Intraspecific Variation in Nickel Tolerance and Hyperaccumulation among Serpentine and Limestone Populations of Odontarrhena serpyllifolia (Brassicaceae: Alysseae) from the Iberian Peninsula
Abstract
:1. Introduction
2. Results
2.1. Characteristics of Field Sites and Populations
2.2. Growth Differences among Accessions in Low-Nickel Solution
2.3. Variation in Nickel Tolerance
2.4. Variation in Nickel Hyperaccumulation
2.5. Effects of Ni on Concentrations of Other Cations—Univariate Analyses
2.6. Effects of Ni on Concentrations of Other Cations—Multivariate Analysis
3. Discussion
4. Materials and Methods
4.1. Sample Collection
4.2. Germination and Hydroponic Cultivation
4.3. Sample Preparation and Analysis
4.4. Data Analysis
5. Conclusions
- Accessions of Odontarrhena serpyllifolia from serpentine soil were substantially more Ni-tolerant than those from limestone soil; however, intermediate degrees of tolerance exist on both soil types, suggesting a spectrum of continuous variation in this character.
- All accessions of O. serpyllifolia, whether from serpentine or limestone, were capable of hyperaccumulating Ni in hydroponic cultivation. There was variation among accessions in ability to hyperaccumulate, but once again, it was continuous rather than discrete.
- Hyperaccumulators are best defined based on metal concentrations in natural self-sustaining populations. In laboratory studies, we propose that physiological ability to hyperaccumulate can be tentatively identified based on foliar metal concentrations exceeding a threshold criterion in plants cultivated in substrates with metal availability at or below the maximum No Observed Effect Concentration (mNOEC), thus providing a safeguard against artifactual “breakthrough” of metals in plants experiencing metal toxicity.
- Accessions of O. serpyllifolia from serpentine substrates maintained relatively constant concentrations of essential nutrient elements in their tissues when grown in a range of Ni concentrations, whereas those from limestone substrates showed an inability to maintain elemental homeostasis when grown in elevated Ni.
- Clypeola jonthlaspi, closely phylogenetically related to Odontarrhena, showed some physiological characteristics of hyperaccumulators, although it has not been observed to hyperaccumulate Ni in nature. Further studies of this species are needed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Hyperaccumulators of metal and metalloid trace elements: Facts and fiction. Plant Soil 2013, 362, 319–334. [Google Scholar] [CrossRef]
- Reeves, R.D.; Baker, A.J.M.; Jaffré, T.; Erskine, P.; Echevarria, G.; van der Ent, A. A global database of hyperaccumulator plants for metal and metalloid trace elements. New Phytol. 2017, 218, 407–411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Global Hyperaccumulator Database. Available online: http://hyperaccumulators.smi.uq.edu.au/collection/ (accessed on 20 February 2021).
- Brooks, R.R. Serpentine and Its Vegetation: A Multidisciplinary Approach; Dioscorides Press: Portland, OR, USA, 1987; ISBN 978-0931146046. [Google Scholar]
- Coleman, R.G.; Jove, C. Geological origin of serpentinites. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Limited: Andover, UK, 1992; pp. 1–17. ISBN 978-0946707621. [Google Scholar]
