Terrestrial Morphotypes of Aquatic Plants Display Improved Seed Germination to Deal with Dry or Low-Rainfall Periods
Abstract
:1. Introduction
2. Methods
2.1. Study Area
2.2. Study Species
2.3. Experimental Work
2.4. Simulated Seed Production under Different Pond Inundation Conditions
2.5. Statistical Analyses
3. Results
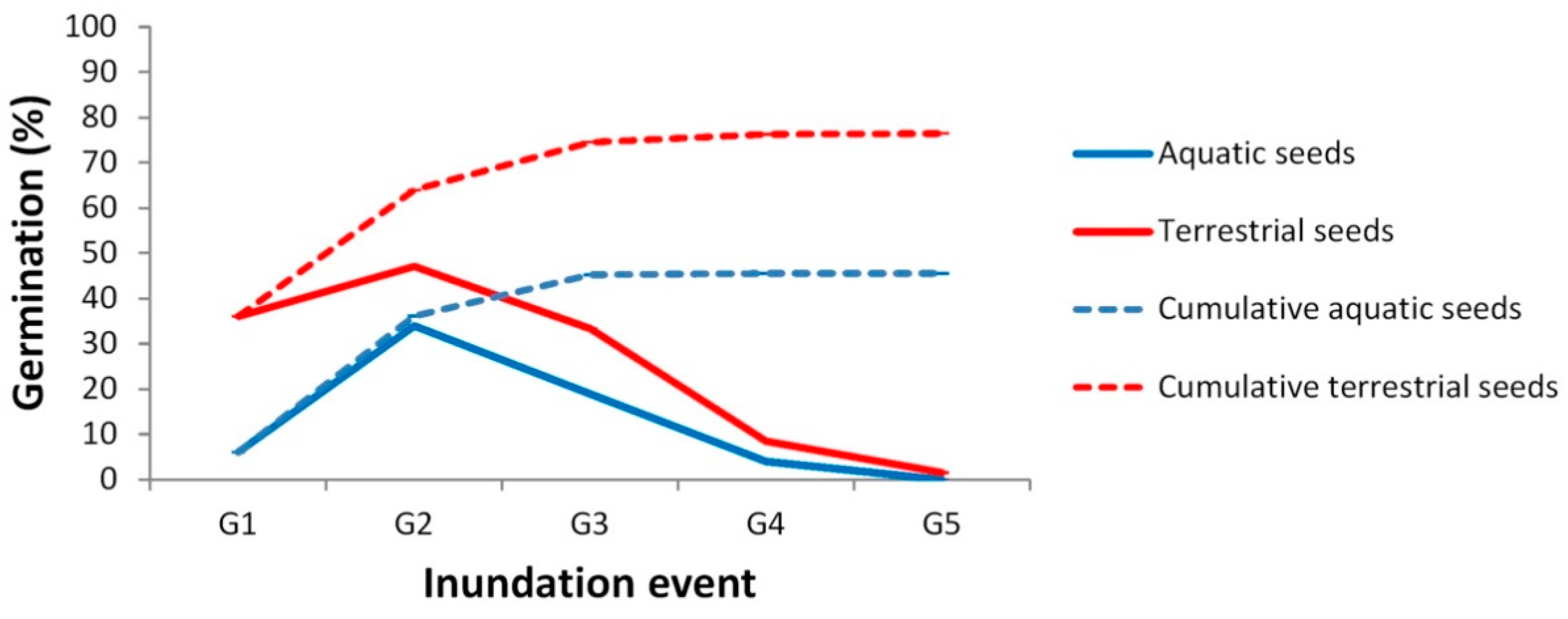
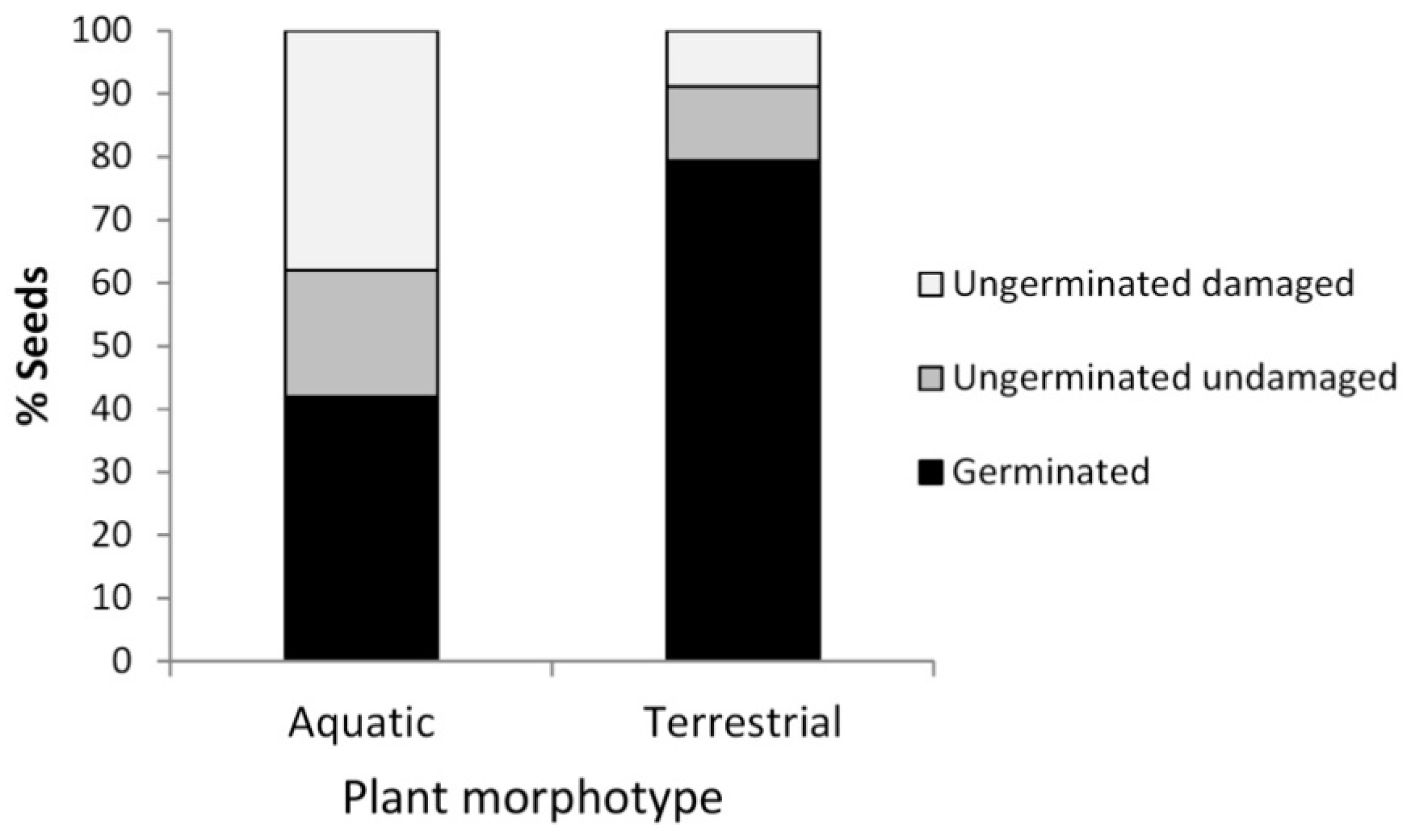
3.1. Germination Patterns of Ranunculus peltatus subsp. saniculifolius
3.2. Germination Patterns in Callitriche brutia
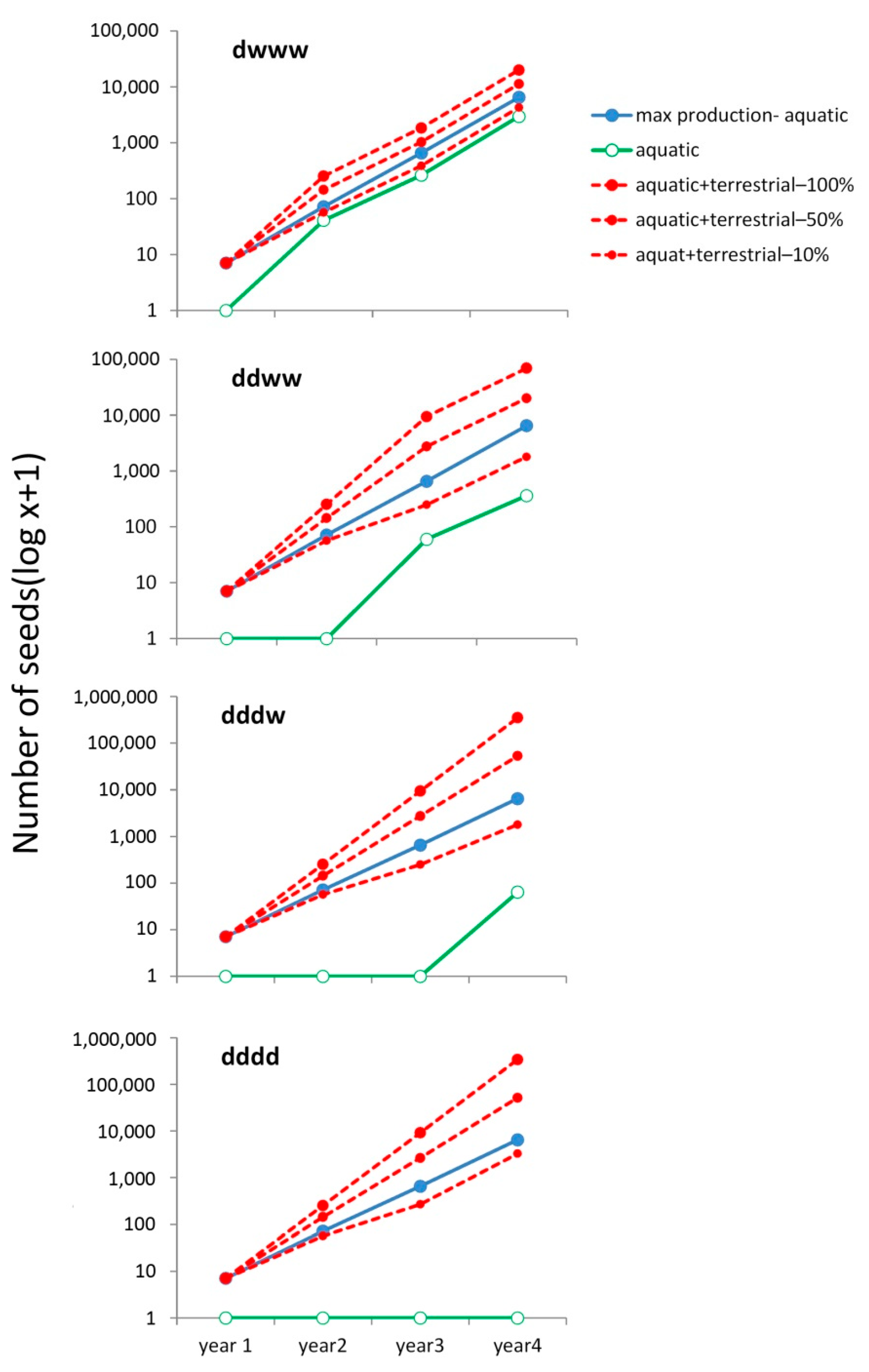
3.3. Simulated Seed Production under Different Pond Inundation Conditions
4. Discussion
4.1. Seed Banks
4.2. Terrestrial Plant Morphotypes
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barrett, S.C.H.; Eckert, C.G.; Husband, B.C. Evolutionary processes in aquatic plant populations. Aquat. Bot. 1993, 44, 105–145. [Google Scholar] [CrossRef]
- Schulthorpe, C.D. The Biology of Aquatic Vascular Plants; Edward Arnold Ltd.: London, UK, 1967. [Google Scholar]
- Cronk, J.K. , Fennessy, M.S. Wetland Plants: Biology and Ecology; CRC Press: Boca Raton, FL, USA, 2001. [Google Scholar]
- Díaz-Paniagua, C.; Fernández-Zamudio, R.; Florencio, M.; García-Murillo, P.; Gómez-Rodríguez, C.; Portheault, A.; Serrano, L.; Siljeström, P. Temporary ponds from Doñana National Park: A system of natural habitats for the preservation of aquatic flora and fauna. Limnetica 2010, 29, 41–58. [Google Scholar]
- Díaz-Paniagua, C.; Fernandez-Zamudio, R.; Serrano, L.; Florencio, M.; Gómez-Rodríguez, C.; Sousa, A.; Sánchez Castillo, P.; García-Murillo, P.; Siljeström, P. El Sistema de Lagunas Temporales de Doñana, una Red de Hábitats Acuáticos Singulares; Organismo Autónomo de Parques Nacionales; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2015. [Google Scholar]
