Photorespiration: The Futile Cycle?
Abstract
:1. Introduction
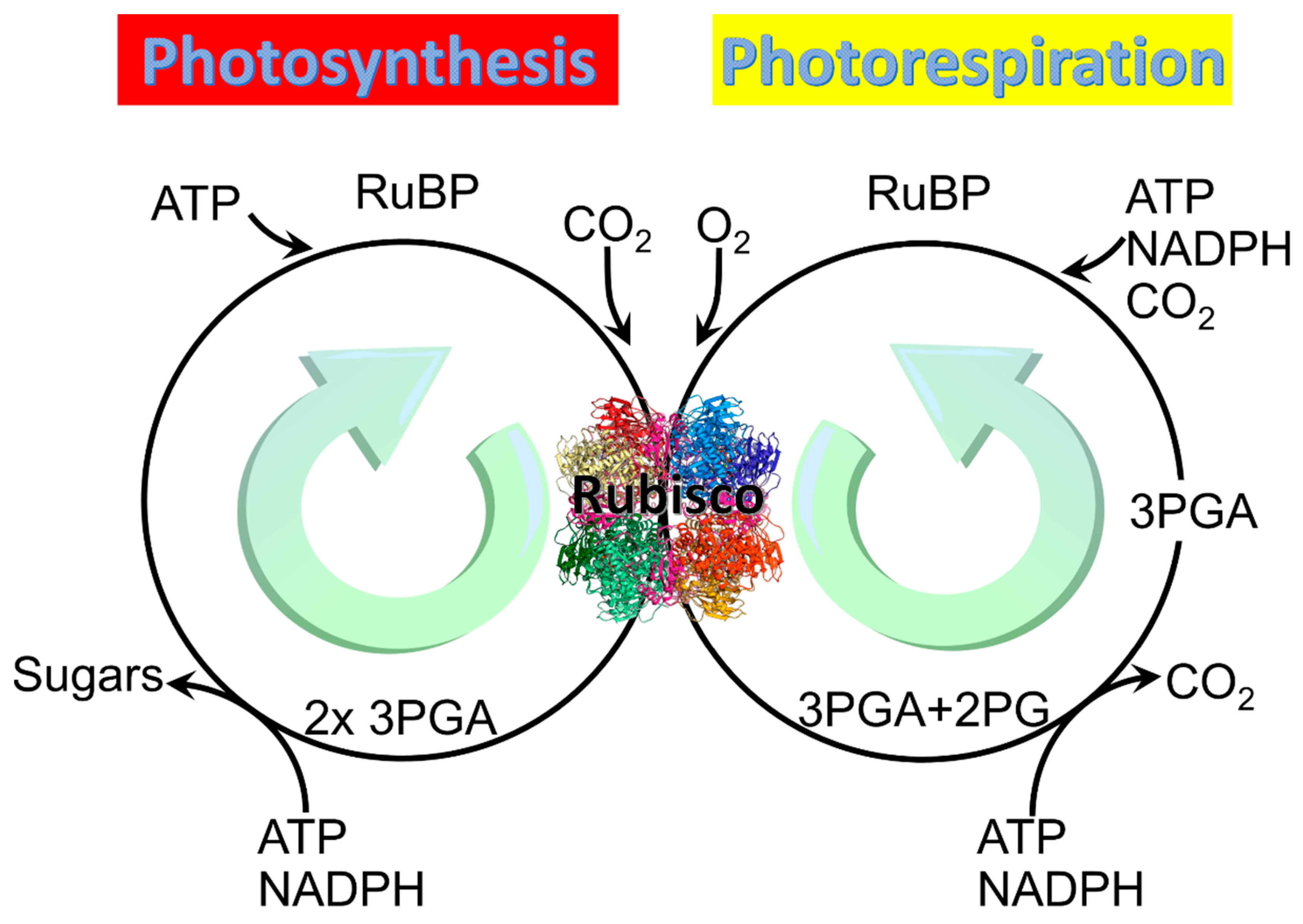
2. Photosynthesis vs. Photorespiration
2.1. Rubisco
2.2. Balance between Carboxylation and Oxygenation and Metal Cofactors
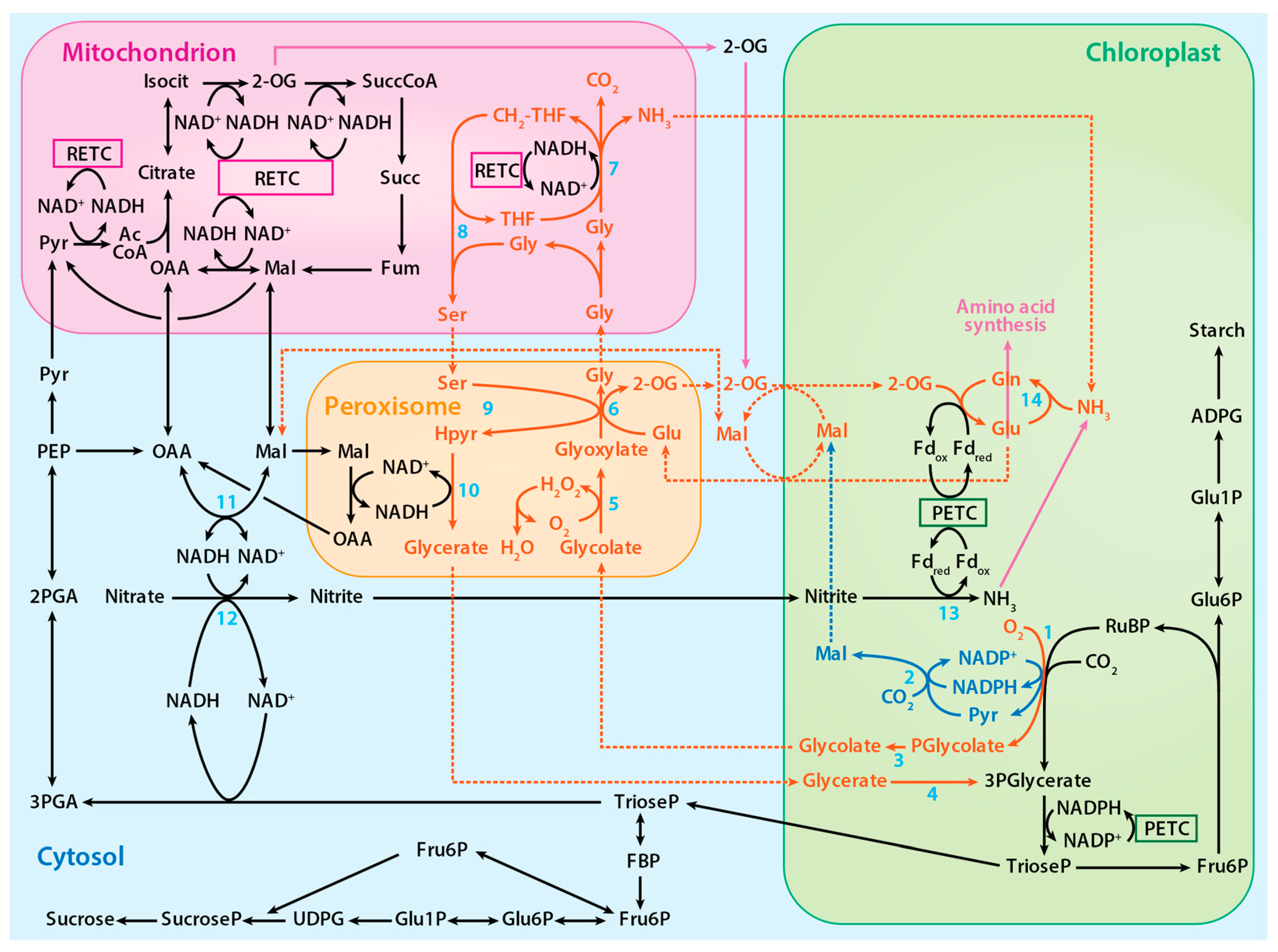
2.3. The Photorespiratory Pathway
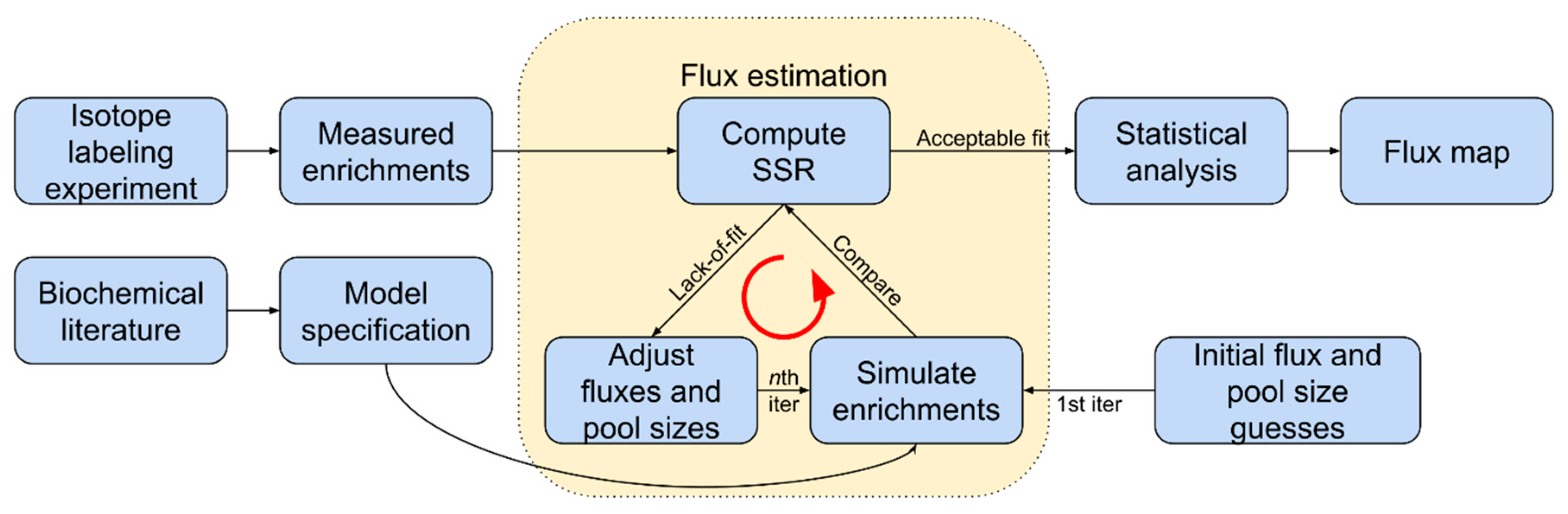
3. Estimating Rates of Photorespiration
3.1. Traditional Methods for Estimating Photorespiration
3.1.1. Post Illumination CO2 Burst
3.1.2. O2 Inhibition of Net CO2 Assimilation
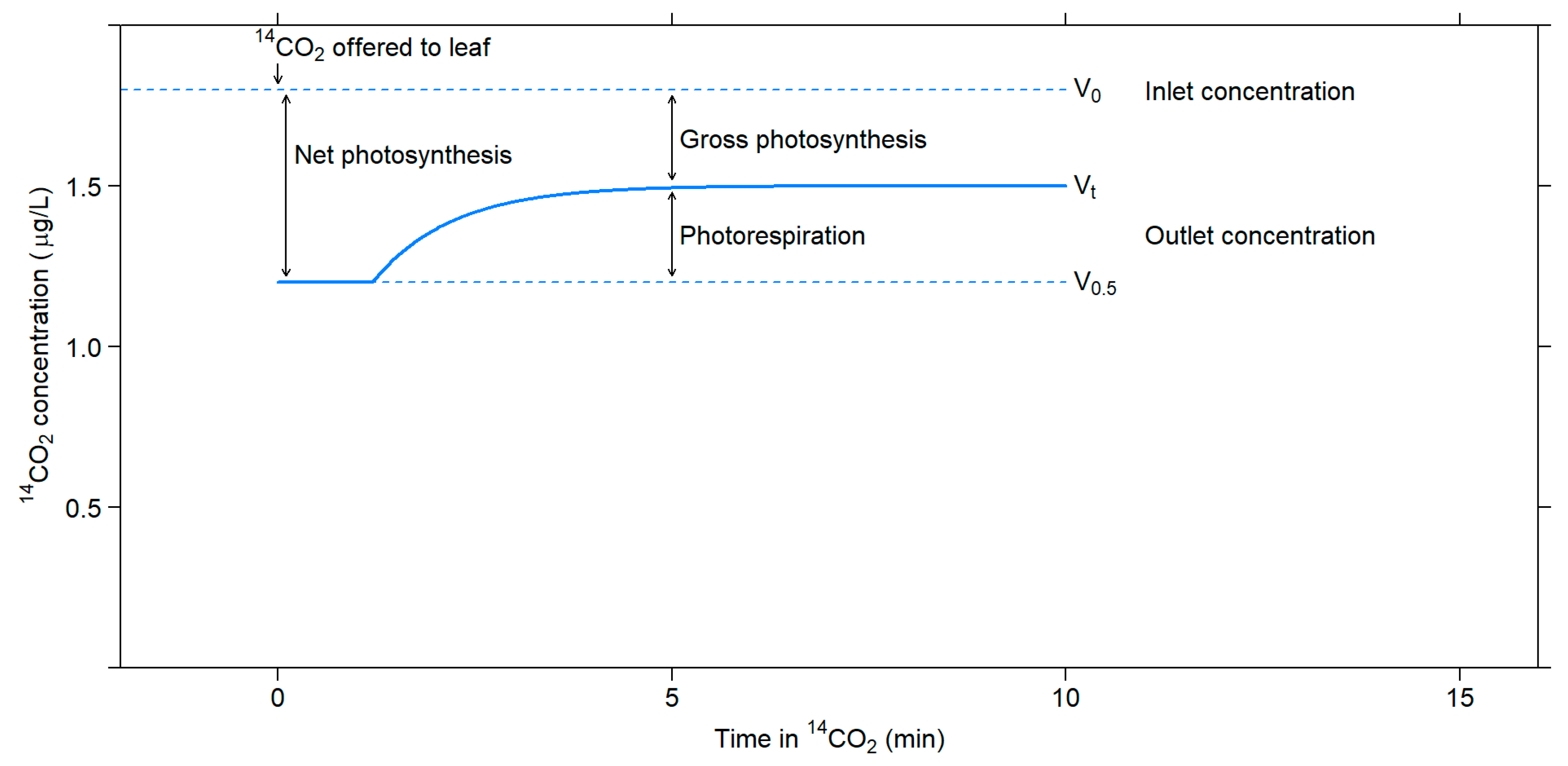
3.1.3. Photorespiration CO2 Efflux into CO2-Free Air
3.1.4. Ratio of 14CO2 to 12CO2 Uptake
3.2. Recent Methods for Estimating Photorespiration
3.2.1. Calculation from Kinetics Models
3.2.2. CO2 Efflux into 13CO2-Air
3.2.3. Labelling of Photosynthates with 14C
3.2.4. Measuring Photorespiratory Ammonia
3.2.5. Measuring 18O2 Consumption and Labeled Metabolites
3.2.6. NMR Measurements on 13C-Labeled Metabolites
3.2.7. Quantification of 2-Phosphoglycolate (2PG) and Photorespiratory Metabolites by Mass Spectrometry
3.2.8. CO2 Labeling and MS Analysis
3.2.9. Micro-Optode Measurement of O2 Consumption
4. Photorespiration and Other Metabolic Pathways
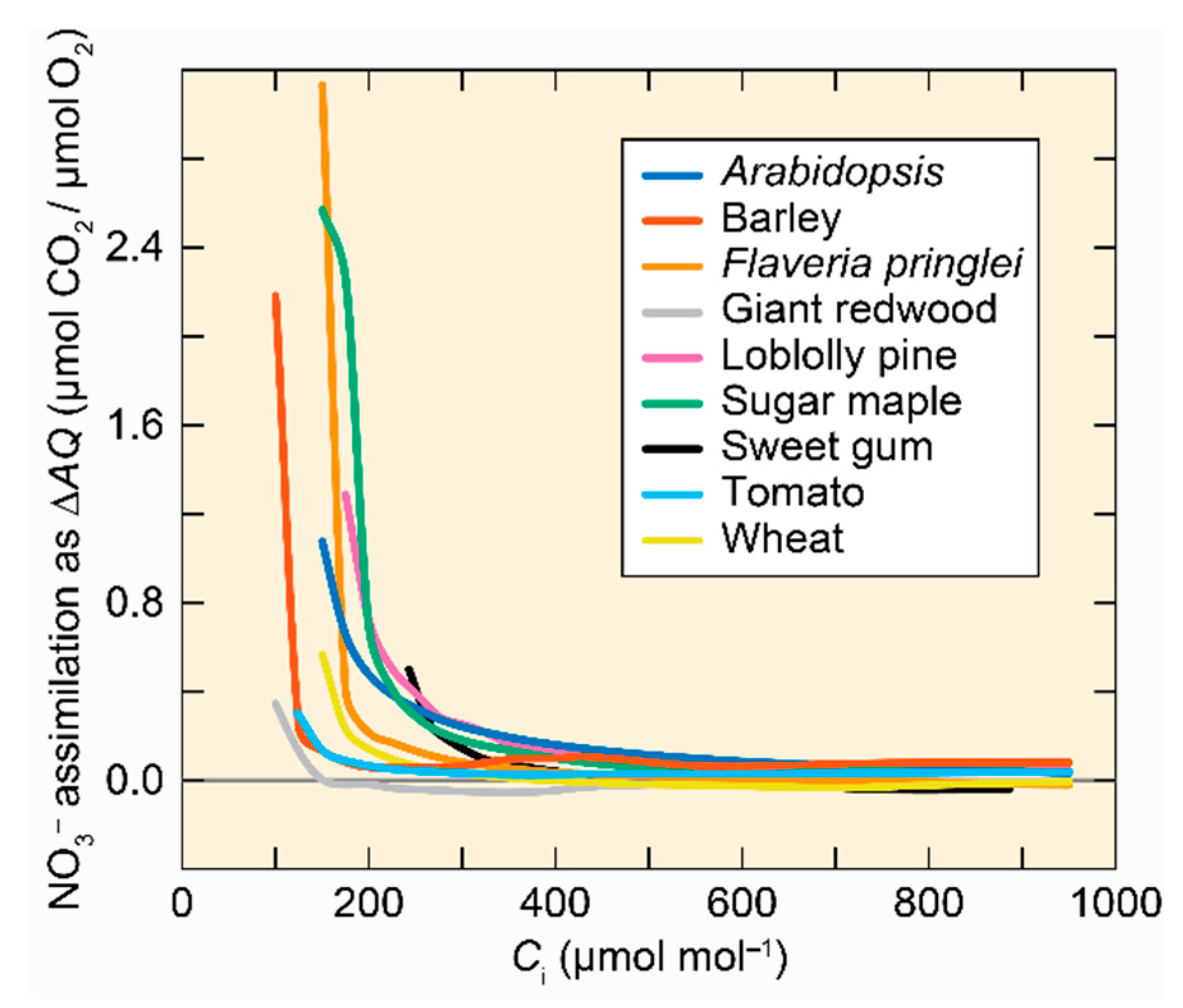
4.1. NO3− Assimilation
4.2. C1 Metabolism
4.3. Sulfur Assimilation
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Zelitch, I. Photorespiration: Studies with Whole Tissues. Photosynthesis II 1979, 28, 353–354. [Google Scholar]
- Canvin, D.T. Photorespiration: Comparison Between C3 and C4 Plants. Photosynthesis II 1979, 29, 368–396. [Google Scholar]
- Husic, D.W.; Husic, H.D.; Tolbert, N.E.; Black, C.C. The oxidative photosynthetic carbon cycle or C2 cycle. Crit. Rev. Plant Sci. 1987, 5, 45–99. [Google Scholar] [CrossRef]
- Sharkey, T.D. Estimating the rate of photorespiration in leaves. Physiol. Plant 1988, 73, 147–152. [Google Scholar] [CrossRef]
- Ogren, W.L. Photorespiration: Pathways, Regulation, and Modification. Annu. Rev. Plant Physiol. 1984, 35, 415–442. [Google Scholar] [CrossRef]