- Moores, E.M. Serpentinites and other ultramafic rocks. In Serpentine: The Evolution and Ecology of a Model System; Harrison, S.P., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 3–28. ISBN 978-0520268357. [Google Scholar]
- Proctor, J.; Woodell, S.R.J. The ecology of serpentine soils. Adv. Ecol. Res. 1975, 9, 255–366. [Google Scholar]
- Brady, K.U.; Kruckeberg, A.R.; Bradshaw, H.D. Evolutionary ecology of plant adaptation to serpentine soils. Ann. Rev. Ecol. Evol. Syst. 2005, 36, 243–266. [Google Scholar] [CrossRef]
- Kazakou, E.; Dimitrakopoulos, P.G.; Baker, A.J.M.; Reeves, R.D.; Troumbis, A.Y. Hypotheses, mechanisms and trade-offs of tolerance and adaptation to serpentine soils: From species to ecosystem level. Biol. Rev. 2008, 83, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Anacker, B.L.; Whittall, J.B.; Goldberg, E.E.; Harrison, S.P. Origins and consequences of serpentine endemism in the California flora. Evolution 2010, 65, 365–376. [Google Scholar] [CrossRef]
- Pollard, A.J.; Reeves, R.D.; Baker, A.J.M. Facultative hyperaccumulation of metals and metalloids. Plant Sci. 2014, 217–218, 8–17. [Google Scholar] [CrossRef]
- Bothe, H.; Słomke, A. Divergent biology of facultative heavy metal plants. J. Plant Physiol. 2017, 219, 45–61. [Google Scholar] [CrossRef]
- Pollard, A.J.; Powell, K.D.; Harper, F.A.; Smith, J.A.C. The genetic basis of metal hyperaccumulation in plants. Crit. Rev. Plant Sci. 2002, 21, 539–566. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Krämer, U. Metal hyperaccumulation in plants. Ann. Rev. Plant Biol. 2010, 61, 517–534. [Google Scholar] [CrossRef]
- Hanikenne, M.; Nouet, C. Metal hyperaccumulation and hypertolerance: A model for plant evolutionary genomics. Curr. Opin. Plant Biol. 2011, 14, 252–259. [Google Scholar] [CrossRef]
- Wójcik, M.; Gonnelli, C.; Selvi, F.; Dresler, S.; Rostanski, A.; Vangronsveld, J. Metallophytes of serpentine and calamine soils—their unique ecophysiology and potential for phytoremediation. Adv. Bot. Res. 2017, 83, 1–42. [Google Scholar] [CrossRef]
- Deng, T.H.B.; van der Ent, A.; Tang, Y.T.; Sterckeman, T.; Echevarria, G.; Morel, J.L.; Qiu, R.L. Nickel hyperaccumulation mechanisms: A review on the current state of knowledge. Plant Soil 2018, 423, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Van der Pas, L.; Ingle, R.A. Towards an understanding of the molecular basis of nickel hyperaccumulation in plants. Plants 2019, 8, 11. [Google Scholar] [CrossRef] [Green Version]
- Merlot, S.; Garcia de la Torre, V.S.; Hanikenne, M. Physiology and molecular biology of trace element hyperaccumulation. In Agromining: Farming for Metals, 2nd ed.; van der Ent, A., Baker, A.J.M., Echevarria, G., Simonnot, M.O., Morel, J.L., Eds.; Mineral Resource Reviews; Springer Nature: Cham, Switzerland, 2021; Chapter 8; pp. 155–181. [Google Scholar] [CrossRef]
- Macnair, M.R.; Bert, V.; Huitson, S.B.; Saumitou-Laprade, P.; Petit, D. Zinc tolerance and hyperaccumulation are genetically independent characters. Proc. R. Soc. Lond. B 1999, 266, 2175–2179. [Google Scholar] [CrossRef] [Green Version]