- Bonis, A.; Lepart, J.; Grillas, P. Seed bank dynamics and coexistence of annual macrophytes in a temporary and variable habitat. Oikos 1995, 74, 81–92. [Google Scholar] [CrossRef]
- Brock, M.A.; Nielsen, D.L.; Shiel, S.J.; Green, J.D.; Langley, J.D. Drought and aquatic community resilience: The role of eggs and seeds in sediments of temporary wetlands. Freshw. Biol. 2003, 48, 1207–1218. [Google Scholar] [CrossRef]
- Warwick, N.W.M.; Brock, M.A. Plant reproduction in temporary wetlands: The effects of seasonal timing, depth, and duration of flooding. Aquat. Bot. 2003, 77, 153–167. [Google Scholar] [CrossRef]
- Deil, U. A review on habitats, plant traits and vegetation of ephemeral wetlands—A global perspective. Phytocoenologia 2005, 35, 533–706. [Google Scholar] [CrossRef]
- Aponte, C.; Kazakis, G.; Ghosn, D.; Papanastasis, V.P. Characteristics of the soil seed bank in Mediterranean temporary ponds and its role in ecosystem dynamics. Wetl. Ecol. Manag. 2010, 18, 243–253. [Google Scholar] [CrossRef] [Green Version]
- Brock, M.A. Persistence of seed banks in Australian temporary wetlands. Freshw. Biol. 2011, 56, 1312–1327. [Google Scholar] [CrossRef]
- Carta, A. Seed regeneration in Mediterranean temporary ponds: Germination ecophysiology and vegetation processes. Hydrobiologia 2016, 782, 23–35. [Google Scholar] [CrossRef]
- Cross, A.T.; Turner, S.R.; Renton, M.; Baskin, J.M.; Dixon, K.W.; Merritt, D.J. Seed dormancy and persistent sediment seed banks of ephemeral freshwater rock pools in the Australian monsoon tropics. Ann. Bot. 2015, 115, 847–859. [Google Scholar] [CrossRef] [Green Version]