- Somerville, C.R.; Ogren, W.L. A phosphoglycolate phosphatase-deficient mutant of Arabidopsis. Nature 1979, 280, 833–836. [Google Scholar] [CrossRef]
- Busch, F.A. Photorespiration in the context of Rubisco biochemistry, CO2 diffusion and metabolism. Plant J. 2020, 101, 919–939. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J.; Lancaster, K.M. Manganese binding to Rubisco could drive a photorespiratory pathway that increases the energy efficiency of photosynthesis. Nat. Plants 2018, 4, 414–422. [Google Scholar] [CrossRef]
- Bloom, A.J.; Smart, D.R.; Nguyen, D.T.; Searles, P.S. Nitrogen assimilation and growth of wheat under elevated carbon dioxide. Proc. Natl. Acad. Sci. USA 2002, 99, 1730–1735. [Google Scholar] [CrossRef] [Green Version]
- McFadden, G.I. Origin and Evolution of Plastids and Photosynthesis in Eukaryotes. Cold Spring Harb. Perspect. Biol. 2014, 6, a016105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raven, J.A. Rubisco: Still the most abundant protein of Earth? New Phytol. 2013, 198, 1–3. [Google Scholar] [CrossRef]
- Badger, M.R.; Bek, E.J. Multiple Rubisco forms in proteobacteria: Their functional significance in relation to CO2 acquisition by the CBB cycle. J. Exp. Bot. 2008, 59, 1525–1541. [Google Scholar] [CrossRef] [Green Version]
- Kono, T.; Mehrotra, S.; Endo, C.; Kizu, N.; Matusda, M.; Kimura, H.; Mizohata, E.; Inoue, T.; Hasunuma, T.; Yokota, A.; et al. A RuBisCO-mediated carbon metabolic pathway in methanogenic archaea. Nat. Commun. 2017, 8, 14007. [Google Scholar] [CrossRef] [Green Version]
- Tabita, F.R.; Satagopan, S.; Hanson, T.E.; Kreel, N.E.; Scott, S.S. Distinct form I, II, III, and IV Rubisco proteins from the three kingdoms of life provide clues about Rubisco evolution and structure/function relationships. J. Exp. Bot. 2007, 59, 1515–1524. [Google Scholar] [CrossRef]
- Ogawa, S.; Suzuki, Y.; Yoshizawa, R.; Kanno, K.; Makino, A. Effect of individual suppression of RBCS multigene family on Rubisco contents in rice leaves. Plant Cell Environ. 2011, 35, 546–553. [Google Scholar] [CrossRef] [PubMed]
