Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.)
Abstract
:1. Introduction
2. Results
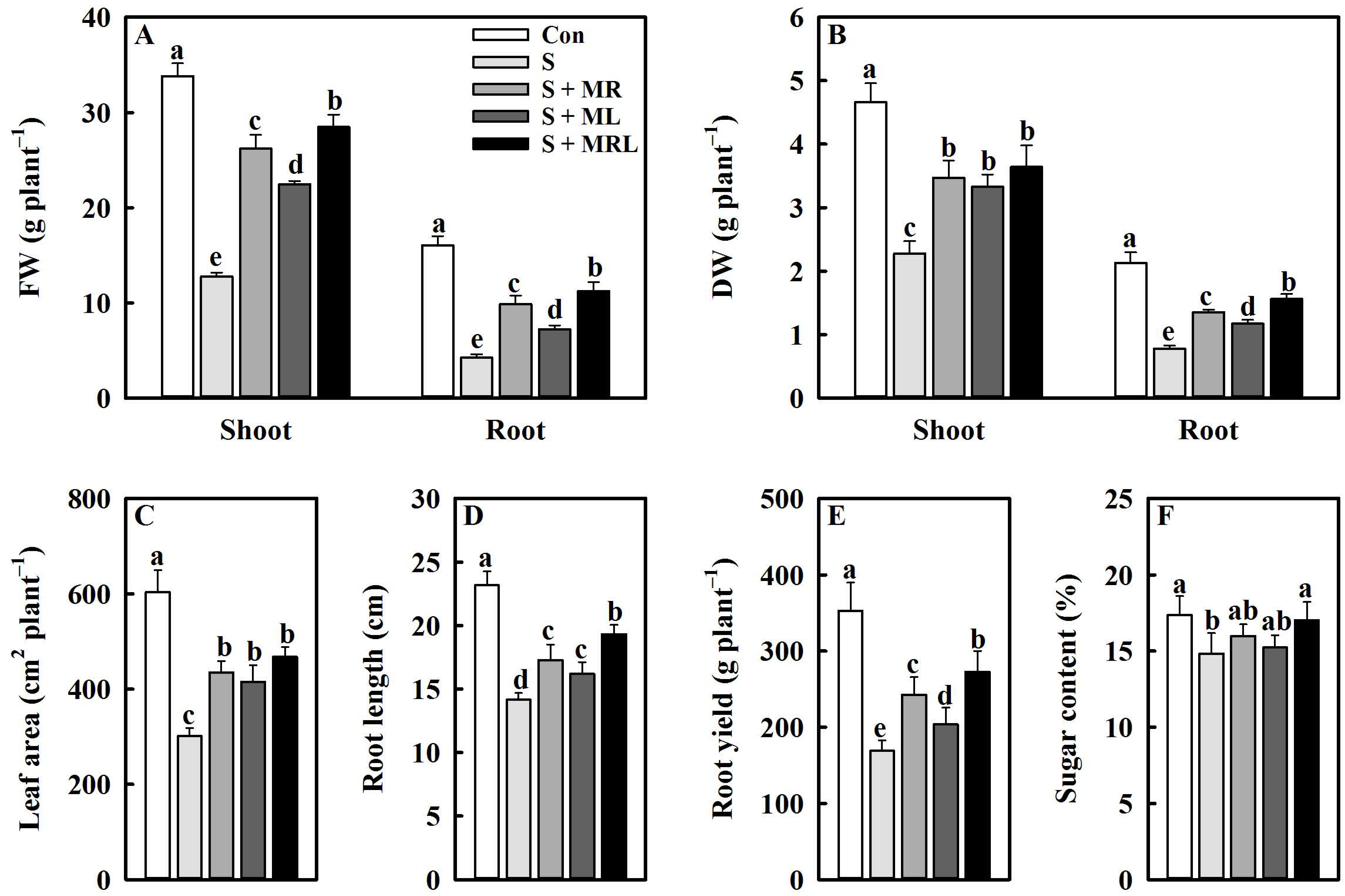
2.1. Melatonin Improved the Growth of Sugar Beet Seedlings and the Yield and Sugar Content of Roots under Salt Stress
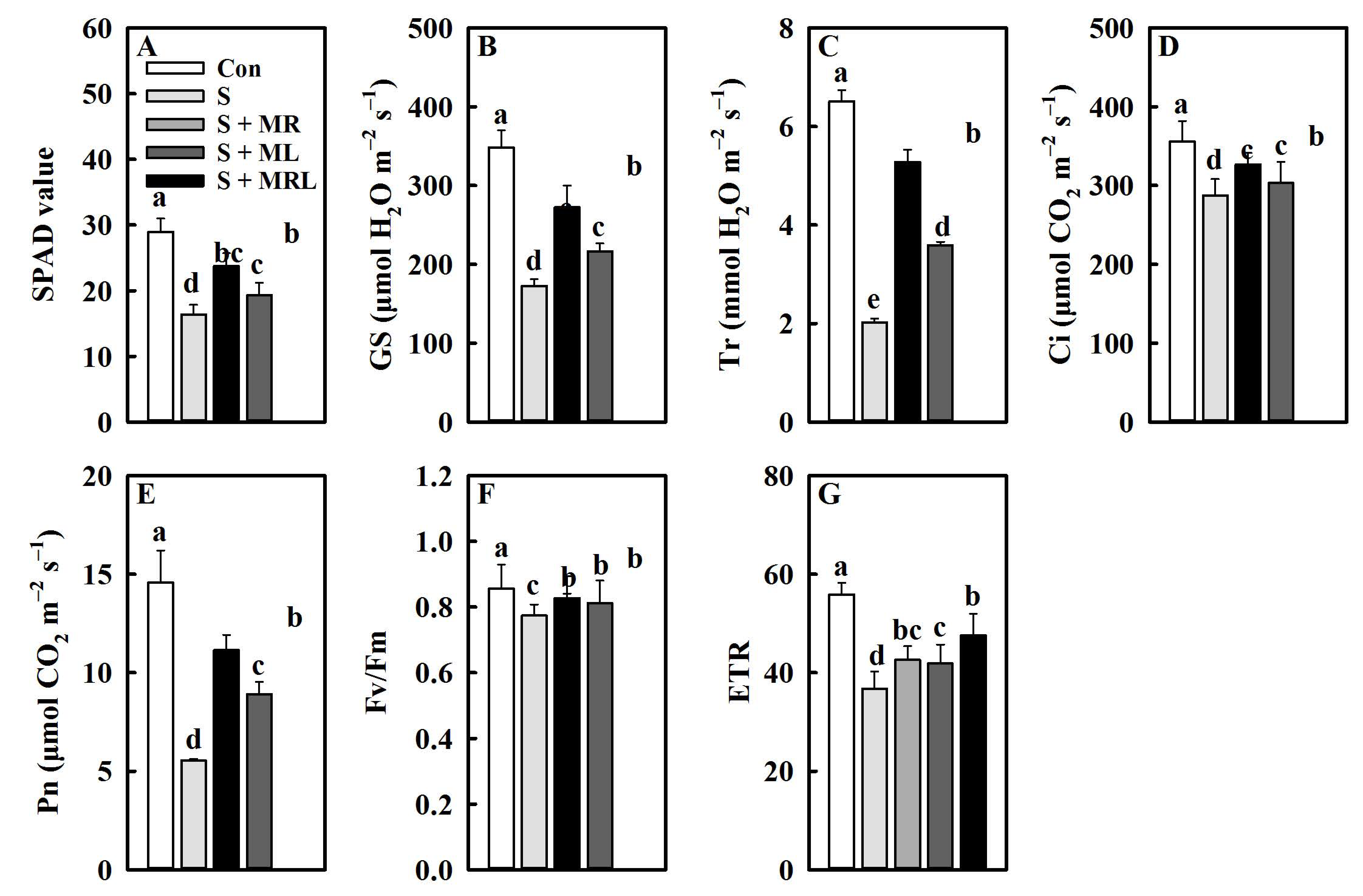
2.2. Melatonin Improved the Chlorophyll Content, Gas Exchange Parameters, and Chlorophyll Fluorescence Parameters of Sugar Beet Seedlings under Salt Stress
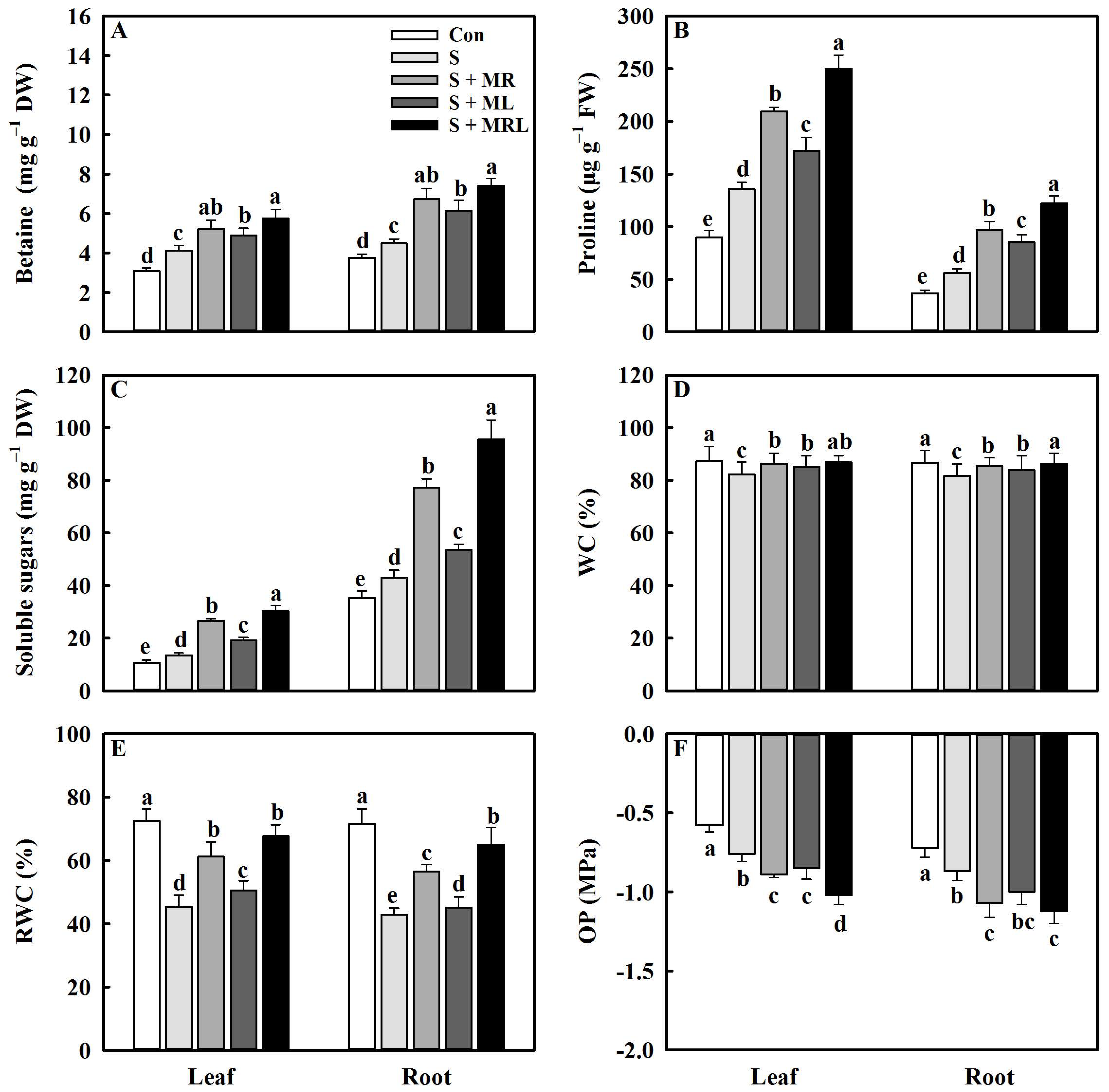
2.3. Melatonin Improved the Accumulation of Osmolytes and the Water Status of Sugar Beet Seedlings under Salt Stress
2.4. Melatonin Regulated the Ion Homeostasis of Sugar Beet Seedlings under Salt Stress
2.5. Melatonin Regulated H+, Na+, and K+ Fluxes and H+-Pump Activities in the Roots of Sugar Beet Seedlings under Salt Stress
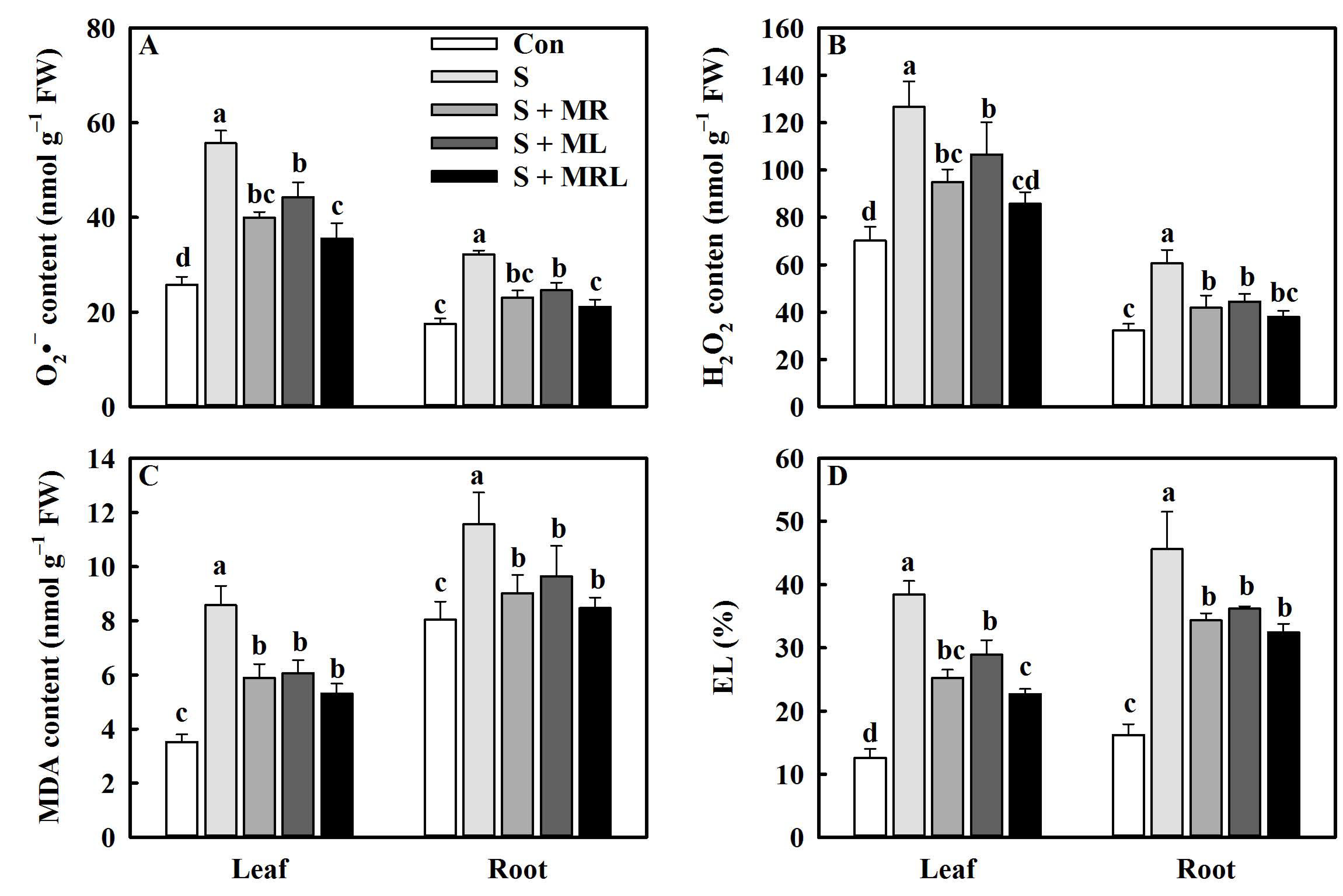
2.6. Melatonin Inhibited ROS Generation and Promoted Membrane Stability in the Sugar Beets under Salt Stress
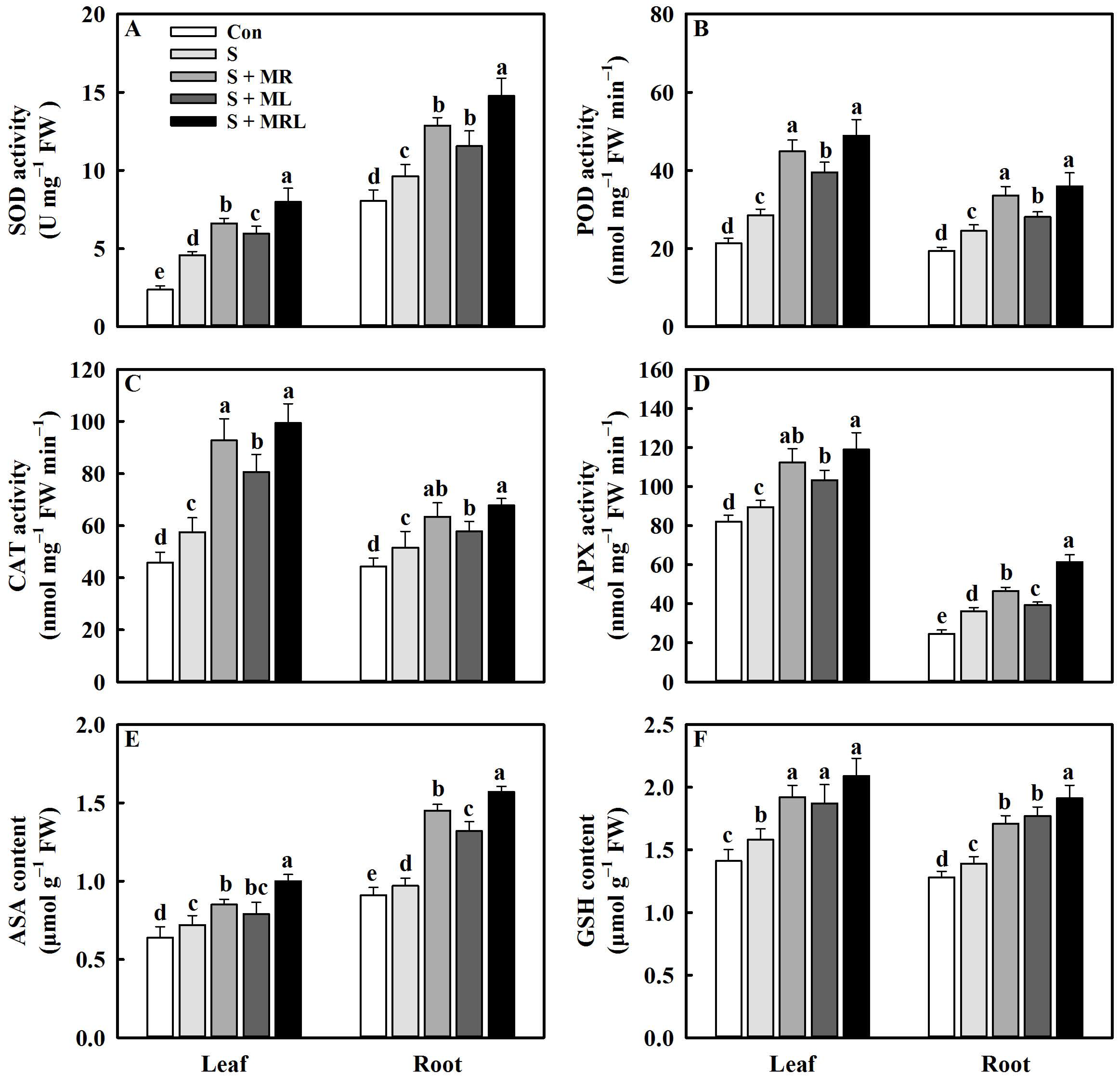
2.7. Melatonin Regulated the Antioxidative System of Sugar Beets under Salt Stress
2.8. Melatonin Increased the Contents of Polyphenolic Compounds in Sugar Beets under Salt Stress
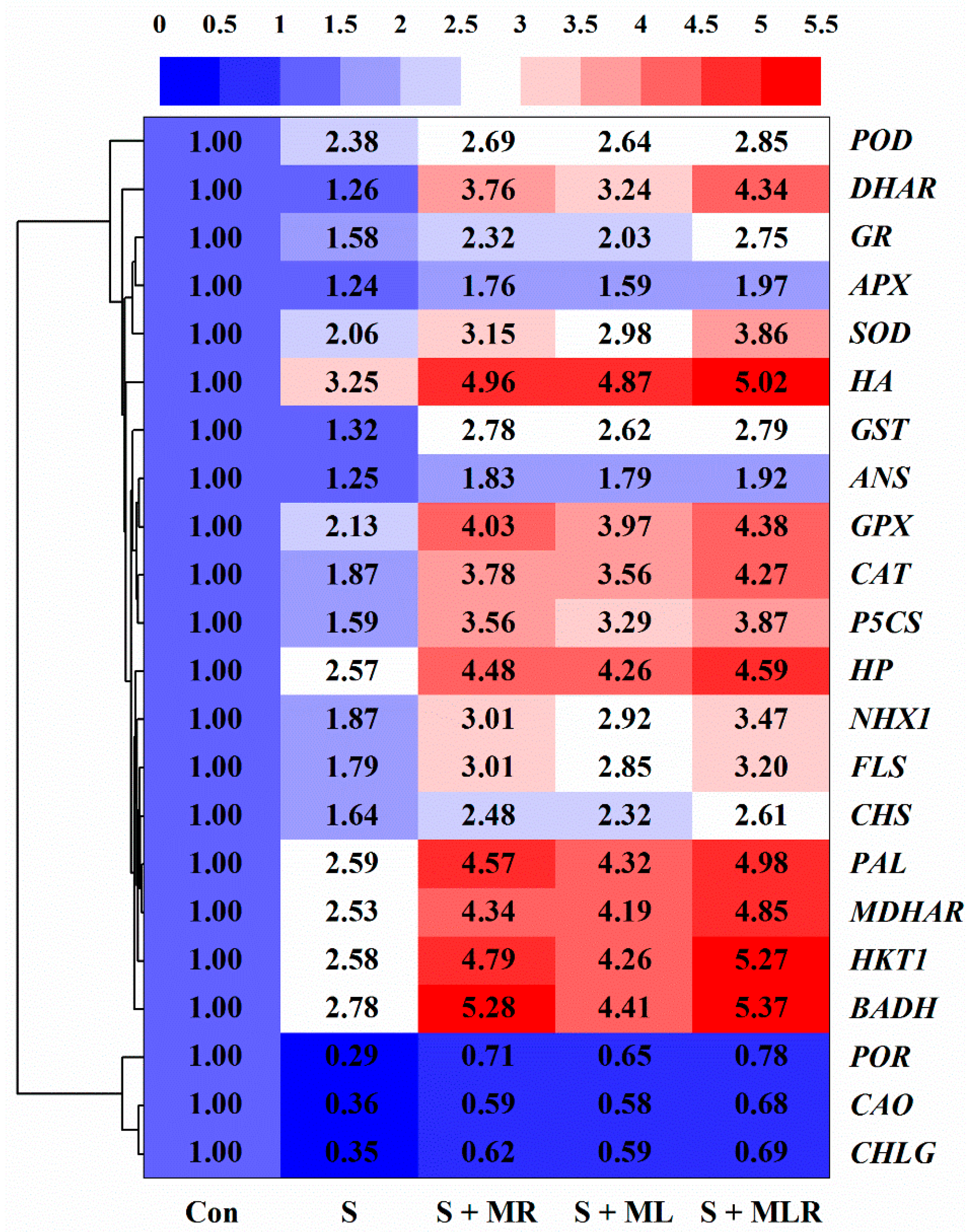
2.9. Melatonin Regulated the Expression Level of Genes in Sugar Beets under Salt Stress
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growing Conditions
4.2. Treatments and Experimental Layout
4.3. Determination of Morphological Parameters and Root Yield and Sugar Content
4.4. Determination of Chlorophyll Content and Gas Exchange and Chlorophyll Fluorescence Parameters
4.5. Determination of Osmolyte Contents
4.6. Determination of Water Status Parameters
4.7. Determination of Na+ and K+ Contents
4.8. Determination of H+, Na+, and K+ Fluxes and H+-Pump Activities of the Roots
4.9. Determination of Reactive Oxygen Species (ROS) Generation
4.10. Determination of MDA and EL
4.11. Determination of Antioxidant Enzyme Activities
4.12. Determination of Antioxidant Contents
4.13. Determination of Contents of Phenolic Compounds
4.14. Determination of Relative Gene Expression
4.15. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Land and Plant Nutrition Management Service. 2008. Available online: http://www.fao.org/ag/agl/agll/spush/ (accessed on 4 August 2020).