- Grillas, P.; Garcia-Murillo, P.; Geertz-Hansen, O.; Marbá, N.; Montes, C.; Duarte, C.M.; Tan Ham, L.; Grossmann, A. Submerged macrophyte seed bank in a Mediterranean temporary marsh: Abundance and relationship with established vegetation. Oecologia 1993, 94, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rhazi, L.; Grillas, P.; Tan Ham, L.; El Khyari, D. The seed bank and the between years dynamics of the vegetation of a Mediterranean temporary pool (NW Morocco). Ecol. Mediterr. 2001, 27, 68–88. [Google Scholar] [CrossRef]
- Thompson, K. The functional ecology of seed banks. In Seeds, the Ecology of Regeneration in Plant Communities; Fenner, M., Ed.; CAB International: Wallingford, UK, 1992; pp. 231–258. [Google Scholar]
- Volder, A.; Bonis, A.; Grillas, P. Effects of drought and flooding on the reproduction of an amphibious plant, Ranunculus peltatus. Aquat. Bot. 1997, 58, 113–120. [Google Scholar] [CrossRef]
- Brock, M.A.; Casanova, M.T. Plant life at the edges of wetlands: Ecological responses to wetting and drying patterns. In Frontiers in Ecology: Building the Links; Klomp, N., Lunt, I., Eds.; Elsevier Science: Oxford, UK, 1997; pp. 181–192. [Google Scholar]
- Brock, M.A. Mechanisms for maintaining persistent populations of Myriophyllum variifolium in a fluctuating Australian shallow lake. Aquat. Bot. 1991, 39, 211–219. [Google Scholar] [CrossRef]
- Cox, J. Terrestrial form of Myriophyllum alterniflorum. Bot. Soc. Brit. Isles News 1997, 77, 36–37. [Google Scholar]
- Ritter, N.P.; Crow, G.E. Myriophyllum quitense Kunth (Haloragaceae) in Bolivia: A terrestrial growth-form with bisexual flowers. Aquat. Bot. 1998, 60, 389–395. [Google Scholar] [CrossRef]
- Lansdown, R.V. A terrestrial form of Callitriche truncata Guss. subsp. occidentalis (ROUY) BRAUN-BLANQUET (Callitrichaceae). Watsonia 1999, 22, 283–286. [Google Scholar]