- Assunção, A.G.L.; Ten Bookum, W.M.; Nelissen, H.J.M.; Voojis, R.; Schat, H.; Ernst, W.H.O. A cosegregation analysis of zinc (Zn) accumulation and Zn tolerance in the Zn hyperaccumulator Thlaspi caerulescens. New Phytol. 2003, 159, 383–390. [Google Scholar] [CrossRef]
- Richau, K.H.; Schat, H. Intraspecific variation of nickel and zinc accumulation and tolerance in the hyperaccumulator Thlaspi caerulescens. Plant Soil 2009, 314, 253–262. [Google Scholar] [CrossRef] [Green Version]
- Jaffré, T.; Brooks, R.R.; Lee, J.; Reeves, R.D. Sebertia acuminata: A hyperaccumulator of nickel from New Caledonia. Science 1976, 193, 579–580. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brooks, R.R.; Lee, J.; Reeves, R.D.; Jaffré, T. Detection of nickeliferous rocks by analysis of herbarium specimens of indicator plants. J. Geochem. Explor. 1977, 7, 49–57. [Google Scholar] [CrossRef]
- Baker, A.J.M.; Walker, P.L. Physiological responses of plants to heavy metals and the quantification of tolerance and toxicity. Chem. Spec. Bioavailab. 1989, 1, 7–17. [Google Scholar] [CrossRef]
- Warwick, S.I.; Sauder, C.A.; Al-Shehbaz, I.A. Phylogenetic relationships in the tribe Alysseae (Brassicaceae) based on nuclear ribosomal ITS DNA sequences. Botany 2008, 86, 315–336. [Google Scholar] [CrossRef]
- Cecchi, L.; Gabbrielli, R.; Arnetoli, M.; Gonnelli, C.; Hasko, A.; Selvi, F. Evolutionary lineages of nickel hyperaccumulation and systematics in European Alysseae (Brassicaceae): Evidence from nrDNA sequence data. Ann. Bot. 2010, 106, 751–767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rešetnik, I.; Satovic, Z.; Schneeweiss, G.M.; Liber, Z. Phylogenetic relationships in Brassicaceae tribe Alysseae inferred from nuclear ribosomal and chloroplast DNA sequence data. Mol. Phylogenet. Evol. 2013, 69, 772–786. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Lv, G.; Liu, B.; Qi, A. The phylogeny of Alyssum (Brassicaceae) inferred from molecular data. Nord. J. Bot. 2015, 33, 715–721. [Google Scholar] [CrossRef]
- Španiel, S.; Kempa, M.; Salmerón-Sánchez, E.; Fuertes-Aguiar, J.; Mota, J.F.; Al-Shehbaz, I.A.; German, D.A.; Olšavská, K.; Šingliarová, B.; Zozomová-Lihová, J.; et al. AlyBase: Database of names, chromosome numbers, and ploidy levels of Alysseae (Brassicaceae), with a new generic concept of the tribe. Plant Syst. Evol. 2015, 301, 2463–2491. [Google Scholar] [CrossRef]
- Brooks, R.R.; Radford, C.C. Nickel accumulation by European species of the genus Alyssum. Proc. R. Soc. Lond. B 1978, 200, 217–224. [Google Scholar] [CrossRef]
- Brooks, R.R.; Morrison, R.S.; Reeves, R.D.; Dudley, T.R.; Akman, Y. Hyperaccumulation of nickel by Alyssum Linnaeus (Cruciferae). Proc. R. Soc. Lond. B 1979, 203, 387–403. [Google Scholar] [CrossRef]
- Reeves, R.D.; Brooks, R.R.; Dudley, T.R. Uptake of nickel by species of Alyssum, Bornmuellera, and other genera of Old World tribus Alysseae. Taxon 1983, 32, 184–192. [Google Scholar] [CrossRef]
- Reeves, R.D.; Adigüzel, N. The nickel hyperaccumulating plants of the serpentines of Turkey and adjacent areas: A review with new data. Turk. J. Biol. 2008, 32, 143–153. [Google Scholar]