- FAO. 2019. Available online: www.fao.org/faostat/en/#data (accessed on 12 October 2019).
- Da Silva, C.J.; Fontes, E.P.B.; Modolo, L.V. Salinity-induced accumulation of endogenous H2S and NO is associated with modulation of the antioxidant and redox defense systems in Nicotiana tabacum L. cv. Havana. Plant Sci. 2017, 256, 148–159. [Google Scholar] [CrossRef]
- Bor, M.; Ozdemir, F.; Turkan, I. The effect of salt stress on lipid peroxidation and antioxidants in leaves of sugar beet (Beta vulgaris L.) and wild beet (Beta maritima L.). Plant Sci. 2003, 164, 77–84. [Google Scholar] [CrossRef]
- Liu, H.; Wang, Q.; Yu, M.; Zhang, Y.; Wu, Y.; Zhang, H. Transgenic salt-tolerant sugar beet (Beta vulgaris L.) constitutively expressing an Arabidopsis thaliana vacuolar Na+/H+ antiporter gene, AtNHX3, accumulates more soluble sugar but less salt in storage roots. Plant Cell Environ. 2008, 31, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.S.; Persicke, M.; ElSayed, A.I.; Kalinowski, J.; Dietz, K.J. Metabolite profiling at the cellular and subcellular level reveals metabolites associated with salinity tolerance in sugar beet. J. Exp. Bot. 2017, 68, 5961–5976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.K. Plant salt tolerance. Trends Plant Sci. 2001, 6, 66–71. [Google Scholar] [CrossRef]
- Munns, R.; Tester, M. Mechanisms of salinity tolerance. Annu. Rev. Plant Biol. 2008, 59, 651–681. [Google Scholar] [CrossRef] [Green Version]
- Hasanuzzaman, M.; Alam, M.M.; Rahman, A.; Hasanuzzaman, M.; Nahar, K.; Fujita, M. Exogenous proline and glycine betaine mediated upregulation of antioxidant defense and glyoxalase systems provides better protection against salt-induced oxidative stress in two rice (Oryza sativa L.) varieties. Biomed Res. Int. 2014, 2014, 757219. [Google Scholar] [CrossRef] [Green Version]
- Per, T.S.; Khan, N.A.; Reddy, P.S.; Masood, A.; Hasanuzzaman, M.; Khan, M.I.R.; Anjum, N.A. Approaches in modulating proline metabolism in plants for salt and drought stress tolerance: Phytohormones, mineral nutrients and transgenics. Plant Physiol. Biochem. 2017, 115, 126–140. [Google Scholar] [CrossRef]
- Anschutz, U.; Becker, D.; Shabala, S. Going beyond nutrition: Regulation of potassium homoeostasis as a common denominator of plant adaptive responses to environment. J. Plant Physiol. 2014, 171, 670–687. [Google Scholar] [CrossRef]