- Leck, M.A.; Brock, M.A. Ecological and evolutionary trends in wetlands: Evidence from seeds and seed banks in New South Wales, Australia and New Jersey, USA. Plant Species Biol. 2000, 15, 97–112. [Google Scholar] [CrossRef]
- Kaplan, Z. Phenotypic plasticity in Potamogeton (Potamogetonaceae). Folia Geobot. 2002, 37, 141–170. [Google Scholar] [CrossRef]
- Fernández-Zamudio, R.; García-Murillo, P.; Díaz-Paniagua, C. Physical and chemical features and water permanence determine aquatic plant distribution in a temporary pond network (Doñana National Park). Hydrobiologia 2016, 774, 123–135. [Google Scholar] [CrossRef]
- García-Murillo, P.; Fernández-Zamudio, R. Las plantas de las lagunas temporales de Doñana. In El Sistema de Lagunas Temporales de Doñana, una Red de Hábitats Acuáticos Singulares; Díaz-Paniagua, C., Coord, Eds.; Organismo Autónomo de Parques Nacionales; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2015. [Google Scholar]
- Cook, C.D.K. A monographic study of Ranunculus subgenus Batrachium (DC.) A.Gray. Mitt. Bot. Staatssummlung Mȕnchen 1966, 6, 37–47. [Google Scholar]
- Preston, C.D.; Croft, J.M. Aquatic Plants in Britain and Ireland; Harley Books; Colchester, UK, 1997. [Google Scholar]
- Lansdown, R.V. Water-starworts (Callitriche) of Europe; BSBI Handbook No 11; Botanical Society of the British Isles: London, UK, 2008. [Google Scholar]
- García-Murillo, P. Callitriche. In Flora Iberica; Castroviejo, S., Morales, R., Quintanar, A., Cabezas, F., Pujadas, A.J., Cirujano, S., Eds.; Real Jardín Botánico CSIC: Madrid, Spain, 2010; Volume XII, pp. 497–513. [Google Scholar]
- Wiegleb, G.; Bobrov, A.A.; Zalewska-Gałosz, J. A taxonomic account of Ranunculus section Batrachium (Ranunculaceae). Phytotaxa 2017, 319, 1–55. [Google Scholar] [CrossRef]
