Application of Hyperspectral Imaging in the Assessment of Drought and Salt Stress in Magneto-Primed Triticale Seeds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Seeds
2.2. Magnetic Treatment
2.3. Salt and Drought Stress Generation
2.4. Hyperspectral Images
2.5. Data Pre-Processing and Processing
3. Results
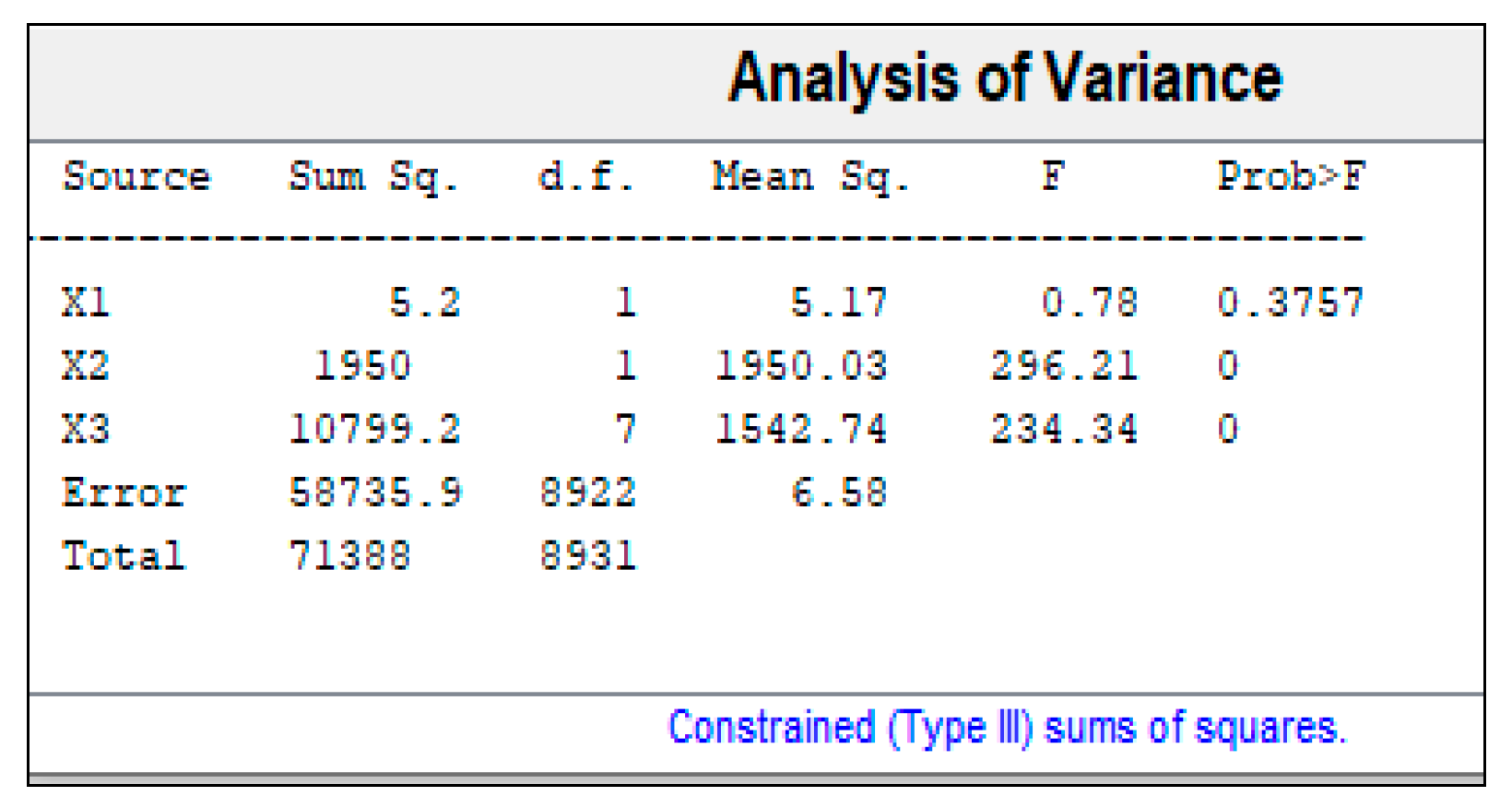
3.1. Analysis of Variance
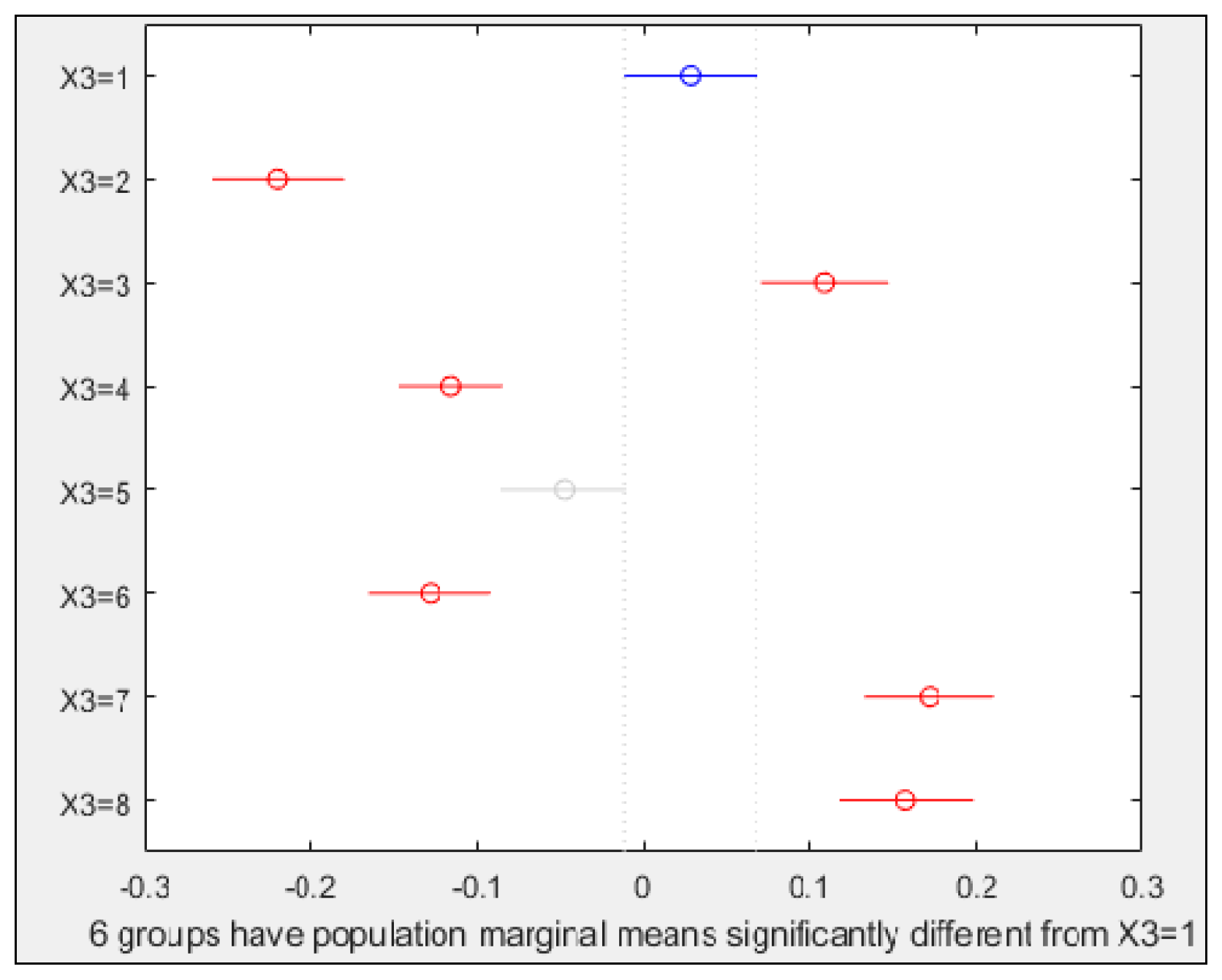
3.2. Multiple Comparison Test
3.3. Mean Spectrum
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivorra, E. Desarrollo de Técnicas de Visión Hiperespectral y Tridimensional para el Sector Agroalimentario. Ph.D. Thesis, Universitat Politècnica de València, Valencia, Spain, 2015. [Google Scholar]
- Passioura, J. The drought environment: Physical, biological and agricultural perspectives. J. Exp. Bot. 2007, 58, 113–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- James, R.A.; Blake, C.; Byrt, C.S.; Munns, R. Major genes for Na+ exclusion, Nax1 and Nax2 (wheat HKT1; 4 and HKT1; 5), decrease Na+ accumulation in bread wheat leaves under saline and waterlogged conditions. J. Exp. Bot. 2011, 62, 2939–2947. [Google Scholar] [CrossRef] [Green Version]
- Sytar, O.; Brestic, M.; Zivcak, M.; Olsovska, K.; Kovar, M.; Shao, H.; He, X. Applying hyperspectral imaging to explore natural plant diversity towards improving salt stress tolerance. Sci. Total Environ. 2017, 578, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U. Priming of induced plant defense responses. Adv. Bot. Res. 2009, 51, 361–395. [Google Scholar] [CrossRef]