- Chinnusamy, V.; Jagendorf, A.; Zhu, J.K. Understanding and improving salt tolerance in plants. Crop Sci. 2005, 45, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Ramon Acosta-Motos, J.; Fernanda Ortuno, M.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Jesus Sanchez-Blanco, M.; Antonio Hernandez, J. Plant responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, A.; Kumar, V.; Shahzad, B.; Ramakrishnan, M.; Sidhu, G.P.S.; Bali, A.S.; Handa, N.; Kapoor, D.; Yadav, P.; Khanna, K.; et al. Photosynthetic response of plants under different abiotic stresses: A review. J. Plant Growth Regul. 2020, 39, 509–531. [Google Scholar] [CrossRef]
- Khanna-Chopra, R. Leaf senescence and abiotic stresses share reactive oxygen species-mediated chloroplast degradation. Protoplasma 2012, 249, 469–481. [Google Scholar] [CrossRef]
- Negrao, S.; Schmockel, S.M.; Tester, M. Evaluating physiological responses of plants to salinity stress. Ann. Bot. 2017, 119, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Farhangi-Abriz, S.; Torabian, S. Antioxidant enzyme and osmotic adjustment changes in bean seedlings as affected by biochar under salt stress. Ecotoxicol. Environ. Saf. 2017, 137, 64–70. [Google Scholar] [CrossRef]
- Iqbal, N.; Umar, S.; Khan, N.A.; Khan, M.I.R. A new perspective of phytohormones in salinity tolerance: Regulation of proline metabolism. Environ. Exp. Bot. 2014, 100, 34–42. [Google Scholar] [CrossRef]
- Mansour, M.M.F.; Ali, E.F. Evaluation of proline functions in saline conditions. Phytochemistry 2017, 140, 52–68. [Google Scholar] [CrossRef]
- Tester, M.; Davenport, R. Na+ tolerance and Na+ transport in higher plants. Ann. Bot. 2003, 91, 503–527. [Google Scholar] [CrossRef]
- Apse, M.P.; Aharon, G.S.; Snedden, W.A.; Blumwald, E. Salt tolerance conferred by overexpression of a vacuolar Na+/H+ antiport in Arabidopsis. Science 1999, 285, 1256–1258. [Google Scholar] [CrossRef] [PubMed]
- Navrot, N.; Rouhier, N.; Gelhaye, E.; Jacquot, J.P. Reactive oxygen species generation and antioxidant systems in plant mitochondria. Physiol. Plant. 2007, 129, 185–195. [Google Scholar] [CrossRef]
- Karuppanapandian, T.; Moon, J.-C.; Kim, C.; Manoharan, K.; Kim, W. Reactive oxygen species in plants: Their generation, signal transduction, and scavenging mechanisms. Aust. J. Crop Sci. 2011, 5, 709–725. [Google Scholar]
