The Effect of Temperature on the Hypersensitive Response (HR) in the Brassica napus–Leptosphaeria maculans Pathosystem
Abstract
:1. Introduction
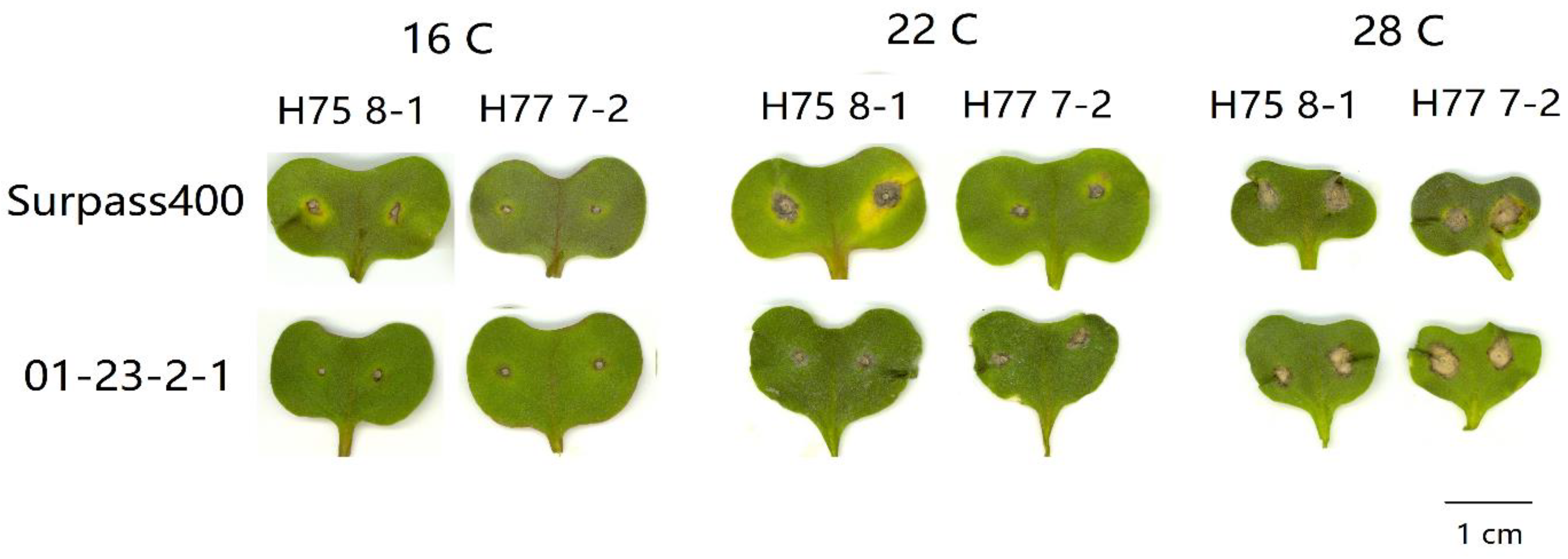
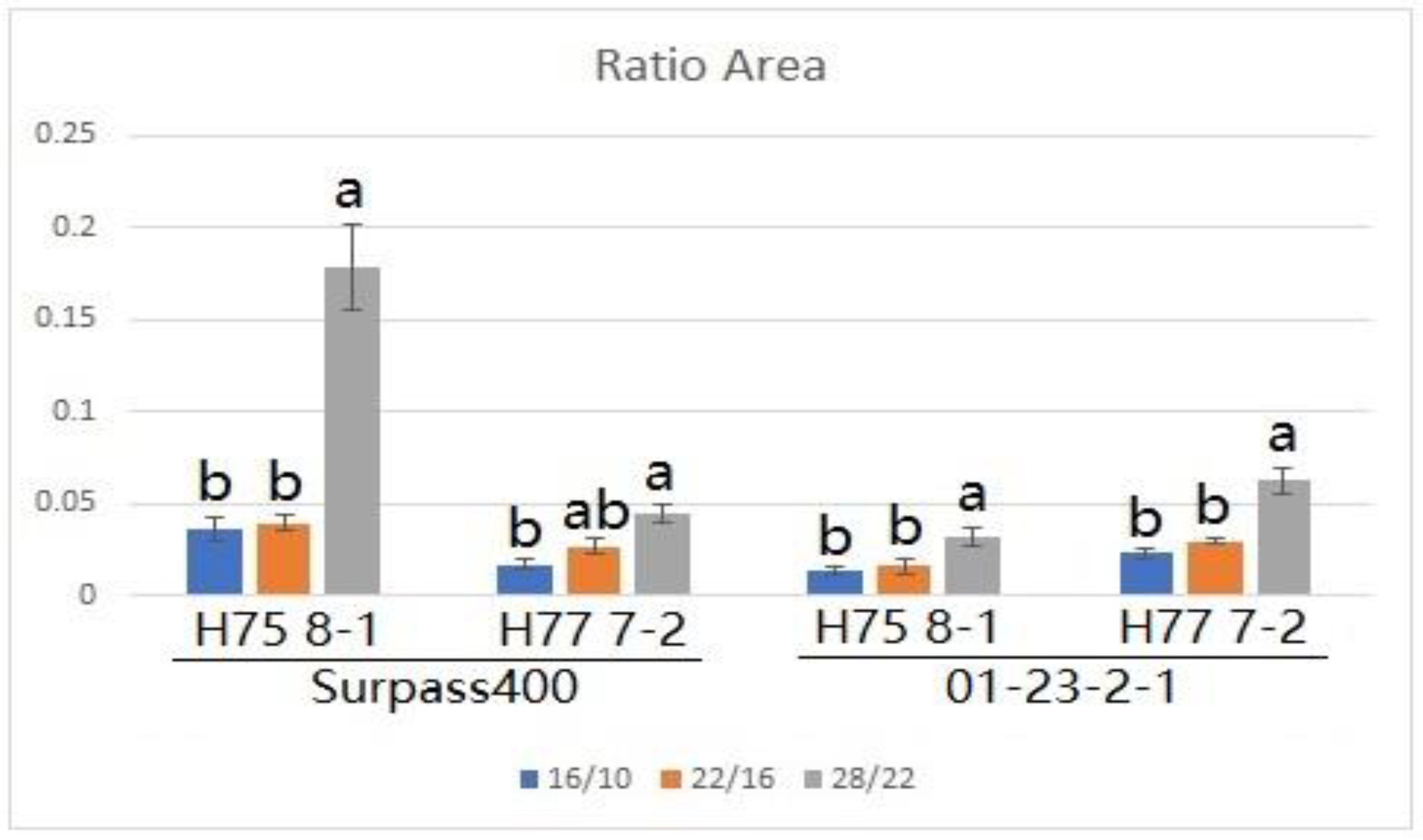
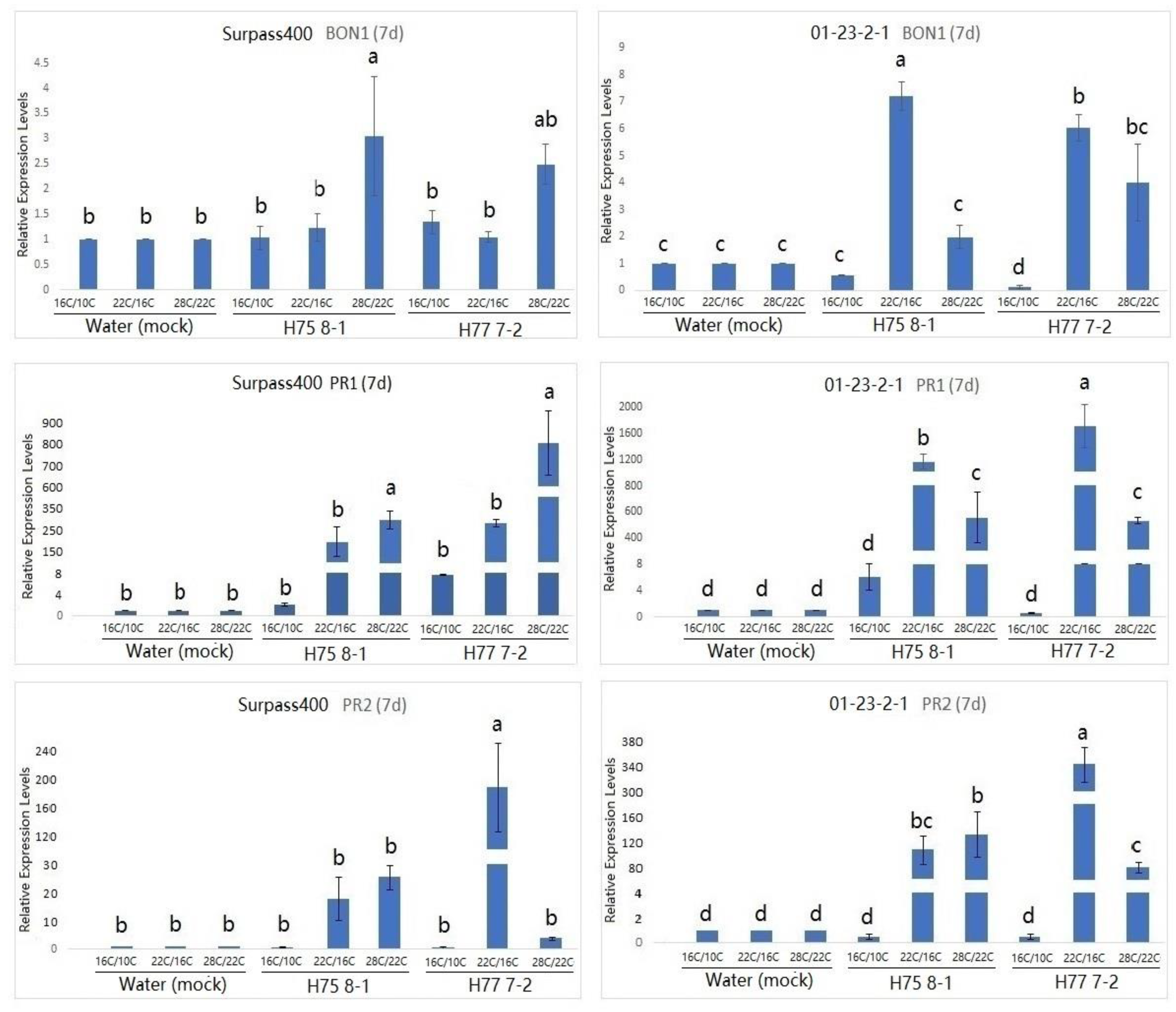
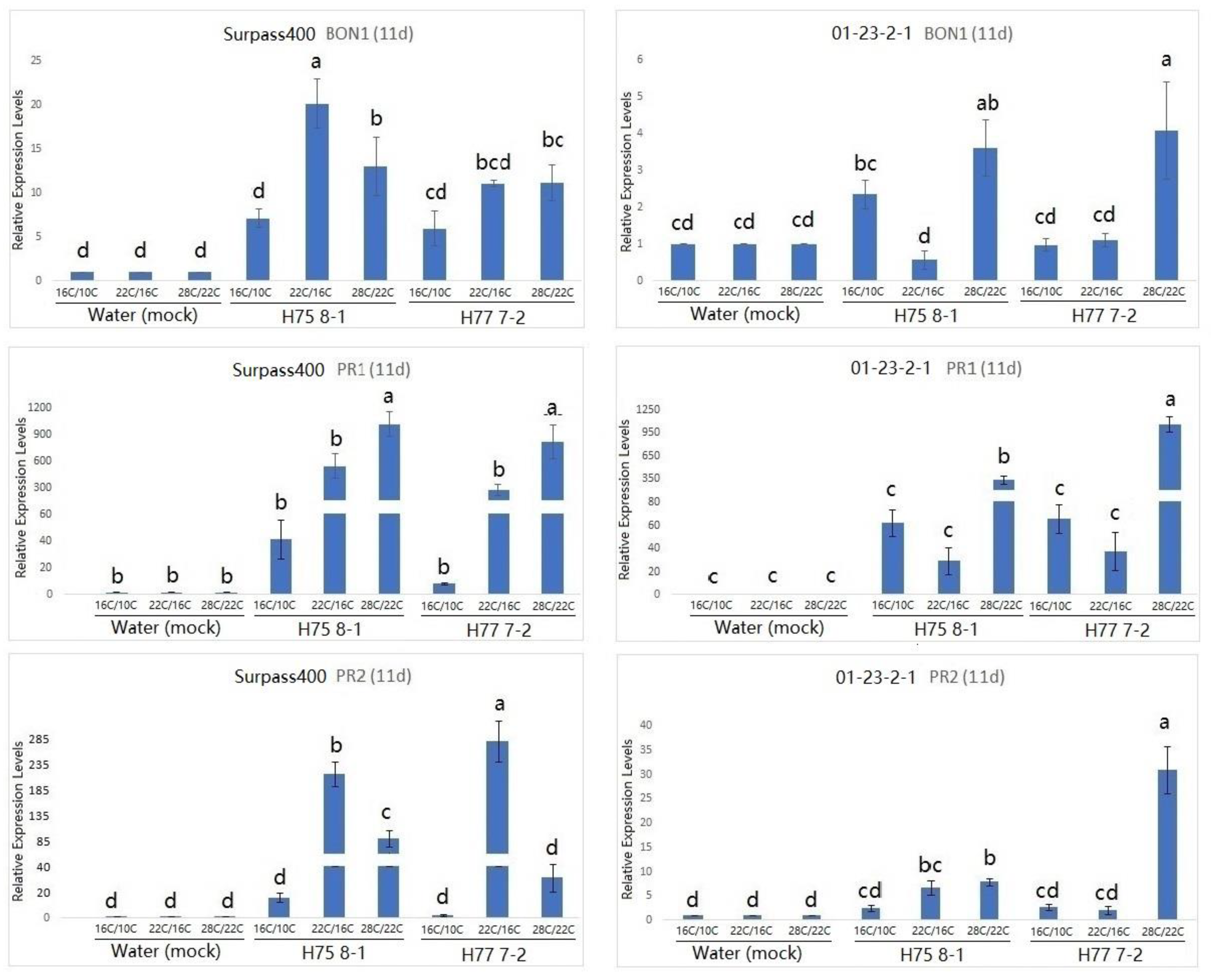
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Cultivation and Temperature Treatments
4.2. Pathogen Inoculation
4.3. Lesion Measurement
4.4. Gene Expression Analysis
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martin-Rivilla, H.; Garcia-Villaraco, A.; Ramos-Solano, B.; Gutierrez-Mañero, F.J.; Lucas, J.A. Bioeffectors as biotechnological tools to boost plant innate immunity signal transduction pathways involved. Plants 2020, 9, 1731. [Google Scholar] [CrossRef] [PubMed]
- Martin-Rivilla, H.; Gutierrez-Mañero, F.J.; Gradillas, A.; Navarro, M.O.P.; Andrade, G.; Lucas, J.A. Identifying the compounds of the metabolic elicitors of Pseudomonas fluorescens N 21.4 responsible for their ability to induce plant resistance. Plants 2020, 9, 1020. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.G.; Zhang, X.; Walker, P.L.; Wan, J.C.; Millar, J.L.; Khan, D.; Granger, M.J.; Cavers, J.D.; Chan, A.C.; Fernando, D.W.G.; et al. Transcriptome analysis of the Brassica napus—Leptosphaeria maculans pathosystem identifies receptor, signaling and structural genes underlying plant resistance. Plant J. 2017, 90, 573–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, R.G.; Cassin, A.; Grandaubert, J.; Clark, B.L.; Van