Harnessing the Rhizosphere of the Halophyte Grass Aeluropus littoralis for Halophilic Plant-Growth-Promoting Fungi and Evaluation of Their Biostimulant Activities
Abstract
:1. Introduction
2. Results
2.1. Characterization and Identification of Isolated Fungi
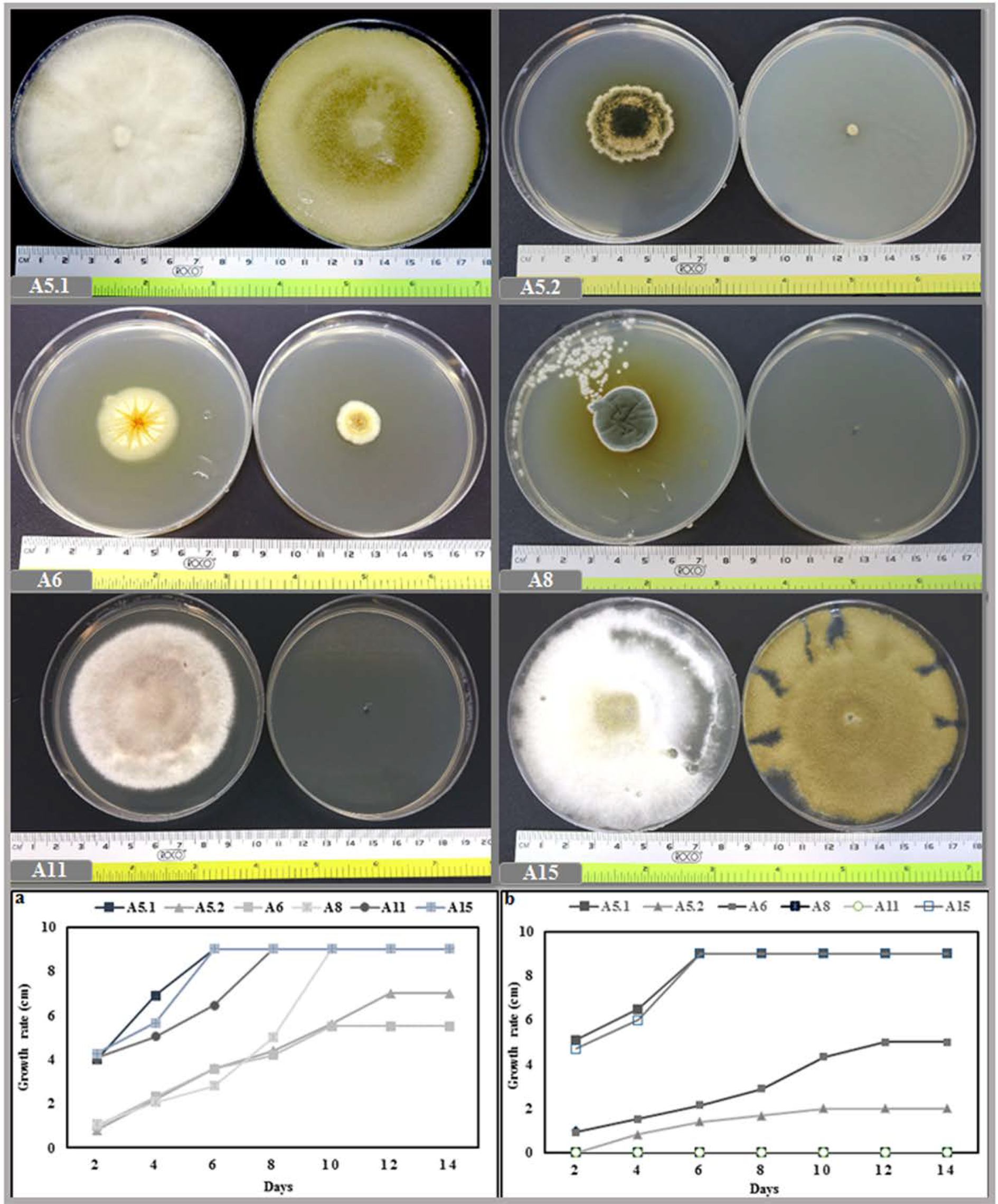
2.1.1. Microscopic and Molecular Characterization
2.1.2. Effect of Temperature and NaCl on Growth
2.2. Evaluation of Fungi on Plant Growth Promoting Activity
2.2.1. Evaluation of the Fungi Effect
2.2.2. Evaluation of the CFFs Effect
2.3. Presence of Auxin in Culture Filtrates
2.4. Effect of Fungal Culture Filtrates on the Expression Profiles of Growth Related Genes
3. Discussion
4. Materials and Methods
4.1. Isolation of Fungi
4.2. Microscopic and Molecular Characterization
4.3. Effect of Temperature and NaCl on Fungal Growth
4.4. Evaluation of Fungi on Plant Growth Promoting Activity
4.4.1. Evaluation of the Fungi Effect
In Solid MS Medium
In Liquid MS Medium
4.4.2. Evaluation of the CFFs Effect
Preparing the Cell-Free Culture Filtrate (CFF)
Adding the Fungal Cell-Free Culture Filtrates (CFF) to Nutrient Solution
4.5. Total Chlorophyll Estimation
4.6. Estimation of Auxin Concentration in Culture Filtrate
4.7. Analysis of Gene Expression
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Den Herder, G.; Van Isterdael, G.; Beeckman, T.; De Smet, I. The roots of a new green revolution. Trends Plant Sci. 2010, 15, 600–607. [Google Scholar] [CrossRef]
- Tuteja, N.; Gill, S.S. Abiotic Stress Response in Plants; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016. [Google Scholar]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil 2014, 383, 3–41. [Google Scholar] [CrossRef] [Green Version]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Abhilash, P.; Dubey, R.K.; Tripathi, V.; Gupta, V.K.; Singh, H.B. Plant growth-promoting microorganisms for environmental sustainability. Trends Biotechnol. 2016, 34, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Babu, A.G.; Kim, S.W.; Yadav, D.R.; Hyum, U.; Adhikari, M.; Lee, Y.S. Penicillium menonorum: A novel fungus to promote growth and nutrient management in cucumber plants. Mycobiology 2015, 43, 49–56. [Google Scholar] [CrossRef] [Green Version]
- Rahi, P.; Vyas, P.; Sharma, S.; Gulati, A.; Gulati, A. Plant growth promoting potential of the fungus Discosia sp. FIHB 571 from tea rhizosphere tested on chickpea, maize and pea. Indian J. Microbiol. 2009, 49, 128–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, V.; Kumar, M.; Sharma, S.; Prasad, R. Probiotics in Agroecosystem; Springer Nature Singapore Pte Ltd.: Singapore, 2017. [Google Scholar]
- Zhou, L.; Tang, K.; Guo, S. The plant growth-promoting fungus (PGPF) Alternaria sp. A13 markedly enhances Salvia miltiorrhiza root growth and active ingredient accumulation under greenhouse and field conditions. Int. J. Mol. Sci. 2018, 19, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergara, C.; Araujo, K.E.C.; Alves, L.S.; de Souza, S.R.; Santos, L.A.; Santa-Catarina, C.; da Silva, K.; Pereira, G.M.D.; Xavier, G.R.; Zilli, J.É. Contribution of dark septate fungi to the nutrient uptake and growth of rice plants. Braz. J. Microbiol. 2018, 49, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Kim, Y.C. Tobacco Growth Promotion by the Entomopathogenic Fungus, Isaria javanica pf185. Mycobiology 2019, 47, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Rajauria, G.; Sahai, V.; Bisaria, V. Culture filtrate of root endophytic fungus Piriformospora indica promotes the growth and lignan production of Linum album hairy root cultures. Process Biochem. 2012, 47, 901–907. [Google Scholar] [CrossRef]