- Bettarini, I.; Colzi, I.; Coppi, A.; Falsini, S.; Echevarria, G.; Pazzagli, L.; Selvi, F.; Gonnelli, C. Unravelling soil and plant metal relationships in Albanian nickel hyperaccumulators in the genus Odontarrhena (syn. Alyssum sect. Odontarrhena, Brassicaceae). Plant Soil 2019, 440, 135–149. [Google Scholar] [CrossRef]
- Bettarini, I.; Colzi, I.; Gonnelli, C.; Pazzagli, L.; Reeves, R.D.; Selvi, F. Inability to accumulate Ni in a genus of hyperaccumulators: The paradox of Odontarrhena sibirica (Brassicaceae). Planta 2020, 252, 99. [Google Scholar] [CrossRef] [PubMed]
- Jalas, J.; Suominen, J.; Lampinen, R. (Eds.) Atlas Florae Europaeae 11: Cruciferae (Ricotia to Raphanus); Committee for Mapping the Flora of Europe & Societas Biologica Fennica Vanamo: Helsinki, Finland, 1996. [Google Scholar]
- Dudley, T.R. A new nickelophilous species of Alyssum (Cruciferae) from Portugal: Alyssum pintodasilvae T.R. Dudley. Feddes Reppert. 1986, 97, 135–138. [Google Scholar]
- Dudley, T.R. A nickel hyperaccumulating species of Alyssum (Cruciferae) from Spain: Alyssum malacitanum (Rivas Goday) T.R. Dudley, comb. and stat. nov. Feddes Reppert. 1986, 97, 139–141. [Google Scholar]
- Mengoni, A.; Baker, A.J.M.; Bazzicalupo, M.; Reeves, R.D.; Adigüzel, N.; Chianni, E.; Galardi, F.; Gabbrielli, R.; Gonnelli, C. Evolutionary dynamics of nickel hyperaccumulation in Alyssum revealed by ITS nrDNA analysis. New Phytol. 2003, 159, 691–699. [Google Scholar] [CrossRef]
- Ball, P.W.; Dudley, T.R. Alyssum L. In Flora Europaea, 2nd ed.; Tutin, T.G., Burges, N.A., Chater, A.O., Edmondson, J.R., Moore, D.M., Valentine, D.H., Walters, S.M., Webb, D.A., Eds.; Cambridge University Press: Cambridge, UK, 1993; Volume 1, pp. 359–369. ISBN 978-0521153669. [Google Scholar]
- Küpfer, P.; Nieto-Feliner, G. Alyssum. In Flora Iberica; Castroviejo, S., Ed.; Real Jardín Botánico, CSIC: Madrid, Spain, 1993; Volume IV, pp. 167–184. ISBN 978-84-00-07385-5. [Google Scholar]
- Sobczyk, M.K.; Smith, J.A.C.; Pollard, A.J.; Filatov, D.A. Evolution of nickel hyperaccumulation and serpentine adaptation in the Alyssum serpyllifolium species complex. Heredity 2017, 118, 31–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, J.W.; Stanton, M.L. Local adaptation in heterogeneous landscapes: Reciprocal transplant experiments and beyond. In Serpentine: The Evolution and Ecology of a Model System; Harrison, S.P., Rajakaruna, N., Eds.; University of California Press: Berkeley, CA, USA, 2011; pp. 97–137. ISBN 978-0520268357. [Google Scholar]
- Zozomová-Lihová, J.; Marhold, K.; Španiel, S. Taxonomy and evolutionary history of Alyssum montanum (Brassicaceae) and related taxa in southwestern Europe and Morocco: Diversification driven by polyploidy, geographic and ecological isolation. Taxon 2014, 63, 562–591. [Google Scholar] [CrossRef] [Green Version]
- Krämer, U.; Cotter-Howells, J.D.; Charnock, J.M.; Baker, A.J.M.; Smith, J.A.C. Free histidine as a metal chelator in plants that accumulate nickel. Nature 1996, 379, 635–638. [Google Scholar] [CrossRef]
- Ingle, R.A.; Mugford, S.T.; Rees, J.D.; Campbell, M.M.; Smith, J.A.C. Constitutively high expression of the histidine biosynthetic pathway contributes to nickel tolerance in hyperaccumulator plants. Plant Cell 2005, 17, 2089–2106. [Google Scholar] [CrossRef] [Green Version]