de Wouw, A.P.; Rouxel, T.; Howlett, B.J. Genomes and transcriptomes of partners in plant-fungal-interactions between canola (Brassica napus) and two Leptosphaeria species. PLoS ONE 2014, 9, e103098. [Google Scholar] [CrossRef]
- Sašek, V.; Nováková, M.; Jindřichová, B.; Bóka, K.; Valentová, O.; Burketová, L. Recognition of avirulence gene AvrLm1 from hemibiotrophic ascomycete Leptosphaeria maculans triggers salicyclic acid and ethylene signaling in Brassica napus. Mol. Plant Microbe Interact. 2012, 25, 1238–1250. [Google Scholar] [CrossRef] [Green Version]
- Shayka, S.K.; Goss, E.M.; Dufault, N.S.; van Bruggen, A.H. Potential effects of diurnal temperature oscillations on potato late blight with special reference to climate change. Phytopathology 2015, 105, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Iglesias, I.; Escuredo, O.; Seijo, C.; Méndez, J. Phytophthora infestans prediction for a potato crop. Am. J. Potato Res. 2010, 87, 32–40. [Google Scholar] [CrossRef]
- Menna, A.; Nguyen, D.; Guttman, D.S.; Desveaux, D. Elevated temperature differentially influences effector-triggered immunity outputs in Arabidopsis. Front. Plant Sci. 2015, 6, 995. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.-J.; Evans, N.; Li, Z.-Q.; Eckert, M.; Chèvre, A.-M.; Renard, M.; Fitt, B.D.L. Temperature and leaf wetness duration affect phenotypic expression of Rlm6—mediated resistance to Leptosphaeria maculans in Brassica napus. N. Phytol. 2006, 170, 129–141. [Google Scholar] [CrossRef]