- Bracher, A.; Whitney, S.M.; Hartl, F.U.; Hayer-Hartl, M. Biogenesis and Metabolic Maintenance of Rubisco. Annu. Rev. Plant Biol. 2007, 68, 29–60. [Google Scholar] [CrossRef] [PubMed]
- Aigner, H.; Wilson, R.H.; Bracher, A.; Calisse, L.; Bhat, J.Y.; Hartl, F.U.; Hayer-Hartl, M. Plant RuBisCo assembly in E. coli with five chloroplast chaperones including BSD2. Science 2017, 358, 1272–1278. [Google Scholar] [CrossRef] [Green Version]
- Foyer, C.H.; Bloom, A.J.; Queval, G.; Noctor, G. Photorespiratory metabolism: Genes, mutants, energetics, and redox signaling. Annu. Rev. Plant Biol. 2009, 60, 455–484. [Google Scholar] [CrossRef]
- Betti, M.; Bauwe, H.; Busch, F.A.; Fernie, A.R.; Keech, O.; Levey, M.; Ort, D.R.; Parry MA, J.; Sage, R.; Timm, S.; et al. Manipulating photorespiration to increase plant productivity: Recent advances and perspectives for crop improvement. J. Exp. Bot. 2016, 67, 2977–2988. [Google Scholar] [CrossRef]
- Walker, B.J.; VanLoocke, A.; Bernacchi, C.J.; Ort, D.R. The Costs of Photorespiration to Food Production Now and in the Future. Annu. Rev. Plant Biol. 2016, 67, 107–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pierce, J.; Andrews, T.J.; Lorimer, G.H. Reaction intermediate partitioning by ribulose-bisphosphate carboxylases with differing substrate specificities. J. Biol. Chem. 1986, 261, 10248–10256. [Google Scholar] [CrossRef]
- Miziorko, H.M.; Sealy, R.C. Characterization of the ribulosebisphosphate carboxylase-carbon dioxide-divalent cation-carboxypentitol bisphosphate complex. Biochemistry 1980, 19, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Stec, B. Structural mechanism of RuBisCO activation by carbamylation of the active site lysine. Proc. Natl. Acad. Sci. USA 2012, 109, 18785–18790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, T.J.; Lorimer, G.H. The Biochemistry of Plants: A Comprehensive Treatise; Photosynthesis; Academic Press: San Diego, CA, USA, 1987; Volume 10. [Google Scholar]