- Cirujano, S.; Meco, A.; García-Murillo, P. Flora Acuática Española. Hidrófitos Vasculares; Real Jardín Botánico CSIC: Madrid, Spain, 2014. [Google Scholar]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and Evolution of Dormancy and Germination; Academic Press: San Diego, CA, USA, 1998. [Google Scholar]
- Velayos, M. Acotaciones a Ranunculus subgénero Batrachium (DC) A. Gray: Tratamiento taxonómico general y estudio de la variabilidad de R. peltatus. An. Jard. Bot. Madr. 1988, 45, 103–119. [Google Scholar]
- Garbey, C.; Thiébaut, G.; Muller, S. Morphological plasticity of a spreading aquatic macrophyte, Ranunculus peltatus, in response to environmental variables. Plant Ecol. 2004, 173, 125–137. [Google Scholar] [CrossRef]
- Porter, R.; Durrell, M.; Romm, H. The use of 2,3,5-triphenyl-tetrazolium chlorid as a measure of seed germinability. Plant Physiol. 1947, 22, 149. [Google Scholar] [CrossRef] [Green Version]
- R Core Development Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. [Google Scholar]
- Venable, D.L.; Lawlor, L. Delayed germination and dispersal in desert annuals—Escape in space and time. Oecologia 1980, 46, 272–282. [Google Scholar] [CrossRef]
- Gremer, J.R.; Venable, D.L. Bet hedging in desert winter annual plants: Optimal germination strategies in a variable environment. Ecol. Let. 2014, 17, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Zamudio, R.; García-Murillo, P.; Díaz-Paniagua, C. Effect of the filling season on aquatic plants in Mediterraneantemporary ponds. J. Plant Ecol. 2018, 11, 502–510. [Google Scholar] [CrossRef] [Green Version]


| Species | Inundation Event | Seed Storage Conditions | Start Date | End Date |
|---|---|---|---|---|