- Malamy, J.; Hennig, J.; Klessig, D.F. Temperature-dependent induction of salicylic acid and its conjugates during the resistance response to tobacco mosaic virus infection. Plant Cell 1992, 4, 359–366. [Google Scholar] [CrossRef]
- Jambunathan, N.; Siani, J.M.; McNellis, T.W. A humidity-sensitive Arabidopsis copine mutant exhibits precocious cell death and increased disease resistance. Plant Cell 2001, 13, 2225–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jambunathan, N.; McNellis, T.W. Regulation of Arabidopsis COPINE 1 gene expression in response to pathogens and abiotic stimuli. Plant Physiol. 2003, 132, 1370–1381. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jambunathan, N.; McNellis, T.W. Transgenic expression of the von Willebrand a domain of the BONZAI 1/COPINE 1 protein triggers a lesion-mimic phenotype in Arabidopsis. Planta 2005, 221, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Grisafi, P.; Cheng, S.-H.; Fink, G.R. Plant growth homeostasis is controlled by the Arabidopsis BON1 and BAP1 genes. Genes Dev. 2001, 15, 2263–2272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.; Yang, H.; Grisafi, P.; Sanchatjate, S.; Fink, G.R.; Sun, Q.; Hua, J. The BON/CPN gene family represses cell death and promotes cell growth in Arabidopsis. Plant J. 2006, 45, 166–179. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pennington, B.O.; Hua, J. Multiple R-like genes negatively regulated by BON1 and BON3 in Arabidopsis. Mol. Plant Microbe Interact. 