- Wildner, G.F.; Henkel, J. The effect of divalent metal ions on the activity of Mg(++) depleted ribulose-1,5-bisphosphate oxygenase. Planta 1979, 146, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Matsumura, H.; Mizohata, E.; Ishida, H.; Kogami, A.; Ueno, T.; Makino, A.; Inoue, T.; Yokota, A.; Mae, T.; Kai, Y. Crystal structure of rice Rubisco and implications for activation induced by positive effectors NADPH and 6-phosphogluconate. Curr. Opin. Plant Biol. 2012, 422, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Chollet, R.; Anderson, L.L. Regulation of ribulose 1,5-bisphosphate carboxylase-oxygenase activities by temperature pretreatment and chloroplast metabolites. Arch. Biochem. Biophys. 1976, 176, 344–351. [Google Scholar] [CrossRef]
- McCurry, S.D.; Pierce, J.; Tolbert, N.E.; Orme-Johnson, W.H. On the mechanism of effector-mediated activation of ribulose bisphosphate carboxylase/oxygenase. J. Biol. Chem. 1981, 256, 6623–6628. [Google Scholar] [CrossRef]
- Chu, D.K.; Bassham, J.A. Activation of ribulose 1,5-diphosphate carboxylase by nicotinamide adenine dinucleotide phosphate and other chloroplast metabolites. Plant Physiol. 1974, 54, 556–559. [Google Scholar] [CrossRef] [Green Version]
- Hanson, T.E.; Satagopan, S.; Witte, B.H.; Kreel, N.E. Phylogenetic and evolutionary relationships of RubisCO and the RubisCO-like proteins and the functional lessons provided by diverse molecular forms. Philos. Trans R. Soc. Lond. B Biol. Sci. 2008, 363, 2629–2640. [Google Scholar]
- Tcherkez GG, B.; Farquhar, G.D.; Andrews, T.J. Despite slow catalysis and confused substrate specificity, all ribulose bisphosphate carboxylases may be nearly perfectly optimized. Proc. Natl. Acad. Sci. USA 2006, 103, 7246–7251. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J.; Kameritsch, P. Relative association of Rubisco with manganese and magnesium as a regulatory mechanism in plants. Physiol. Plant 2017, 161, 545–559. [Google Scholar] [CrossRef]
- Pierce, J.; Reddy, G.S. The sites for catalysis and activation of ribulosebisphosphate carboxylase share a common domain. Arch. Biochem. Biophys. 1986, 245, 483–493. [Google Scholar] [CrossRef]