- Abati, J.; Dunning, G.R.; Arenas, R.; Díaz Garcia, F.; González Cuadra, P.; Martínez Catalán, J.R.; Andonaegui, P. Early Ordovician orogenic event in Galicia (NW Spain): Evidence from U–Pb ages in the uppermost unit of the Ordenes Complex. Earth Planet. Sci. Lett. 1999, 165, 213–228. [Google Scholar] [CrossRef]
- Crespo, E.; Luque, F.J.; Rodas, M.; Wada, H.; Gervilla, F. Graphite-sulfide deposits in Ronda and Beni Bousera peridotites (Spain and Morocco) and the origin of carbon in mantle-derived rocks. Gondwana Res. 2006, 9, 279–290. [Google Scholar] [CrossRef]
- Roosens, N.; Verbruggen, N.; Meerts, P.; Ximénez-Embún, P.; Smith, J.A.C. Natural variation in cadmium tolerance and its relationship to metal hyperaccumulation for seven populations of Thlaspi caerulescens from western Europe. Plant Cell Environ. 2003, 26, 1657–1672. [Google Scholar] [CrossRef] [Green Version]
- Wilkins, D.A. A technique for the measurement of lead tolerance in plants. Nature 1957, 180, 37–38. [Google Scholar] [CrossRef]
- Wilkins, D.A. The measurement of tolerance to edaphic factors by means of root growth. New Phytol. 1978, 80, 623–633. [Google Scholar] [CrossRef]
- Crane, M.; Newman, M.C. What level of effect is a no observed effect? Environ. Toxicol. Chem. 2000, 19, 516–519. [Google Scholar] [CrossRef]
- Murado, M.A.; Prieto, M.A. NOEC and LOEC as merely concessive expedients: Two unambiguous alternatives and some criteria to maximize the efficiency of dose-response experimental designs. Sci. Total Environ. 2013, 461, 576–586. [Google Scholar] [CrossRef] [Green Version]
- Macnair, M.R. The hyperaccumulation of metals by plants. Adv. Bot. Res. 2003, 40, 63–105. [Google Scholar] [CrossRef]
- Quintela-Sabarís, C.; Marchand, L.; Smith, J.A.C.; Kidd, P.S. Using AFLP genome scanning to explore serpentine adaptation and nickel hyperaccumulation in Alyssum serpyllifolium. Plant Soil 2017, 416, 391–408. [Google Scholar] [CrossRef]
- Brooks, R.R.; Shaw, S.; Asensi Marfil, A. Some observations on the ecology, metal uptake and nickel tolerance of Alyssum serpyllifolium subspecies from the Iberian Peninsula. Vegetatio 1981, 45, 183–188. [Google Scholar] [CrossRef]
- Morrison, R.S.; Brooks, R.R.; Reeves, R.D. Nickel uptake by Alyssum species. Plant Sci. Lett. 1980, 17, 451–457. [Google Scholar] [CrossRef]
- Antonovics, J.; Bradshaw, A.D.; Turner, R.J. Heavy metal tolerance in plants. Adv. Ecol. Res. 1971, 7, 1–85. [Google Scholar]
- Vicić, D.D.; Stoiljković, M.M.; Bojat, N.Č.; Sabovljević, M.S.; Stevanović, B.M. Physiological tolerance mechanisms of serpentine tolerant plants from Serbia. Rev. Écol. 2014, 69, 185–195. [Google Scholar]
- Galardi, F.; Mengoni, A.; Pucci, S.; Barletti, L.; Massi, L.; Barzanti, R.; Gabbrielli, R.; Gonnelli, C. Intra-specific differences in mineral element composition in the Ni-hyperaccumulator Alyssum bertolonii: A survey of populations in nature. Environ. Exp. Bot. 2007, 60, 50–56. [Google Scholar] [CrossRef]
- Adamidis, G.C.; Aloupi, M.; Kazakou, E.; Dimitrakopoulos, P.G. Intra-specific variation in Ni tolerance, accumulation and translocation patterns in the Ni-hyperaccumulator Alyssum lesbiacum. Chemosphere 2014, 95, 496–502. [Google Scholar] [CrossRef]