- Kaya, M.D.; Okçu, G.; Atak, M.; Cıkılı, Y.; Kolsarıcı, Ö. Seed treatments to overcome salt and drought stress during germination in sunflower (Helianthus annuus L.). Eur. J. Agron. 2006, 24, 291–295. [Google Scholar] [CrossRef]
- Alvarez, J.; Martinez, E.; Carbonell, V.; Florez, M. Effects of polyethylene glycol and sodium chloride stress on water absorption of magneto-primed triticale seeds. Rom. Rep. Phys. 2020, 72, 708. [Google Scholar]
- Alvarez, J.; Martinez, E.; Florez, M.; Carbonell, V. Germination Performance and Hydro-Time Model for Magneto-Primed and Osmotic-Stressed Triticale Seeds. Rom. J. Phys. 2021, 66, 801. [Google Scholar]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Oliveira Santos, L.; Menegatti, R.D.; Jain, M.; Ihtisham, M.; Liu, S. Magnetic field (MF) applications in plants: An overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E. Magnetic field effects on plant growth, development, and evolution. Front. Plant Sci. 2014, 5, 445. [Google Scholar] [CrossRef] [Green Version]
- Ayala Martini, D. Automatización del Análisis de Imágenes Hiperespectrales para Identificación de Aptitud de Patatas. Bachelor’s Thesis, Universidad Pública de Navarra, Navarra, Spain, 2018. [Google Scholar]
- Hong, T.; Li, Z.; Wu, C.; Liu, M.; Qiao, J.; Wang, N. Review of hyperspectral image technology for non-destructive inspection of fruit quality. Trans. Chin. Soc. Agric. Eng. 2007, 23, 280–285. [Google Scholar]
- Wang, J.; Nakano, K.; Ohashi, S.; Kubota, Y.; Takizawa, K.; Sasaki, Y. Detection of external insect infestations in jujube fruit using hyperspectral reflectance imaging. Biosyst. Eng. 2011, 108, 345–351. [Google Scholar] [CrossRef]