- Khan, A.R.; Ullah, I.; Waqas, M.; Shahzad, R.; Hong, S.-J.; Park, G.-S.; Jung, B.K.; Lee, I.-J.; Shin, J.-H. Plant growth-promoting potential of endophytic fungi isolated from Solanum nigrum leaves. World J. Microbiol. Biotechnol. 2015, 31, 1461–1466. [Google Scholar] [CrossRef]
- Shimizu, K.; Hossain, M.M.; Kato, K.; Kubota, M.; Hyakumachi, M. Induction of defense responses in cucumber plants by using the cell-free filtrate of the plant growth-promoting fungus Penicillium simplicissimum GP17-2. J. Oleo Sci. 2013, 62, 613–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, A.; Verma, S.; Sahay, N.; Bütehorn, B.; Franken, P. Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl. Environ. Microbiol. 1999, 65, 2741–2744. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- González-Pérez, E.; Ortega-Amaro, M.A.; Salazar-Badillo, F.B.; Bautista, E.; Douterlungne, D.; Jiménez-Bremont, J.F. The Arabidopsis-Trichoderma interaction reveals that the fungal growth medium is an important factor in plant growth induction. Sci. Rep. 2018, 8, 16427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermosa, R.; Viterbo, A.; Chet, I.; Monte, E. Plant-beneficial effects of Trichoderma and of its genes. Microbiology 2012, 158, 17–25. [Google Scholar] [CrossRef] [Green Version]
- Heckman, J. Effect of an organic biostimulant on cabbage yield. J. Home Consum. Hortic. 1993, 1, 111–113. [Google Scholar] [CrossRef]
- Masunaka, A.; Hyakumachi, M.; Takenaka, S. Plant growth-promoting fungus, Trichoderma koningi suppresses isoflavonoid phytoalexin vestitol production for colonization on/in the roots of Lotus japonicus. Microbes Environ. 2009. [Google Scholar] [CrossRef] [Green Version]
- Bent, E. Induced systemic resistance mediated by plant growth-promoting rhizobacteria (PGPR) and fungi (PGPF). In Multigenic and Induced Systemic Resistance in Plants; Tuzun, S., Bent, E., Eds.; Springer: Boston, MA, USA, 2006. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Islam, S. Plant growth-promoting fungi (PGPF): Phytostimulation and induced systemic resistance. In Plant-Microbe Interactions in Agro-Ecological Perspectives; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: New Delhi, India, 2017; pp. 135–191. [Google Scholar]
- Hyakumachi, M. Plant-growth-promoting fungi from turfgrass rhizosphere with potential for disease suppression. Soil Microorg. 1994, 44, 53–68. [Google Scholar]
- Young-Hyun, Y.; Yoon, H.; Kang, S.-M.; Shin, J.-H.; Choo, Y.-S.; Lee, I.-J.; Lee, J.-M.; Kim, J.-G. Fungal Diversity and Plant Growth Promotion of Endophytic Fungi from Six Halophytes in Suncheon Bay. J. Microbiol. Biotechnol. 2012, 22, 1549–1556. [Google Scholar]