- Ghasemi, R.; Ghaderian, S.M. Responses of two populations of an Iranian nickel-hyperaccumulating serpentine plant, Alyssum inflatum Nyar., to substrate Ca/Mg quotient and nickel. Environ. Exp. Bot. 2009, 67, 260–268. [Google Scholar] [CrossRef]
- Reeves, R.D.; Laidlaw, W.S.; Doronila, A.; Baker, A.J.M.; Batianoff, G.N. Erratic hyperaccumulation of nickel, with particular reference to the Queensland serpentine endemic Pimelea leptospermoides. Aust. J. Bot. 2015, 63, 119–127. [Google Scholar] [CrossRef]
- Pauwels, M.; Saumitou-Laprade, P.; Holl, A.C.; Petit, D.; Bonnin, I. Multiple origin of metallicolous populations of the pseudometallophyte Arabidopsis halleri (Brassicaceae) in central Europe: The cpDNA testimony. Mol. Ecol. 2005, 14, 4403–4414. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, R.; Ghaderian, S.M.; Krämer, U. Interference of nickel with copper and iron homeostasis contributes to metal toxicity symptoms in the nickel hyperaccumulator plant Alyssum inflatum. New Phytol. 2009, 184, 566–580. [Google Scholar] [CrossRef]
- Salt, D.E.; Baxter, I.; Lahner, B. Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol. 2008, 59, 709–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- van der Ent, A.; Baker, A.J.M.; Reeves, R.D.; Pollard, A.J.; Schat, H. Commentary: Toward a more physiologically and evolutionarily relevant definition of metal hyperaccumulation in plants. Front. Plant Sci. 2015, 6, 554. [Google Scholar] [CrossRef] [Green Version]
- Ghaderian, S.M.; Baker, A.J.M. Geobotanical and biogeochemical reconnaissance of the ultramafics of central Iran. J. Geochem. Exp. 2007, 92, 34–42. [Google Scholar] [CrossRef]
- Ghaderian, S.M.; Fattahi, H.; Khosravi, A.R.; Noghreian, M. Geobotany and biogeochemistry of serpentine soils of Neyriz, Iran. Northeast. Nat. 2009, 16, 8–20. [Google Scholar] [CrossRef]
- Reeves, R.D. Hyperaccumulation of nickel by serpentine plants. In The Vegetation of Ultramafic (Serpentine) Soils; Baker, A.J.M., Proctor, J., Reeves, R.D., Eds.; Intercept Limited: Andover, UK, 1992; pp. 253–277. ISBN 978-0946707621. [Google Scholar]
- Assunção, A.G.L.; Pieper, B.; Vromans, J.; Lindhout, P.; Aarts, M.G.M.; Schat, H. Construction of a genetic linkage map of Thlaspi caerulescens and quantitative trait loci analysis of zinc accumulation. New Phytol. 2006, 170, 21–32. [Google Scholar] [CrossRef]
- Bert, V.; Macnair, M.R.; de Laguérie, P.; Saumitou-Laprade, P.; Petit, D. Zinc tolerance and accumulation in metallicolous and nonmetallicolous populations of Arabidopsis halleri (Brassicaceae). New Phytol. 2000, 146, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bert, V.; Bonnin, I.; Saumitou-Laprade, P.; de Laguérie, P.; Petit, D. Do Arabidopsis halleri from nonmetallicolous populations accumulate zinc and cadmium more effectively than those from metallicolous populations? New Phytol. 2002, 155, 47–57. [Google Scholar] [CrossRef]
- Boyd, R.S. Ecology of metal hyperaccumulation. New Phytol. 2004, 162, 563–567. [Google Scholar] [CrossRef]
- Fones, H.N.; Preston, G.M.; Smith, J.A.C. Variation in defence strategies in the metal hyperaccumulator plant Noccaea caerulescens is indicative of synergies and trade-offs between forms of defence. R. Soc. Open Sci. 2019, 6, 172418. [Google Scholar] [CrossRef] [Green Version]