- Cogdill, R.P.; Hurburgh, C.R.; Rippke, G.R.; Bajic, S.J.; Jones, R.W. Single-kernel maize analysis by near-infrared hyperspectral imaging. Trans. ASAE 2004, 47, 311. [Google Scholar] [CrossRef] [Green Version]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Rekha, P.N.; Gangadharan, R.; Pillai, S.M.; Ramanathan, G.; Panigrahi, A. Hyperspectral image processing to detect the soil salinity in coastal watershed. In Proceedings of the Fourth International Conference on Advanced Computing (ICoAC), Chennai, India, 13–15 December 2012; pp. 1–5. [Google Scholar]
- Lara, M.Á.; Diezma, B.; Lleó, L.; Roger, J.M.; Garrido, Y.; Gil, M.I.; Ruiz-Altisent, M. Hyperspectral imaging to evaluate the effect of irrigation water salinity in lettuce. App. Sci. 2016, 6, 412. [Google Scholar] [CrossRef]
- Sun, D.W. Hyperspectral Imaging for Food Quality Analysis and Control; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Giunta, F.; Motzo, R.; Deidda, M. Effect of drought on yield and yield components of durum wheat and triticale in a Mediterranean environment. Field Crop. Res. 1993, 33, 399–409. [Google Scholar] [CrossRef]
- Alvarez, J.; Carbonell, V.; Martinez, E.; Florez, M. The use of Peleg’s equation to model water absorption in triticale (X Triticosecale Wittmack) seeds magnetically treated before soaking. Rom. J. Phys. 2019, 64, 810. [Google Scholar]
- Michel, B.E.; Kaufmann, M.R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973, 51, 914–916. [Google Scholar] [CrossRef] [PubMed]
- Blum, A. Use of PEG to Induce and Control Plant Water Deficit in Experimental Hydroponics’ Culture. Methods. Plantstress. Available online: https://plantstress.com/use-of-peg/ (accessed on 3 January 2021).