- Das, S.K.; Patra, J.K.; Thatoi, H. Antioxidative response to abiotic and biotic stresses in mangrove plants: A review. Int. Rev. Hydrobiol. 2016, 101, 3–19. [Google Scholar] [CrossRef]
- Agati, G.; Azzarello, E.; Pollastri, S.; Tattini, M. Flavonoids as antioxidants in plants: Location and functional significance. Plant Sci. 2012, 196, 67–76. [Google Scholar] [CrossRef]
- Gill, S.S.; Anjum, N.A.; Hasanuzzaman, M.; Gill, R.; Trivedi, D.K.; Ahmad, I.; Pereira, E.; Tuteja, N. Glutathione and glutathione reductase: A boon in disguise for plant abiotic stress defense operations. Plant Physiol. Biochem. 2013, 70, 204–212. [Google Scholar] [CrossRef]
- Desoky, E.S.M.; Merwad, A.R.M.; Rady, M.M. Natural biostimulants improve saline soil characteristics and salt stressed-sorghum performance. Commun. Soil Sci. Plant Anal. 2018, 49, 967–983. [Google Scholar] [CrossRef]
- Qin, H.; Li, Y.; Huang, R. Advances and challenges in the breeding of salt-tolerant rice. Int. J. Mol. Sci. 2020, 21, 8385. [Google Scholar] [CrossRef]
- Bahcesular, B.; Yildirim, E.D.; Karacocuk, M.; Kulak, M.; Karaman, S. Seed priming with melatonin effects on growth, essential oil compounds and antioxidant activity of basil (Ocimum basilicum L.) under salinity stress. Ind. Crop. Prod. 2020, 146, 112165. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.; Wang, Z.; Zou, C.; Wang, B.; Zhang, H.; Gai, Z.; Zhang, P.; Wang, Y.; Li, C. Exogenous allantoin improves the salt tolerance of sugar beet by increasing putrescine metabolism and antioxidant activities. Plant Physiol. Biochem. 2020, 154, 699–713. [Google Scholar] [CrossRef]
- Kubatka, P.; Zubor, P.; Busselberg, D.; Kwon, T.K.; Adamek, M.; Petrovic, D.; Opatrilova, R.; Gazdikova, K.; Caprnda, M.; Rodrigo, L.; et al. Melatonin and breast cancer: Evidences from preclinical and human studies. Crit. Rev. Oncol. Hematol. 2018, 122, 133–143. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernandez-Ruiz, J. Melatonin: A New Plant Hormone and/or a Plant Master Regulator? Trends Plant Sci. 2019, 24, 38–48. [Google Scholar] [CrossRef]