- Hossain, M.M.; Sultana, F.; Miyazawa, M.; Hyakumachi, M. The plant growth-promoting fungus Penicillium spp. GP15-1 enhances growth and confers protection against damping-off and anthracnose in the cucumber. J. Oleo Sci. 2014, 63, 391–400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Priyadharsini, P.; Muthukumar, T. The root endophytic fungus Curvularia geniculata from Parthenium hysterophorus roots improves plant growth through phosphate solubilization and phytohormone production. Fungal Ecol. 2017, 27, 69–77. [Google Scholar] [CrossRef]
- Pryce, T.; Palladino, S.; Kay, I.; Coombs, G. Rapid identification of fungi by sequencing the ITS1 and ITS2 regions using an automated capillary electrophoresis system. Med. Mycol. 2003, 41, 369–381. [Google Scholar] [CrossRef]
- Raja, H.A.; Miller, A.N.; Pearce, C.J.; Oberlies, N.H. Fungal identification using molecular tools: A primer for the natural products research community. J. Nat. Prod. 2017, 80, 756–770. [Google Scholar] [CrossRef]
- Samson, R.; Houbraken, J.; Varga, J.; Frisvad, J.C. Polyphasic taxonomy of the heat resistant ascomycete genus Byssochlamys and its Paecilomyces anamorphs. Persoonia 2009, 22, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houbraken, J.; Varga, J.; Rico-Munoz, E.; Johnson, S.; Samson, R.A. Sexual reproduction as the cause of heat resistance in the food spoilage fungus Byssochlamys spectabilis (anamorph Paecilomyces variotii). Appl. Environ. Microbiol. 2008, 74, 1613–1619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 2014, 3, 641. [Google Scholar] [CrossRef] [Green Version]
- Hasan, H. Gibberellin and auxin production by plant root-fungi and their biosynthesis under salinity-calcium interaction. Acta Microbiol Immunol Hung. 2002, 49, 105–118. [Google Scholar] [CrossRef]
- Khan, A.L.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Jung, H.-Y.; Lee, J.-H.; Lee, I.-J. Endophytic fungal association via gibberellins and indole acetic acid can improve plant growth under abiotic stress: An example of Paecilomyces formosus LHL10. BMC Microbiol. 2012, 12, 3. [Google Scholar] [CrossRef] [Green Version]
- Karnwal, A. Isolation and identification of plant growth promoting rhizobacteria from maize (Zea mays L.) rhizosphere and their plant growth promoting effect on rice (Oryza sativa L.). J. Plant Prot. Res. 2017, 57. [Google Scholar] [CrossRef]
- Shah, S.; Shrestha, R.; Maharjan, S.; Selosse, M.-A.; Pant, B. Isolation and characterization of plant growth-promoting endophytic fungi from the roots of Dendrobium moniliforme. Plants 2019, 8, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santamaria, O.; Lledó, S.; Rodrigo, S.; Poblaciones, M.J. Effect of fungal endophytes on biomass yield, nutritive value and accumulation of minerals in Ornithopus compressus. Microb. Ecol. 2017, 74, 841–852. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Gavíra, A.; Diánez, F.; Sánchez-Montesinos, B.; Santos, M. Paecilomyces variotii as A Plant-Growth Promoter in Horticulture. Agronomy 2020, 10, 597. [Google Scholar] [CrossRef]
- Zhai, X.; Luo, D.; Li, X.; Han, T.; Jia, M.; Kong, Z.; Ji, J.; Rahman, K.; Qin, L.; Zheng, C. Endophyte Chaetomium globosum D38 promotes bioactive constituents accumulation and root production in Salvia miltiorrhiza. Front. Microbiol. 2018, 8, 2694. [Google Scholar] [CrossRef] [PubMed]