- Salisbury, F.B.; Ross, C.W. Plant Physiology, 4th ed.; Wadsworth Publishing Company: Belmont, CA, USA, 1992. [Google Scholar]
- Murillo-Amador, B.; López-Aguilar, R.; Kaya, C.; Larrinaga-Mayoral, J.; Flores-Hernández, A. Comparative effects of NaCl and polyethylene glycol on germination, emergence and seedling growth of cowpea. J. Agron. Crop Sci. 2002, 188, 235–247. [Google Scholar] [CrossRef]
- Pietruszewski, S.; Martínez, E. Magnetic field as a method of improving the quality of sowing material: A review. Int. Agrophysics 2015, 29, 377–389. [Google Scholar] [CrossRef]
- Ambrose, A.; Kandpal, L.M.; Kim, M.S.; Lee, W.H.; Cho, B.K. High speed measurement of corn seed viability using hyperspectral imaging. Infrared Phys. Technol. 2016, 75, 173–179. [Google Scholar] [CrossRef]
- Zhang, T.; Wei, W.; Zhao, B.; Wang, R.; Li, M.; Yang, L.; Wang, J.; Sun, Q. A reliable methodology for determining seed viability by using hyperspectral data from two sides of wheat seeds. Sensors 2018, 18, 813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clevers, J.G.; Kooistra, L.; Schaepman, M.E. Estimating canopy water content using hyperspectral remote sensing data. Int. J. Appl. Earth Obs. 2010, 12, 119–125. [Google Scholar] [CrossRef]
- Mahesh, S.; Jayas, D.S.; Paliwal, J.; White, N.D. Comparing two statistical discriminant models with a back-propagation neural network model for pairwise classification of location