *** | 100 | 95.1 ± 5.2 | 82.5 ± 6.8 | 65.3 ± 6.4 | 48.8 ± 6.9 | 42.8 ± 5.4 |
| No. | Sample | IC50 (µg/mg Protein, Mean + SD) | ||
|---|---|---|---|---|
| Individual Extract Mean | In Combination with Black Tea | In Combination with Green Tea | ||
| 1 | Origanum vulgare | 5.5 ± 0.9 | 8.9 ± 3.5 | 6.0 ± 0.8 |
| 2 | Thymus serpyllum | 3.3 ± 0.7 | 2.75 ± 0.4 | 4.3 ± 1.1 |
| 3 | Salvia officinalis | 9.2 ± 2.8 | 8.2 ± 2.1 | 7.3 ± 1.6 |
| 4 | Mentha piperita | 5.8 ± 0.8 | 7.3 ± 1.8 | 8.5 ± 2.3 |
| 5 | Ziziphora bungeana, | 11.5 ± 2.5 | 7.5 ± 0.9 | 9.1 ± 3.1 |
| 6 | Dracocephalum integrifolium | 7.1 ± 1.5 | 10.3 ± 3.5 | 9.3 ± 3.8 |
| 7 | Green tea | 9.7 ± 3.1 | - | - |
| 8 | Black tea | 14.8 ± 4.5 | - | - |
| No. | Sample | IC50 (µg/mL of RBC, mean + SD) | ||
|---|---|---|---|---|
| Individual Extract Mean | In Combination with Black Tea | In Combination with Green Tea | ||
| 1 | Origanum vulgare | 75.1 ± 8.1 | 113.0 ± 8.5 | 79.2 ± 6.8 |
| 2 | Thymus serpyllum | 194 ± 6.5 | 178.1 ± 9.8 | 143.0 ± 7.9 |
| 3 | Salvia officinalis | 75.8 ± 4.8 | 70.0 ± 4.8 | 65.8 ± 5.6 |
| 4 | Mentha piperita | >200 | >200 | 118.0 ± 12.3 |
| 5 | Ziziphora bungeana, | 194 ± 11.6 | 176.1 ± 8.5 | 161.0 ± 9.8 |
| 6 | Dracocephalum integrifolium | >200 | > 200 | 173.4 ± 11.5 |
| 7 | Green tea | 114.3 ± 9.5 | - | - |
| 8 | Black tea | >200 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ydyrys, A.; Zhaparkulova, N.; Aralbaeva, A.; Mamataeva, A.; Seilkhan, A.; Syraiyl, S.; Murzakhmetova, M. Systematic Analysis of Combined Antioxidant and Membrane-Stabilizing Properties of Several Lamiaceae Family Kazakhstani Plants for Potential Production of Tea Beverages. Plants 2021, 10, 666. https://doi.org/10.3390/plants10040666
Ydyrys A, Zhaparkulova N, Aralbaeva A, Mamataeva A, Seilkhan A, Syraiyl S, Murzakhmetova M. Systematic Analysis of Combined Antioxidant and Membrane-Stabilizing Properties of Several Lamiaceae Family Kazakhstani Plants for Potential Production of Tea Beverages. Plants. 2021; 10(4):666. https://doi.org/10.3390/plants10040666
Chicago/Turabian StyleYdyrys, Alibek, Nazgul Zhaparkulova, Arailym Aralbaeva, Aigul Mamataeva, Ainur Seilkhan, Sayagul Syraiyl, and Maіra Murzakhmetova. 2021. "Systematic Analysis of Combined Antioxidant and Membrane-Stabilizing Properties of Several Lamiaceae Family Kazakhstani Plants for Potential Production of Tea Beverages" Plants 10, no. 4: 666. https://doi.org/10.3390/plants10040666
APA StyleYdyrys, A., Zhaparkulova, N., Aralbaeva, A., Mamataeva, A., Seilkhan, A., Syraiyl, S., & Murzakhmetova, M. (2021). Systematic Analysis of Combined Antioxidant and Membrane-Stabilizing Properties of Several Lamiaceae Family Kazakhstani Plants for Potential Production of Tea Beverages. Plants, 10(4), 666. https://doi.org/10.3390/plants10040666