2009, 7, 840–848. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Hua, J. A Haplotype-Specific Resistance Gene Regulated by BONZAI1 Mediates Temperture-Dependent Growth Control in Arabidopsis. Plant Cell 2004, 16, 1060–1071. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yang, S.; Yang, H.; Hua, J. The TIR—NB—LRR gene SNC1 is regulated at the transcript level by multiple factors. Mol. Plant Microbe Interact. 2007, 20, 1449–1456. [Google Scholar] [CrossRef]
- Chellappan, P.; Vanitharani, R.; Ogbe, F.; Fauquet, C.M. Effect of temperature on Geminivirus-induced RNA silencing in plants. Plant Physiol. 2005, 138, 1828–1841. [Google Scholar] [CrossRef] [Green Version]
- Larkan, N.J.; Lydiate, D.J.; Parkin, I.A.; Nelson, M.N.; Epp, D.J.; Cowling, W.A.; Rimmer, S.R.; Borhan, M.H. The Brassica napus blackleg resistance gene LepR3 encodes a receptor-like protein triggered by the Leptosphaeria maculans effector AVRLM1. N. Phytol. 2013, 197, 595–605. [Google Scholar] [CrossRef]
- Dandena, H.B.; Zhang, Q.; Zhou, T.; Hirani, A.H.; Liu, Z.; Fernando, D.W.G.; Duncan, R.W.; Li, G. Analysis of quantitative adult plant resistance to blackleg in Brassica napus. Mol. Breed. 2019, 39, 124. [Google Scholar] [CrossRef]
- Neik, T.X.; Ghanbarnia, K.; Ollivier, B.; Scheben, A.; Severn-Ellis, A.; Larkan, N.J.; Haddadi, P.; Fernando, W.G.D.; Rouxel, T.; Batley, J.; et al. Two independent approaches converge to the cloning of a new Leptosphaeria maculans avirulence effector