- Tan, D.X.; Manchester, L.C.; Reiter, R.J.; Qi, W.B.; Karbownik, M.; Calvo, J.R. Significance of melatonin in antioxidative defense system: Reactions and products. Biol. Signals Recept. 2000, 9, 137–159. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Sun, X.; Li, C.; Wei, Z.; Liang, D.; Ma, F. Long-term exogenous application of melatonin delays drought-induced leaf senescence in apple. J. Pineal Res. 2013, 54, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Caputo, G.A.; Wadl, P.A.; McCarty, L.; Adelberg, J.; Jennings, K.M.; Cutulle, M. In vitro safening of bentazon by melatonin in sweetpotato (Ipomoea batatas). Hortscience 2020, 55, 1406–1410. [Google Scholar] [CrossRef]
- Jahan, M.S.; Guo, S.; Baloch, A.R.; Sun, J.; Shu, S.; Wang, Y.; Ahammed, G.J.; Kabir, K.; Roy, R. Melatonin alleviates nickel phytotoxicity by improving photosynthesis, secondary metabolism and oxidative stress tolerance in tomato seedlings. Ecotoxicol. Environ. Saf. 2020, 197, 110593. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Wei, H.; Li, W.; Liu, Z.; Tang, S.; Chen, L.; Ding, C.; Jiang, Y.; Ding, Y.; Li, G. Melatonin improves K+ and Na+ homeostasis in rice under salt stress by mediated nitric oxide. Ecotoxicol. Environ. Saf. 2020, 206, 111358. [Google Scholar] [CrossRef]
- Tousi, S.; Zoufan, P.; Ghahfarrokhie, A.R. Alleviation of cadmium-induced phytotoxicity and growth improvement by exogenous melatonin pretreatment in mallow (Malva parviflora) plants. Ecotoxicol. Environ. Saf. 2020, 206, 111403. [Google Scholar] [CrossRef]
- Zhang, T.G.; Shi, Z.F.; Zhang, X.H.; Zheng, S.; Wang, J.; Mo, J.N. Alleviating effects of exogenous melatonin on salt stress in cucumber. Sci. Hortic. 2020, 262, 109070. [Google Scholar] [CrossRef]
- Li, J.; Yuan, F.; Liu, Y.; Zhang, M.; Liu, Y.; Zhao, Y.; Wang, B.; Chen, M. Exogenous melatonin enhances salt secretion from salt glands by upregulating the expression of ion transporter and vesicle transport genes in Limonium bicolor. Bmc Plant Biol. 2020, 20, 493. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.; Kamran, M.; Abbasi, G.H.; Saleem, M.H.; Ahmad, S.; Parveen, A.; Malik, Z.; Afzal, S.; Ahmar, S.; Dawar, K.M.; et al. Melatonin-induced salinity tolerance by ameliorating osmotic and oxidative stress in the seedlings of two tomato (Solanum lycopersicum L.) cultivars. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Kaur, H.; Bhatla, S.C. Melatonin and nitric oxide modulate glutathione content and glutathione reductase activity in sunflower seedling cotyledons accompanying salt stress. Nitric Oxide 2016, 59, 42–53. [Google Scholar] [CrossRef]