- Hamayun, M.; Khan, S.A.; Khan, A.L.; Afzal, M.; Lee, I.-J. Endophytic Cephalotheca sulfurea AGH07 reprograms soybean to higher growth. J. Plant Interact. 2012, 7, 301–306. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Kang, S.-M.; Baek, I.-Y.; Lee, I.-J. Characterization of plant growth-promoting traits of Penicillium species against the effects of high soil salinity and root disease. J. Plant Interact. 2014, 9, 754–762. [Google Scholar] [CrossRef]
- Gómez-Muñoz, B.; Jensen, L.; De Neergaard, A.; Richardson, A.; Magid, J. Effects of Penicillium bilaii on maize growth are mediated by available phosphorus. Plant Soil 2018, 431, 159–173. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, Y.; Wang, L.; Wang, J.; Zhang, C. Bioactive secondary metabolites from Nigrospora sp. LLGLM003, an endophytic fungus of the medicinal plant Moringa oleifera Lam. World J. Microbiol. Biotechnol. 2012, 28, 2107–2112. [Google Scholar] [CrossRef]
- Mmbaga, M.; Gurung, M.A.; Maheshwari, A. Screening of plant endophytes as biological control agents against root rot pathogens of pepper (Capsicum annum L.). J. Plant Pathol. Microbiol. 2018, 9, 2–8. [Google Scholar]
- Saporta, R.; Bou, C.; Frías, V.; Mulet, J.M. A Method for a fast evaluation of the biostimulant potential of different natural extracts for promoting growth or tolerance against abiotic stress. Agronomy 2019, 9, 143. [Google Scholar] [CrossRef] [Green Version]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma species—Opportunistic, avirulent plant symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Naznin, H.A.; Kimura, M.; Miyazawa, M.; Hyakumachi, M. Analysis of volatile organic compounds emitted by plant growth-promoting fungus Phoma sp. GS8-3 for growth promotion effects on tobacco. Microbes Environ. 2013, 28, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakelin, S.; Gupta, V.V.; Harvey, P.; Ryder, M. The effect of Penicillium fungi on plant growth and phosphorus mobilization in neutral to alkaline soils from southern Australia. Can. J. Microbiol. 2007, 53, 106–115. [Google Scholar] [CrossRef] [PubMed]
- Baishya, D.; Deka, P.; Kalita, M.C. In vitro co-cultivation of Piriformospora indica filtrate for improve biomass productivity in Artemisia annua (L.). Symbiosis 2015, 66, 37–46. [Google Scholar] [CrossRef]
- Sherameti, I.; Shahollari, B.; Venus, Y.; Altschmied, L.; Varma, A.; Oelmüller, R. The endophytic fungus Piriformospora indica stimulates the expression of nitrate reductase and the starch-degrading enzyme glucan-water dikinase in tobacco and Arabidopsis roots through a homeodomain transcription factor that binds to a conserved motif in their promoters. J. Biol. Chem. 2005, 280, 26241–26247. [Google Scholar] [PubMed] [Green Version]