- Jordan, D.B.; Ogren, W.L. Species variation in kinetic properties of ribulose 1,5-bisphosphate carboxylase/oxygenase. Arch. Biochem. Biophys. 1983, 227, 425–433. [Google Scholar] [CrossRef]
- Martin, F.; Winspear, M.J.; MacFarlane, J.D.; Oaks, A. Effect of Methionine Sulfoximine on the Accumulation of Ammonia in C3 and C4 Leaves. Plant Physiol. 1983, 71, 177–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christeller, J.T. The effects of bivalent cations on ribulose bisphosphate carboxylase/oxygenase. Biochem. J. 1981, 193, 839–844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christeller, J.T.; Laing, W.A. Effects of manganese ions and magnesium ions on the activity of soya-bean ribulose bisphosphate carboxylase/oxygenase. Biochem. J. 1979, 183, 747–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordan, D.B.; Ogren, W.L. A Sensitive Assay Procedure for Simultaneous Determination of Ribulose-1,5-bisphosphate Carboxylase and Oxygenase Activities. Plant Physiol. 1981, 67, 237–245. [Google Scholar] [CrossRef]
- Lilley, R.M.; Wang, X.; Krausz, E.; Andrews, T.J. Complete spectra of the far-red chemiluminescence of the oxygenase reaction of Mn2+-activated ribulose-bisphosphate carboxylase/oxygenase establish excited Mn2+ as the source. J. Biol. Chem. 2003, 278, 16488–16493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mogel, S.N.; McFadden, B.A. Chemiluminescence of the Mn2+-activated ribulose-1,5-bisphosphate oxygenase reaction: Evidence for singlet oxygen production. Biochemistry 1990, 29, 8333–8337. [Google Scholar] [CrossRef]
- Bock, C.R.; Connor, J.A.; Gutierrez, A.R. Estimation of excited-state redox potentials by electron-transfer quenching. Application of electron-transfer theory to excited-state redox processes. J. Am. Chem. Soc. 1979, 101, 4815–4824. [Google Scholar] [CrossRef]
- Creutz, C.; Sutin, N. Reaction of tris(bipyridine)ruthenium(III) with hydroxide and its application in a solar energy storage system. Proc. Natl. Acad. Sci. USA 1975, 72, 2858–2862. [Google Scholar] [CrossRef] [Green Version]