gene, AvrLmS-Lep2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Stintzi, A.; Heitz, T.; Prasad, V.; Wiedemann-Merdinoglu, S.; Kauffmann, S.; Geoffroy, P.; Legrand, M.; Fritig, B. Plant ‘pathogenesis-related’ proteins and their role in defense against pathogens. Biochimie 1993, 75, 687–706. [Google Scholar] [CrossRef]
- Borad, V.; Sriram, S. Pathogenesis-related proteins for the plan t protection. J. Exp. Sci. 2008, 22, 189–196. [Google Scholar]
- Thibaud, M.C.; Gineste, S.; Nussaume, L.; Robaglia, C. Sucrose increases pathogenesis-related PR-2 gene expression in Arabidopsis thaliana through an SA-dependent but NPR1-independent signaling pathway. C Plant Physiol. Biochem. 2004, 42, 81–88. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of temperature modulation of plant defense against biotrophic microbes. Mol. Plant Microbe Interact. 2009, 22, 498–506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velásquez, A.C.; Castroverde, C.D.M.; He, S.Y. Plant-pathogen warfare under changing climate conditions. Curr. Biol. 2018, 28, R619–R634. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Y.; Qian, W.; Hua, J. Temperature modulates plant defense responses through NB-LRR proteins. PLoS Pathog. 2010, 6, e1000844. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Cui, D.; Liu, J.; Zhao, J.; Liu, C.; Xin, W.; Li, Y.; Liu, N.; Ren, D.; Tang, D.; et al. Arabidopsis ZED1—related kinases mediate the temperature—sensitive intersection of immune response and growth homeostasis. N. Phytol. 