- Alford, É.R.; Pilon-Smits, E.A.H.; Paschke, M.W. Metallophytes—A view from the rhizosphere. Plant Soil 2010, 337, 33–50. [Google Scholar] [CrossRef]
- Benizri, E.; Lopez, S.; Durand, A.; Kidd, P.A. Diversity and role of endophytic and rhizosphere microbes associated with hyperaccumulator plants during metal accumulation. In Agromining: Farming for Metals, 2nd ed.; Van der Ent, A., Baker, A.J.M., Echevarria, G., Simonnot, M.O., Morel, J.L., Eds.; Mineral Resource Reviews; Springer Nature: Cham, Switzerland, 2021; Chapter 12; pp. 239–279. [Google Scholar] [CrossRef]
- Cecchi, L.; Colzi, I.; Coppi, A.; Gonnelli, C.; Selvi, F. Diversity and biogeography of Ni hyperaccumulators of Alyssum section Odontarrhena (Brassicaceae) in the central western Mediterranean: Evidence from karyology, morphology and DNA sequence data. Bot. J. Linn. Soc. 2013, 173, 269–289. [Google Scholar] [CrossRef] [Green Version]
- Španiel, S.; Marhold, K.; Filová, B.; Zozomová-Lihová, J. Genetic and morphological variation in the diploid-polyploid Alyssum montanum in Central Europe: Taxonomic and evolutionary considerations. Plant Syst. Evol. 2011, 294, 1–25. [Google Scholar] [CrossRef]
- Španiel, S.; Zozomová-Lihová, J.; Marhold, K. Revised taxonomic treatment of the Alyssum montanum—A. repens complex in the Balkans: A multivariate morphological analysis. Plant Syst. Evol. 2017, 303, 1413–1442. [Google Scholar] [CrossRef]
- Španiel, S.; Marhold, K.; Zozomová-Lihová, J. The polyploid Alyssum montanum—A. repens complex in the Balkans: A hotspot of species and genetic diversity. Plant Syst. Evol. 2017, 303, 1443–1465. [Google Scholar] [CrossRef]
- Hewitt, E.J.; Smith, T.A. Plant Mineral Nutrition; The English Universities Press: London, UK, 1975; ISBN 978-0470383858. [Google Scholar]
- Brown, P.H.; Welch, R.M.; Cary, E.E. Nickel: A micronutrient essential for higher plants. Plant Physiol. 1987, 85, 801–803. [Google Scholar] [CrossRef] [PubMed]
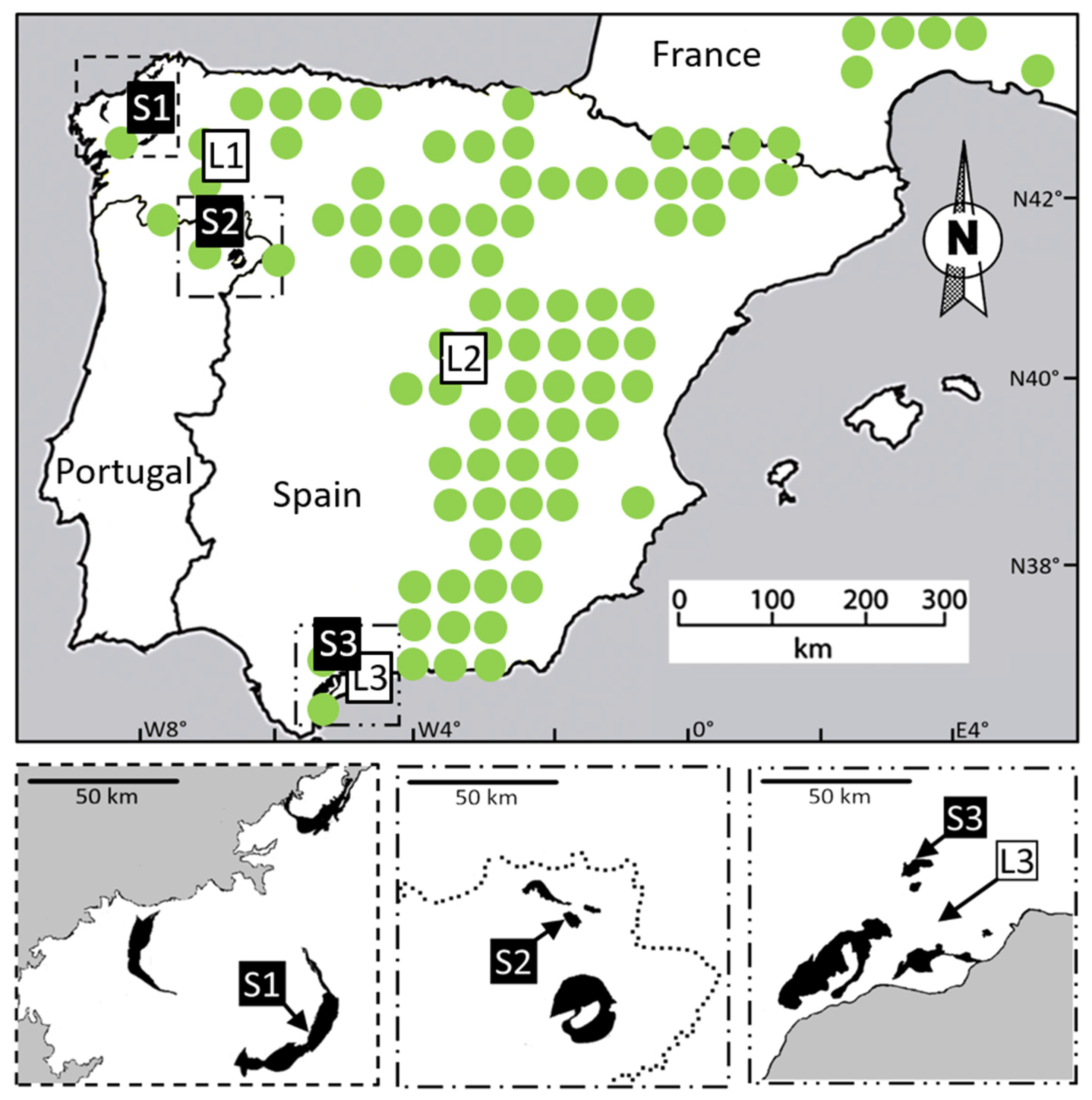
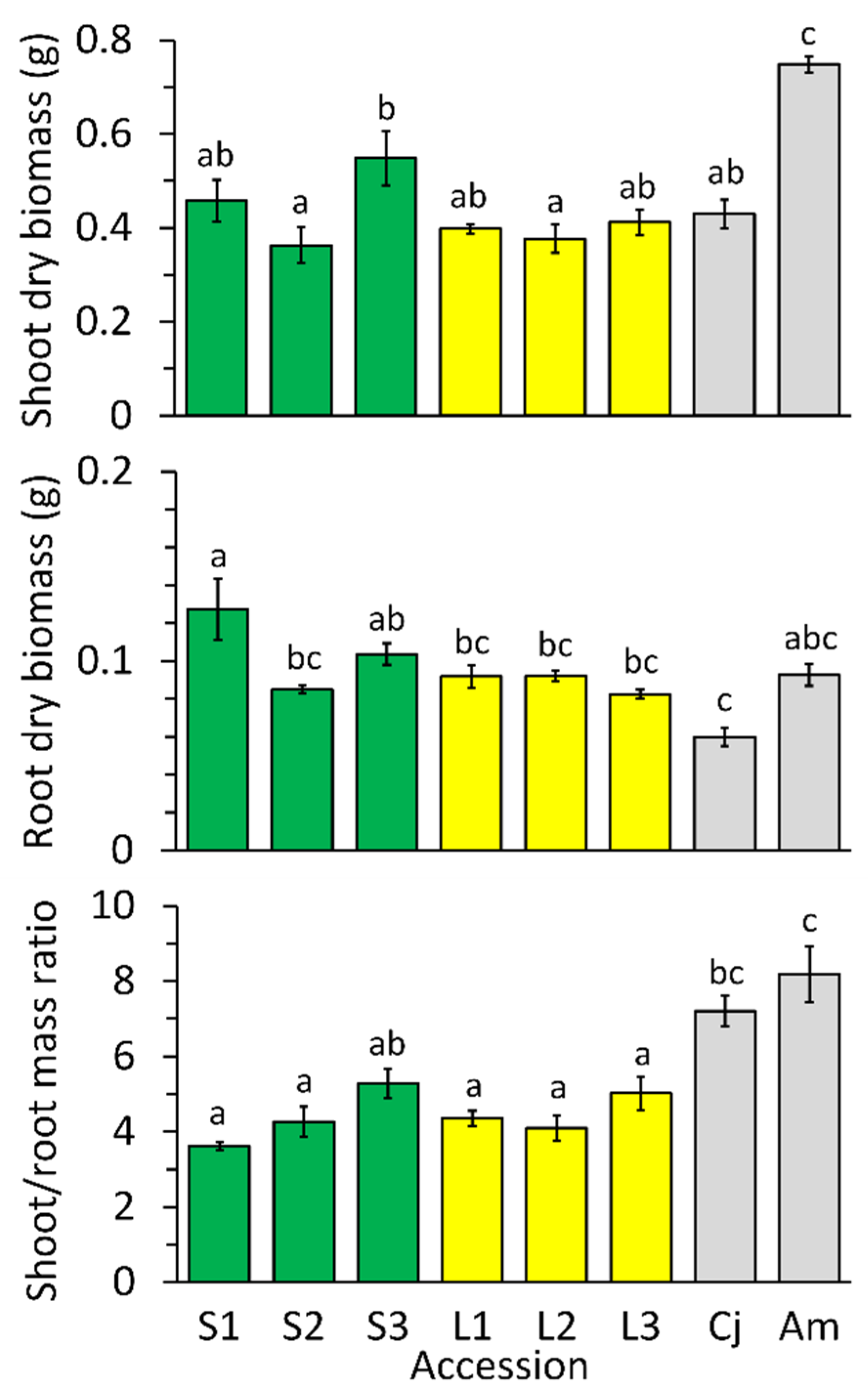







| Accession Code | Substrate | Source | Latitude/Longitude | Year Collected |
|---|---|---|---|---|
| S1 | Serpentine | Barazón (Galicia, Spain) | 42°51.058′ N, 8°00.313′ W | 2011 |
| S2 | Serpentine | Samil (Bragança, Portugal) | 41°46.774′ N, 6°45.106′ W | 1999 |
| S3 | Serpentine | Carratraca (Andalucía, Spain) | 36°51.223′ N, 4°48.736′ W | 2011 |
| L1 | Limestone | Rubiá (Galicia, Spain) | 42°29.260′ N, 6°49.830′ W | 2011 |
| L2 | Limestone | Morata de Tajuña (Madrid, Spain) | 40°14.997′ N, 3°29.071′ W | 2002 |
| L3 | Limestone | Alhaurín de la Torre (Andalucía, Spain) | 36°38.780′ N, 4°36.077′ W | 2002 |
| Cj | N/A | Millennium Seed Bank (Origin: Jordan) | N/A | 2011 * |
| Am | N/A | Purchased from Dobies of Devon, UK | N/A | 2012 * |
| Accession Code | Soil Ni (g kg−1) | Leaf Ni (g kg−1) | ||||
|---|---|---|---|---|---|---|
| N | Mean ± SE | Range | N | Mean ± SE | Range | |
| S1 | 3 | 3.614 ± 0.781 | 2.604–5.150 | 4 | 4.595 ± 2.681 | 0.165–11.189 |
| S2 | 12 | 2.962 ± 0.102 | 2.550–3.841 | 4 | 1.377 ± 1.236 | 0.104–5.084 |
| S3 | 6 | 1.593 ± 0.205 | 1.075–2.426 | 4 | 2.513 ± 0.236 | 2.067–3.127 |
| L1 | 3 | 0.028 ± 0.004 | 0.020–0.034 | 4 | 0.028 ± 0.003 | 0.022–0.035 |