- Ortiz, J.; Soto, J.; Fuentes, A.; Herrera, H.; Meneses, C.; Arriagada, C. The endophytic fungus Chaetomium cupreum regulates expression of genes involved in the tolerance to metals and plant growth promotion in Eucalyptus globulus roots. Microorganisms 2019, 7, 490. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Palma, M.; Salzano, M.; Villano, C.; Aversano, R.; Lorito, M.; Ruocco, M.; Docimo, T.; Piccinelli, A.L.; D’Agostino, N.; Tucci, M. Transcriptome reprogramming, epigenetic modifications and alternative splicing orchestrate the tomato root response to the beneficial fungus Trichoderma harzianum. Hortic. Res. 2019, 6, 1–15. [Google Scholar] [CrossRef]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Cortés-Penagos, C.; López-Bucio, J. Trichoderma virens, a plant beneficial fungus, enhances biomass production and promotes lateral root growth through an auxin-dependent mechanism in Arabidopsis. Plant Physiol. 2009, 149, 1579–1592. [Google Scholar] [CrossRef] [Green Version]
- Meents, A.K.; Furch, A.C.; Almeida-Trapp, M.; Özyürek, S.; Scholz, S.; Kirbis, A.; Lenser, T.; Theißen, G.; Grabe, V.; Hansson, B.S. Beneficial and pathogenic Arabidopsis root-interacting fungi differently affect auxin levels and responsive genes during early infection. Front. Microbiol. 2019, 10, 380. [Google Scholar] [CrossRef]
- Souza-Motta, C.M.d.; Cavalcanti, M.A.d.Q.; Fernandes, M.J.d.S.; Lima, D.M.M.; Nascimento, J.P.; Laranjeira, D. Identification and characterization of filamentous fungi isolated from the sunflower (Helianthus annus L.) rhizosphere according to their capacity to hydrolyse inulin. Braz. J. Microbiol. 2003, 34, 273–280. [Google Scholar] [CrossRef]
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Innis, M.A., Gelfand, D.H., Sninsky, J.J., White, T.J., Eds.; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Arnon, D.I. Copper enzymes in isolated chloroplasts. Polyphenoloxidase in Beta vulgaris. Plant Physiol. 1949, 24, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indoleacetic acid. Plant Physiol. 1951, 26, 192. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarroum, M.; Ben Romdhane, W.; Ali, A.A.M.; Al-Qurainy, F.; Al-Doss, A.; Fki, L.; Hassairi, A. Harnessing the Rhizosphere of the Halophyte Grass Aeluropus littoralis for Halophilic Plant-Growth-Promoting Fungi and Evaluation of Their Biostimulant Activities. Plants 2021, 10, 784. https://doi.org/10.3390/plants10040784
Tarroum M, Ben Romdhane W, Ali AAM, Al-Qurainy F, Al-Doss A, Fki L, Hassairi A. Harnessing the Rhizosphere of the Halophyte Grass Aeluropus littoralis for Halophilic Plant-Growth-Promoting Fungi and Evaluation of Their Biostimulant Activities. Plants. 2021; 10(4):784. https://doi.org/10.3390/plants10040784
Chicago/Turabian StyleTarroum, Mohamed, Walid Ben Romdhane, Ahmed Abdelrahim Mohamed Ali, Fahad Al-Qurainy, Abdullah Al-Doss, Lotfi Fki, and Afif Hassairi. 2021. "Harnessing the Rhizosphere of the Halophyte Grass Aeluropus littoralis for Halophilic Plant-Growth-Promoting Fungi and Evaluation of Their Biostimulant Activities" Plants 10, no. 4: 784. https://doi.org/10.3390/plants10040784
APA StyleTarroum, M., Ben Romdhane, W., Ali, A. A. M., Al-Qurainy, F., Al-Doss, A., Fki, L., & Hassairi, A. (2021). Harnessing the Rhizosphere of the Halophyte Grass Aeluropus littoralis for Halophilic Plant-Growth-Promoting Fungi and Evaluation of Their Biostimulant Activities. Plants, 10(4), 784. https://doi.org/10.3390/plants10040784