- Sattler, W.; Ener, M.E.; Blakemore, J.D. Generation of powerful tungsten reductants by visible light excitation. J. Am. Chem. Soc. 2013, 135, 10614–10617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, G.L.; Drincovich, M.F.; Andreo, C.S.; Lara, M.V. Nicotiana tabacum NADP-malic enzyme: Cloning, characterization and analysis of biological role. Plant Cell Physiol. 2008, 49, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler MC, G.; Arias, C.L.; Tronconi, M.A.; Maurino, V.G.; Andreo, C.S.; Drincovitch, M.F. Arabidopsis thaliana NADP-malic enzyme isoforms: High degree of identity but clearly distinct properties. Plant Mol. Biol. 2008, 67, 231–242. [Google Scholar] [CrossRef]
- Husic, H.D.; Tolbert, N.E. Anion and divalent cation activation of phosphoglycolate phosphatase from leaves. Arch. Biochem. Biophys. 1984, 229, 64–72. [Google Scholar] [CrossRef]
- Decker, J.P. A Rapid, Postillumination Deceleration of Respiration in Green Leaves. Plant Physiol. 1955, 30, 82–84. [Google Scholar] [CrossRef] [Green Version]
- Peterson, R.B. Estimation of Photorespiration Based on the Initial Rate of Postillumination CO2 Release: I. A Nonsteady State Model for Measurement of CO2 Exchange Transients. Plant Physiol. 1983, 73, 978–982. [Google Scholar] [CrossRef] [Green Version]
- Laisk, A.; Kiirats, O.; Oja, V. Assimilatory Power (Postillumination CO2 Uptake) in Leaves. Plant Physiol. 1984, 76, 723–729. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, T.D.; Seemann, J.R.; Pearcy, R.W. Contribution of Metabolites of Photosynthesis to Postillumination CO2 Assimilation in Response to Lightflects. Plant Physiol. 1986, 82, 1063–1068. [Google Scholar] [CrossRef] [Green Version]
- Azcón-Bieto, J.; Osmond, C.B. Relationship between Photosynthesis and Respiration: The Effect of Carbohydrate Status on the Rate of CO2 Production by Respiration in Darkened and Illuminated Wheat Leaves. Plant Physiol. 1983, 71, 574–581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharkey, T.D. O2-Insensitive Photosynthesis in C3 Plants. Plant Physiol. 