- Kaya, C.; Higgs, D.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. Integrative roles of nitric oxide and hydrogen sulfide in melatonin-induced tolerance of pepper (Capsicum annuum L.) plants to iron deficiency and salt stress alone or in combination. Physiol. Plant. 2020, 168, 256–277. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Chen, Q.; Chen, W.; Guo, Q.; Xia, Y.; Wang, S.; Jing, D.; Liang, G. Physiological and transcription analyses reveal the regulatory mechanism of melatonin in inducing drought resistance in loquat (Eriobotrya japonica Lindl.) seedlings. Environ. Exp. Bot. 2021, 181, 104291. [Google Scholar] [CrossRef]
- Kamiab, F. Exogenous melatonin mitigates the salinity damages and improves the growth of pistachio under salinity stress. J. Plant Nutr. 2020, 43, 1468–1484. [Google Scholar] [CrossRef]
- Borisova-Mubarakshina, M.M.; Ivanov, B.N.; Vetoshkina, D.V.; Lubimov, V.Y.; Fedorchuk, T.P.; Naydov, I.A.; Kozuleva, M.A.; Rudenko, N.N.; Dall’Osto, L.; Cazzaniga, S.; et al. Long-term acclimatory response to excess excitation energy: Evidence for a role of hydrogen peroxide in the regulation of photosystem II antenna size. J. Exp. Bot. 2015, 66, 7151–7164. [Google Scholar] [CrossRef] [Green Version]
- Yin, Z.P.; Lu, J.Z.; Meng, S.D.; Liu, Y.L.; Mostafa, I.; Qi, M.F.; Li, T.L. Exogenous melatonin improves salt tolerance in tomato by regulating photosynthetic electron flux and the ascorbate-glutathione cycle. J. Plant Interact. 2019, 14, 453–463. [Google Scholar] [CrossRef]
- Ding, F.; Liu, B.; Zhang, S. Exogenous melatonin ameliorates cold-induced damage in tomato plants. Sci. Hortic. 2017, 219, 264–271. [Google Scholar] [CrossRef]
- Qiao, Y.; Ren, J.; Yin, L.; Liu, Y.; Deng, X.; Liu, P.; Wang, S. Exogenous melatonin alleviates PEG-induced short-term water deficiency in maize by increasing hydraulic conductance. BMC Plant Biol. 2020, 20, 218. [Google Scholar] [CrossRef]
- Farouk, S.; Elhindi, K.M.; Alotaibi, M.A. Silicon supplementation mitigates salinity stress on Ocimum basilicum L. via improving water balance, ion homeostasis, and antioxidant defense system. Ecotoxicol. Environ. Saf. 2020, 206, 111396. [Google Scholar] [CrossRef]