| L2 | 3 | 0.002 ± 0.001 | 0.002–0.003 | 4 | 0.025 ± 0.006 | 0.013–0.041 |
| L3 | 5 | 0.007 ± 0.003 | 0.000–0.017 | 4 | 0.007 ± 0.003 | 0.001–0.014 |
| Accession | Root Mass | Shoot Mass |
|---|---|---|
| S1 | 7.008 * | 6.825 * |
| S2 | 1.034 | 2.296 |
| S3 | 0.777 | 0.194 |
| L1 | 109.393 *** | 91.234 *** |
| L2 | 32.409 *** | 39.239 *** |
| L3 | 28.557 *** | 62.427 *** |
| Cj | 45.873 *** | 38.514 *** |
| Am | 30.350 *** | 57.051 *** |
| Accession | mNOEC (µM) Based on Shoot Mass | mNOEC (µM) Based on Root Mass | Foliar [Ni] (g kg−1) at mNOEC |
|---|---|---|---|
| S1 | 100 | 100 | 6.14 |
| S2 | ≥300 | ≥300 | 11.52 |
| S3 | ≥300 | ≥300 | 11.68 |
| L1 | 10 | 30 | 2.54 * |
| L2 | 30 | 30 | 2.97 |
| L3 | 30 | 30 | 2.21 |
| Cj | 30 | 30 | 2.78 |
| Am | 0.1 | 0.1 | 0.001 |
| Accession Group | Concentration of Element in Plant Tissue | ||||
|---|---|---|---|---|---|
| Tissue | Ca | Mg | K | Fe | |
| Os-S | Leaf | 0.59 | 0.95 | 1.88 | 0.49 |
| Os-L | Leaf | 4.28 ** | 6.50 *** | 2.05 | 15.39 *** |
| Cj | Leaf | 13.70 ** | 8.94 ** | 17.69 *** | 4.92 * |
| Am | Leaf | 4.88 * | 5.01 * | 1.87 | 2.20 |
| Os-S | Root | 2.23 | 7.68 *** | 3.17 | 51.68 *** |
| Os-L | Root | 16.59 *** | 4.54 ** | 5.73 ** | 56.54 *** |
| Cj | Root | 31.71 *** | 25.65 *** | 15.25 *** | 475.24 *** |
| Am | Root | 15.84 *** | 3.59 | 5.94 * | 9.36 ** |
| Accession Group | Principal Component Axis | ||
|---|---|---|---|
| Tissue | PCA1 | PCA2 | |
| Os-S | Leaf | 1.00 | 1.11 |
| Os-L | Leaf | 5.97 *** | 13.25 *** |
| Cj | Leaf | 15.74 *** | 6.79 * |
| Am | Leaf | 4.89 * | 2.19 |
| Os-S | Root | 2.28 | 6.31 *** |
| Os-L | Root | 28.30 *** | 2.80 * |
| Cj | Root | 242.04 *** | 9.61 ** |
| Am | Root | 9.35 ** | 2.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollard, A.J.; McCartha, G.L.; Quintela-Sabarís, C.; Flynn, T.A.; Sobczyk, M.K.; Smith, J.A.C. Intraspecific Variation in Nickel Tolerance and Hyperaccumulation among Serpentine and Limestone Populations of Odontarrhena serpyllifolia (Brassicaceae: Alysseae) from the Iberian Peninsula. Plants 2021, 10, 800. https://doi.org/10.3390/plants10040800
Pollard AJ, McCartha GL, Quintela-Sabarís C, Flynn TA, Sobczyk MK, Smith JAC. Intraspecific Variation in Nickel Tolerance and Hyperaccumulation among Serpentine and Limestone Populations of Odontarrhena serpyllifolia (Brassicaceae: Alysseae) from the Iberian Peninsula. Plants. 2021; 10(4):800. https://doi.org/10.3390/plants10040800
Chicago/Turabian StylePollard, A. Joseph, Grace L. McCartha, Celestino Quintela-Sabarís, Thomas A. Flynn, Maria K. Sobczyk, and J. Andrew C. Smith. 2021. "Intraspecific Variation in Nickel Tolerance and Hyperaccumulation among Serpentine and Limestone Populations of Odontarrhena serpyllifolia (Brassicaceae: Alysseae) from the Iberian Peninsula" Plants 10, no. 4: 800. https://doi.org/10.3390/plants10040800
APA StylePollard, A. J., McCartha, G. L., Quintela-Sabarís, C., Flynn, T. A., Sobczyk, M. K., & Smith, J. A. C. (2021). Intraspecific Variation in Nickel Tolerance and Hyperaccumulation among Serpentine and Limestone Populations of Odontarrhena serpyllifolia (Brassicaceae: Alysseae) from the Iberian Peninsula. Plants, 10(4), 800. https://doi.org/10.3390/plants10040800