and crop year specific wheat classes at three selected moisture contents using NIR hyperspectral images. Trans. ASABE 2014, 57, 63–74. [Google Scholar] [CrossRef]
- Yang, X.; Ye, Y.; Li, X.; Lau, R.Y.; Zhang, X.; Huang, X. Hyperspectral image classification with deep learning models. IEEE Trans. Geosci. Remote 2018, 56, 5408–5423. [Google Scholar] [CrossRef]
- Chelladurai, V.; Karuppiah, K.; Jayas, D.S.; Fields, P.G.; White, N.D.G. Detection of Callosobruchus maculatus (F.) infestation in soybean using soft X-ray and NIR hyperspectral imaging techniques. J. Stored Prod. Res. 2014, 57, 43–48. [Google Scholar] [CrossRef]
- Jiang, J.; Qiao, X.; He, R. Use of Near-Infrared hyperspectral images to identify moldy peanuts. J. Food Eng. 2016, 169, 284–290. [Google Scholar] [CrossRef]

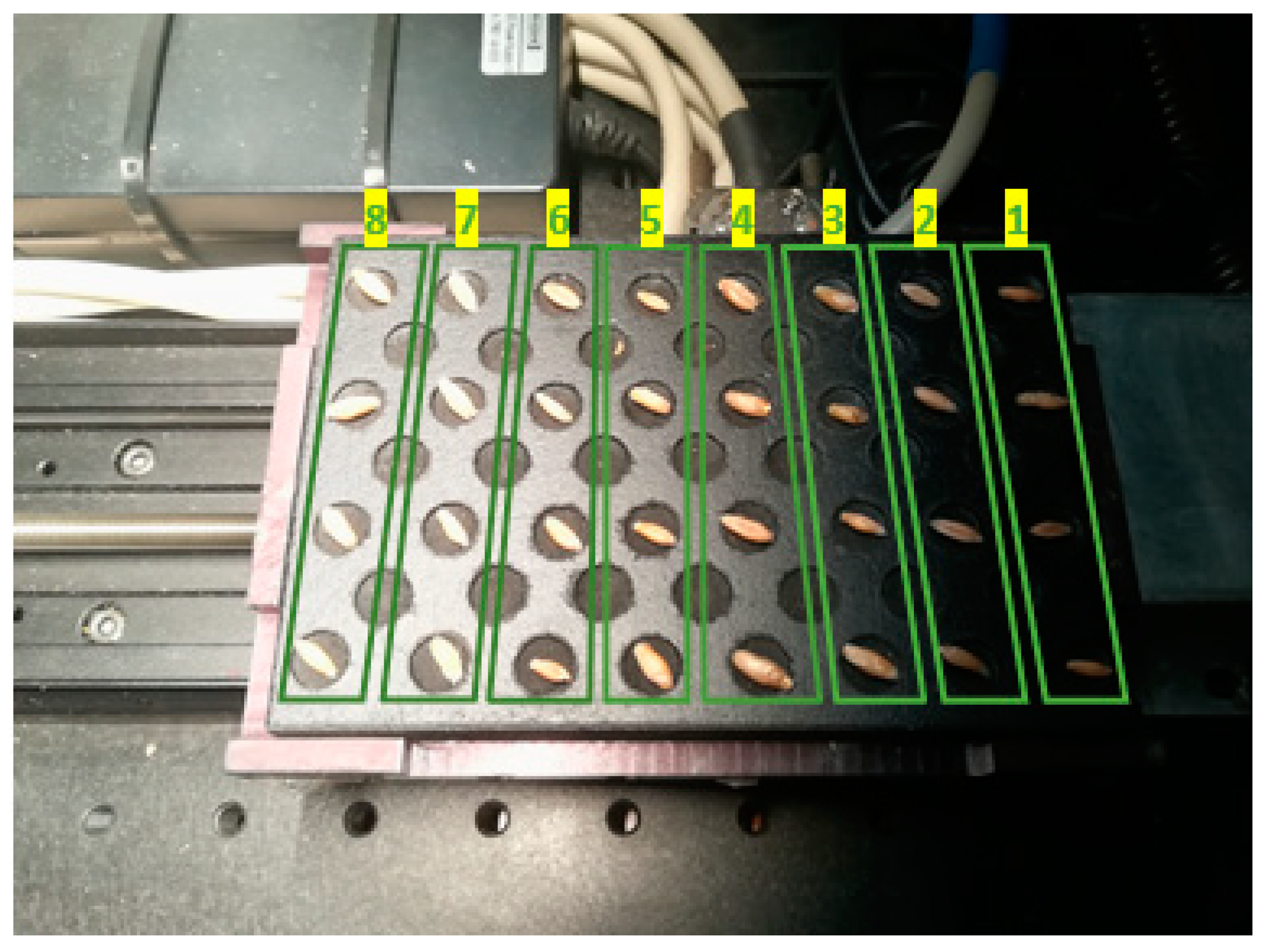




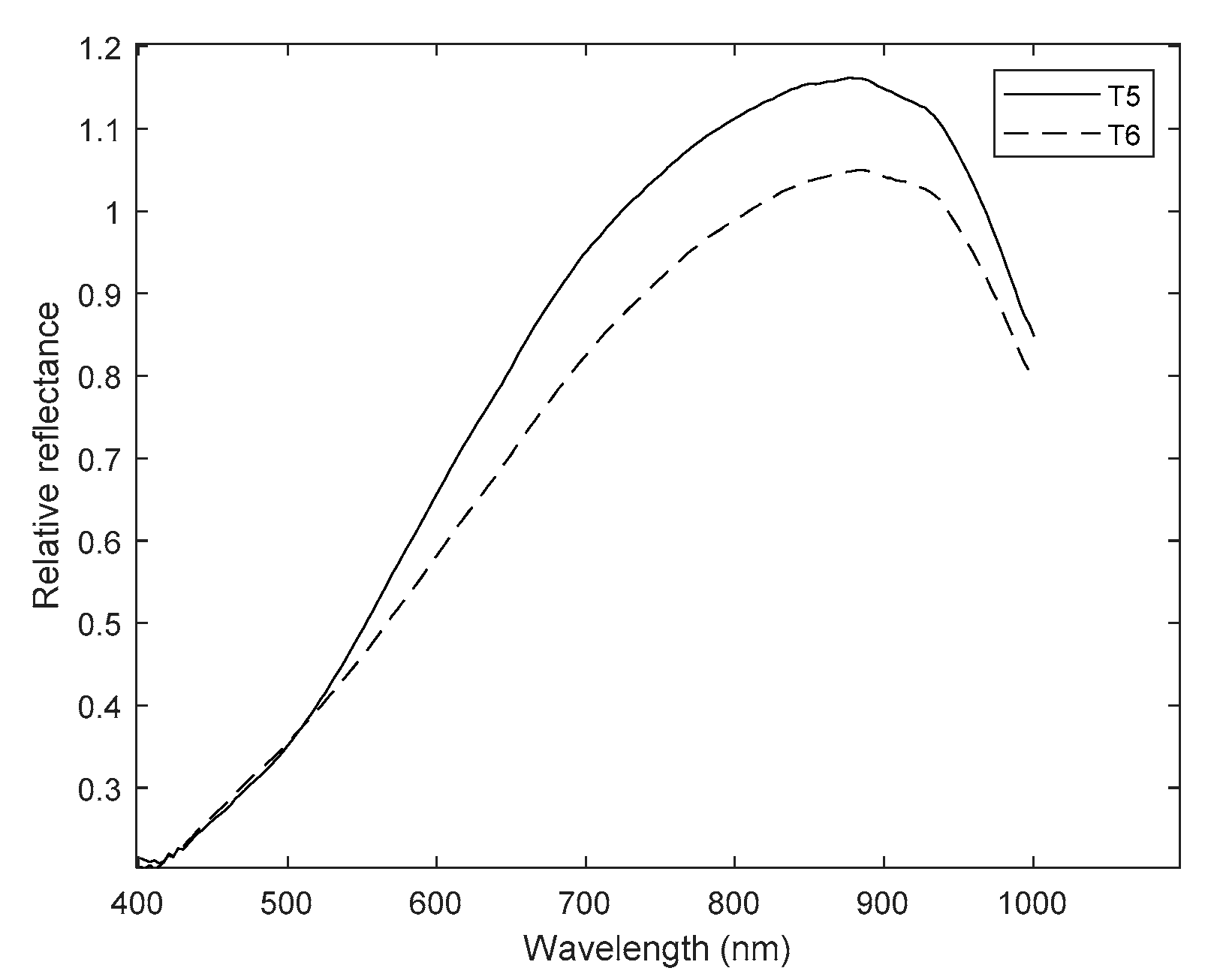
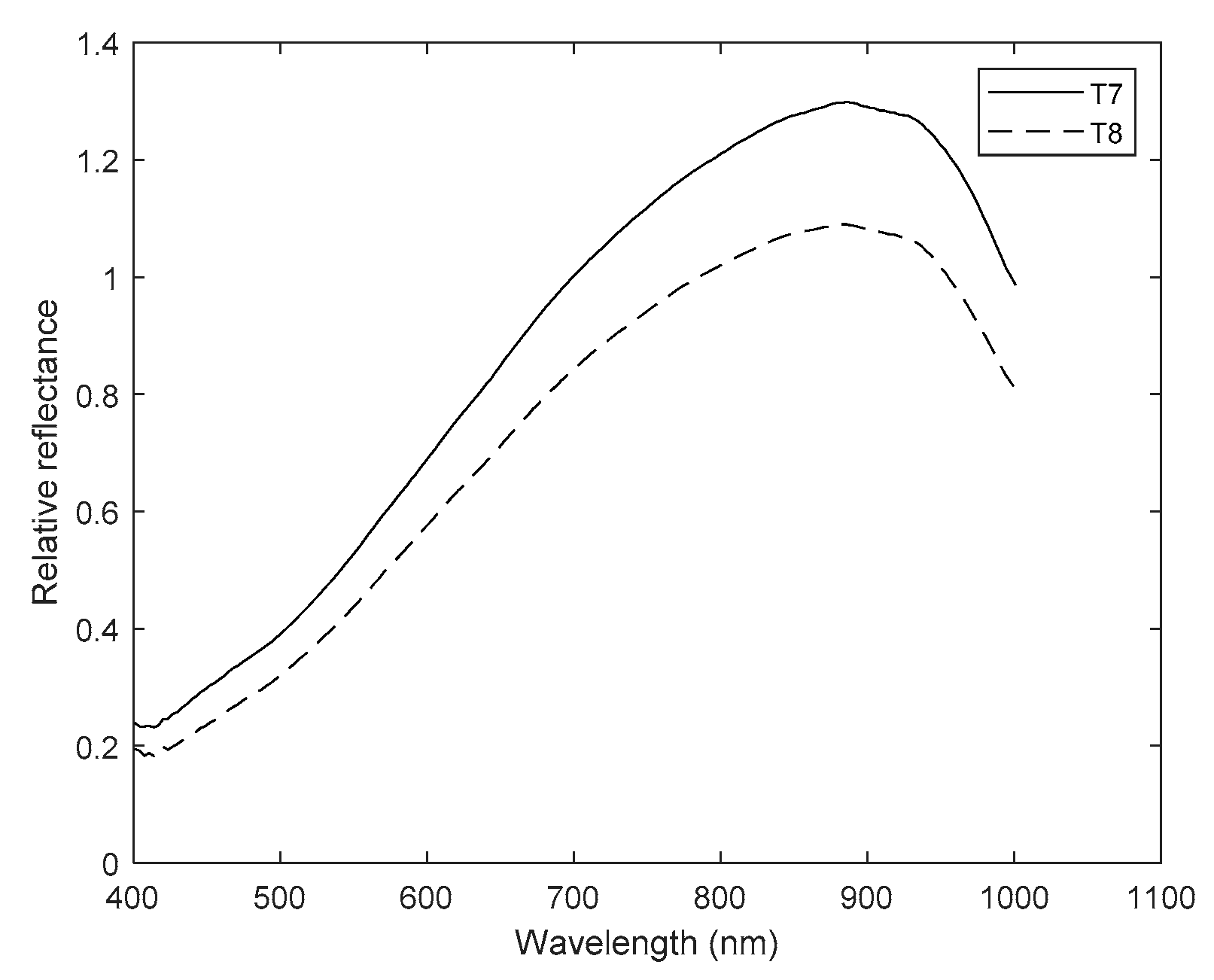
| Treatment | Column Number (Figure 2) | Description |
|---|---|---|
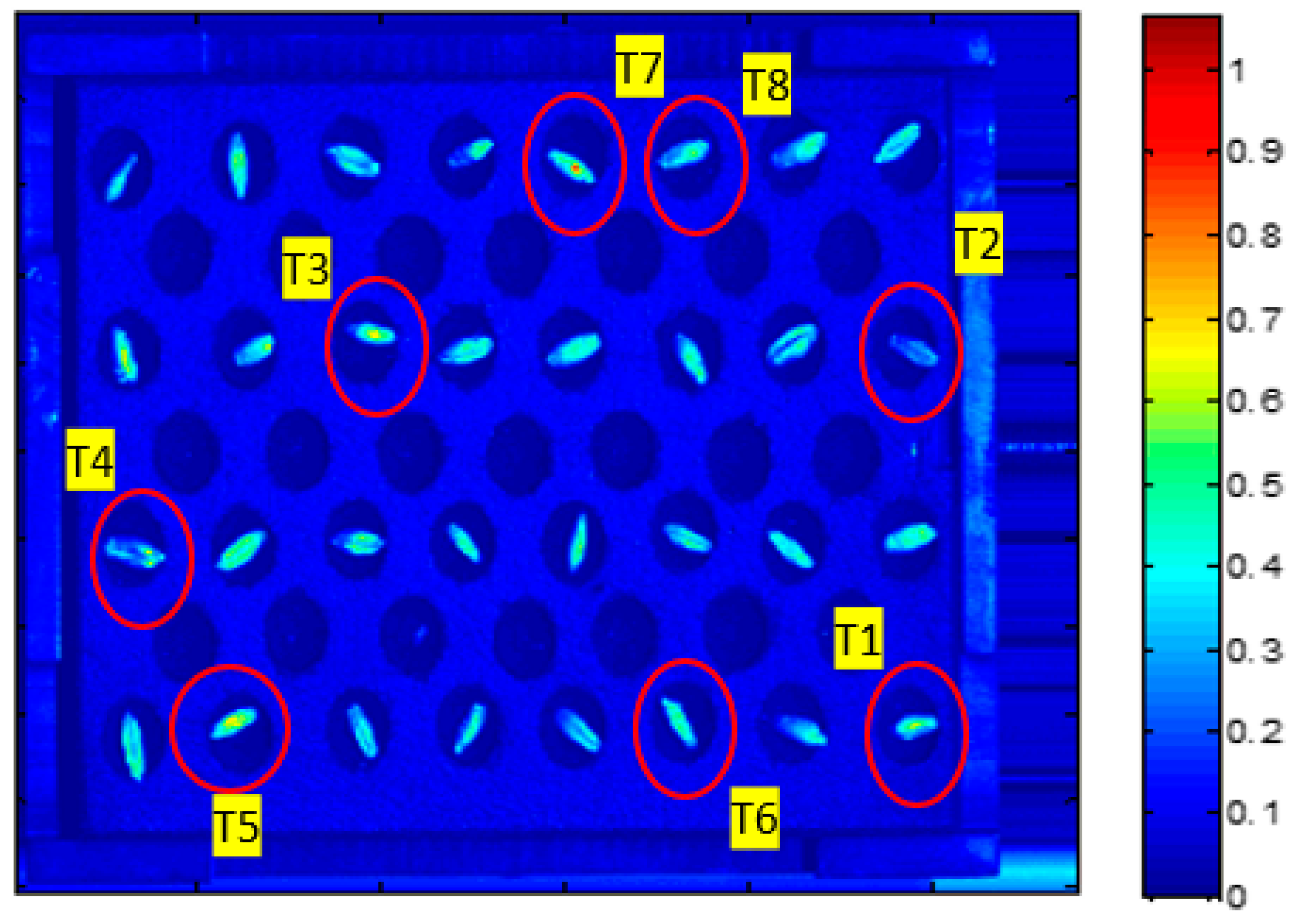
| T1 | 1 | Control |
| T2 | 2 | Magneto-primed seeds |
| T3 | 3 | Soaked in water seeds |
| T4 | 4 | Soaked in water and magneto-primed seeds |
| T5 | 5 | Seeds imbibed in the PEG solution |
| T6 | 6 | Magneto-primed seeds imbibed in the PEG solution |
| T7 | 7 | Seeds imbibed in the NaCl solution |
| T8 | 8 | Magneto-primed seeds imbibed in the NaCl solution |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alvarez, J.; Martinez, E.; Diezma, B. Application of Hyperspectral Imaging in the Assessment of Drought and Salt Stress in Magneto-Primed Triticale Seeds. Plants 2021, 10, 835. https://doi.org/10.3390/plants10050835
Alvarez J, Martinez E, Diezma B. Application of Hyperspectral Imaging in the Assessment of Drought and Salt Stress in Magneto-Primed Triticale Seeds. Plants. 2021; 10(5):835. https://doi.org/10.3390/plants10050835
Chicago/Turabian StyleAlvarez, Jose, Elvira Martinez, and Belén Diezma. 2021. "Application of Hyperspectral Imaging in the Assessment of Drought and Salt Stress in Magneto-Primed Triticale Seeds" Plants 10, no. 5: 835. https://doi.org/10.3390/plants10050835