- Leopoldini, M.; Russo, N.; Chiodo, S.; Toscano, M. Iron chelation by the powerful antioxidant flavonoid quercetin. J. Agric. Food. Chem. 2006, 54, 6343–6351. [Google Scholar] [CrossRef]
- Mukherjee, S. Novel perspectives on the molecular crosstalk mechanisms of serotonin and melatonin in plants. Plant Physiol. Biochem. 2018, 132, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Chung, R.S.; Chen, C.C.; Ng, L.T. Nitrogen fertilization affects the growth performance, betaine and polysaccharide concentrations of Lycium barbarum. Ind. Crop. Prod. 2010, 32, 650–655. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil. 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Spiro, R.G. Analysis of sugars found in glycoproteins. Methods Enzymol. 1966, 8, 3–26. [Google Scholar] [CrossRef]
- Chen, Y.E.; Mao, J.J.; Sun, L.Q.; Huang, B.; Ding, C.B.; Gu, Y.; Liao, J.Q.; Hu, C.; Zhang, Z.W.; Yuan, S.; et al. Exogenous melatonin enhances salt stress tolerance in maize seedlings by improving antioxidant and photosynthetic capacity. Physiol. Plant. 2018, 164, 349–363. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Storey, R. Salt Tolerance, ion relations and the effect of root medium on the response of citrus to salinity. Funct. Plant Biol. 1995, 22, 101–114. [Google Scholar] [CrossRef]
- Kong, X.; Luo, Z.; Dong, H.; Eneji, A.E.; Li, W. Effects of non-uniform root zone salinity on water use, Na+ recirculation, and Na+ and H+ flux in cotton. J. Exp. Bot. 2012, 63, 2105–2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Yu, X.; Li, M.; Lang, D.; Zhang, X.; Xie, Z. Silicon promotes growth and root yield of glycyrrhiza uralensis under salt and drought stresses through enhancing osmotic adjustment and regulating antioxidant metabolism. Crop Prot. 2018, 107, 1–11. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Rajinder, S.D.; Pamela, P.D.; Trevor, A.T. Leaf senescense: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Dionisio-Sese, M.L.; Tobita, S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998, 135, 1–9. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- He, J.; Ren, Y.; Chen, X.; Chen, H. Protective roles of nitric oxide on seed germination and seedling growth of rice (Oryza sativa L.) under cadmium stress. Ecotoxicol. Environ. Saf. 2014, 108, 114–119. [Google Scholar] [CrossRef] [PubMed]