2017, 215, 711–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, Y.; Xie, Z.; Chen, W.; Glazebrook, J.; Chang, H.-S.; Han, B.; Zhu, T.; Zou, G.; Katagiri, F. Quantitative nature of Arabidopsis responses during compatible and incompatible interactions with the bacterial pathogen Pseudomonas syringae. Plant Cell 2003, 15, 317–330. [Google Scholar] [CrossRef] [Green Version]
- McDowell, J.M.; Simon, S.A. Recent insights into R gene evolution. Mol. Plant Pathol. 2006, 7, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)). Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, C.; Zou, Z.; Fernando, W.G.D. The Effect of Temperature on the Hypersensitive Response (HR) in the Brassica napus–Leptosphaeria maculans Pathosystem. Plants 2021, 10, 843. https://doi.org/10.3390/plants10050843
Yang C, Zou Z, Fernando WGD. The Effect of Temperature on the Hypersensitive Response (HR) in the Brassica napus–Leptosphaeria maculans Pathosystem. Plants. 2021; 10(5):843. https://doi.org/10.3390/plants10050843
Chicago/Turabian StyleYang, Cunchun, Zhongwei Zou, and Wannakuwattewaduge Gerard Dilantha Fernando. 2021. "The Effect of Temperature on the Hypersensitive Response (HR) in the Brassica napus–Leptosphaeria maculans Pathosystem" Plants 10, no. 5: 843. https://doi.org/10.3390/plants10050843
APA StyleYang, C., Zou, Z., & Fernando, W. G. D. (2021). The Effect of Temperature on the Hypersensitive Response (HR) in the Brassica napus–Leptosphaeria maculans Pathosystem. Plants, 10(5), 843. https://doi.org/10.3390/plants10050843