1985, 78, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Caemmerer, S.V.; Edmondson, D.L. Relationship Between Steady-State Gas Exchange, in vivo Ribulose Bisphosphate Carboxylase Activity and Some Carbon Reduction Cycle Intermediates in Raphanus sativus. Funct. Plant Biol. 1986, 13, 669–688. [Google Scholar] [CrossRef]
- Badger, M.R.; Sharkey, T.D.; von Caemmerer, S. The relationship between steady-state gas exchange of bean leaves and the levels of carbon-reduction-cycle intermediates. Planta 1984, 160, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, L.J.; Canvin, D.T. An open gas-exchange system for the simultaneous measurement of the CO2 and 14CO2 fluxes from leaves. Can. J. Bot. 1971, 49, 1299–1313. [Google Scholar] [CrossRef]
- Gerbaud, A.; Andre, M. An Evaluation of the Recycling in Measurements of Photorespiration. Plant Physiol. 1987, 83, 933–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Leary, M.H. Carbon isotope fractionation in plants. Phytochemistry 1981, 20, 553–567. [Google Scholar] [CrossRef]
- Farquhar, G.D.; von Caemmerer, S. Modelling of Photosynthetic Response to Environmental Conditions. Physiol. Plant Ecol. II 1982, 12, 549–587. [Google Scholar]
- Busch, F.A. Current methods for estimating the rate of photorespiration in leaves. Plant Biol. 2012, 15, 648–655. [Google Scholar] [CrossRef]
- Busch, F.A.; Sage, T.L.; Cousins, A.B.; Sage, R.F. C3 plants enhance rates of photosynthesis by reassimilating photorespired and respired CO2. Plant Cell Environ. 2012, 36, 200–212. [Google Scholar] [CrossRef]
- Parnik, T.; Keerberg, O. Decarboxylation of primary and end products of photosynthesis at different oxygen concentrations. J. Exp. Bot. 1995, 46, 1439–1477. [Google Scholar] [CrossRef]
- Pärnik, T.; Keerberg, O. Advanced radiogasometric method for the determination of the rates of photorespiratory and respiratory decarboxylations of primary and stored photosynthates under steady-state photosynthesis. Physiol. Plant 2007, 129, 34–44. [Google Scholar] [CrossRef]