| Ranunculus peltatus subsp. saniculifolius | G1 | Dry, room temperature (20–26 °C) | 04/12/2012 | 21/01/2013 |
| G2 | Dry, 4 °C | 16/05/2013 | 25/06/2013 | |
| G3 | Dry, room temperature (20–26 °C) | 08/01/2014 | 19/02/2014 | |
| G4 | Dry, 4 °C | 11/11/2014 | 12/12/2014 | |
| G5 | Dry, 10 °C | 08/05/2015 | 22/06/2015 | |
| Callitriche brutia | G1 | Dry, room temperature (20–26 °C) | 23/01/2013 | 13/03/2013 |
| Inundation Event | Plant Morphotype | Mean | SE | Min | Max | N |
|---|---|---|---|---|---|---|
| G1 | Aquatic | 14.18 | 1.71 | 8 | 40 | 22 |
| Terrestrial | 12.44 | 0.50 | 8 | 37 | 156 | |
| G2 | Aquatic | 6.45 | 0.28 | 5 | 18 | 82 |
| Terrestrial | 7.90 | 0.48 | 5 | 25 | 91 | |
| G3 | Aquatic | 10.69 | 0.87 | 9 | 19 | 13 |
| Terrestrial | 10.21 | 0.69 | 9 | 23 | 28 | |
| G4 | Aquatic | 14.0 | - | - | - | 1 |
| Terrestrial | 14.25 | 2.56 | 7 | 31 | 8 | |
| G5 | Aquatic | - | - | - | - | 0 |
| Terrestrial | 14.0 | 1 | 1 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Zamudio, R.; García-Murillo, P.; Díaz-Paniagua, C. Terrestrial Morphotypes of Aquatic Plants Display Improved Seed Germination to Deal with Dry or Low-Rainfall Periods. Plants 2021, 10, 741. https://doi.org/10.3390/plants10040741
Fernández-Zamudio R, García-Murillo P, Díaz-Paniagua C. Terrestrial Morphotypes of Aquatic Plants Display Improved Seed Germination to Deal with Dry or Low-Rainfall Periods. Plants. 2021; 10(4):741. https://doi.org/10.3390/plants10040741
Chicago/Turabian StyleFernández-Zamudio, Rocío, Pablo García-Murillo, and Carmen Díaz-Paniagua. 2021. "Terrestrial Morphotypes of Aquatic Plants Display Improved Seed Germination to Deal with Dry or Low-Rainfall Periods" Plants 10, no. 4: 741. https://doi.org/10.3390/plants10040741