- Nabih, I.; el-Wassimy, M.T.; Metri, J. Structure and activity in molluscicides. II. Activity assary of catalase and peroxidase in snails. J. Pharm Sci. 1968, 57, 1609–1611. [Google Scholar] [CrossRef] [PubMed]
- Fielding, J.L.; Hall, J.L. A biochemical and cytochemical study of peroxidase activity in poots of Pisum sativum. J. Exp. Bot. 1978, 29, 983–991. [Google Scholar] [CrossRef]
- Logan, B.A.; Grace, S.C.; Adams Iii, W.W.; Demmig-Adams, B. Seasonal differences in xanthophyll cycle characteristics and antioxidants in Mahonia repens growing in different light environments. Oecologia 1998, 116, 9–17. [Google Scholar] [CrossRef]
- Griffith, O.W. Determination of glutathione and glutathione disulfide using glutathione reductase and 2-vinylpyridine. Anal. Biochem. 1980, 106, 207–212. [Google Scholar] [CrossRef]
- Singleton, V.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1964, 16, 144–158. [Google Scholar]
- Zhishen, J.; Mengcheng, T.; Jianming, W. The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals. Food Chem. 1999, 64, 555–559. [Google Scholar] [CrossRef]
- Abdel-Aal, E.S.M.; Hucl, P.A. A Rapid Method for quantifying total anthocyanins in blue aleurone and purple pericarp wheats. Cereal Chem. 1999, 76, 350–354. [Google Scholar] [CrossRef]
- Shen, J.L.; Wang, Y.; Shu, S.; Jahan, M.S.; Zhong, M.; Wu, J.Q.; Sun, J.; Guo, S.R. Exogenous putrescine regulates leaf starch overaccumulation in cucumber under salt stress. Sci. Hortic. 2019, 253, 99–110. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, P.; Liu, L.; Wang, X.; Wang, Z.; Zhang, H.; Chen, J.; Liu, X.; Wang, Y.; Li, C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants 2021, 10, 886. https://doi.org/10.3390/plants10050886
Zhang P, Liu L, Wang X, Wang Z, Zhang H, Chen J, Liu X, Wang Y, Li C. Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.). Plants. 2021; 10(5):886. https://doi.org/10.3390/plants10050886
Chicago/Turabian StyleZhang, Pengfei, Lei Liu, Xin Wang, Ziyang Wang, He Zhang, Jingting Chen, Xinyu Liu, Yubo Wang, and Caifeng Li. 2021. "Beneficial Effects of Exogenous Melatonin on Overcoming Salt Stress in Sugar Beets (Beta vulgaris L.)" Plants 10, no. 5: 886. https://doi.org/10.3390/plants10050886