- Ivanova, H.; Keerberg, O. Photorespiratory and respiratory decarboxylations in leaves of C3 plants under different CO2 concentrations and irradiances. Plant Cell Environ. 2007, 30, 1535–1544. [Google Scholar]
- Tcherkez, G.; Mahé, A.; Guérard, F.; Boex-Fontvieille, E.R.A.; Gout, E.; Lamothe, M.; Barbour, M.M.; Bligny, R. Short-term effects of CO2 and O2 on citrate metabolism in illuminated leaves. Plant Cell Environ. 2012, 35, 2208–2220. [Google Scholar] [CrossRef] [PubMed]
- Bauwe, H.; Hagemann, M.; Fernie, A.R. Photorespiration: Players, partners and origin. Trends Plant Sci. 2010, 15, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Lacuesta, M.; Dever, L.V.; Munoz-Rueda, A.; Lea, P.J. A study of photorespiratory ammonia production in the C4 plant Amaranthus edulis, using mutants with altered photosynthetic capacities. Physiol. Plant 1997, 99, 447–455. [Google Scholar] [CrossRef]
- Wendler, C.; Barniske, M.; Wild, A. Effect of phosphinothricin (glufosinate) on photosynthesis and photorespiration of C3 and C4 plants. Planta 1990, 24, 55–61. [Google Scholar] [CrossRef]
- Husted, S.; Hebbern, C.A.; Mattsson, M. A critical experimental evaluation of methods for determination of NH4+ in plant tissue, xylem sap and apoplastic fluid. Physiol. Plant 2000, 109, 167–179. [Google Scholar] [CrossRef]
- Ruuska, S.A.; Badger, M.R.; Andrews, T.J.; Von Caemmerer, S. Photosynthetic electron sinks in transgenic tobacco with reduced amounts of Rubisco: Little evidence for significant Mehler reaction. J. Exp. Bot. 2000, 51, 357–368. [Google Scholar] [CrossRef] [Green Version]
- Canvin, D.T.; Berry, J.A.; Badger, M.R.; Fock, H.; Osmond, C.B. Oxygen Exchange in Leaves in the Light. Plant Physiol. 1980, 66, 302–307. [Google Scholar] [CrossRef]
- De Veau, E.J.; Burris, J.E. Photorespiratory rates in wheat and maize as determined by o-labeling. Chem. Rev. 1989, 90, 500–511. [Google Scholar] [CrossRef] [Green Version]
- Berry, J.A.; Osmond, C.B.; Lorimer, G.H. Fixation of O2 during Photorespiration: Kinetic and Steady-State Studies of the Photorespiratory Carbon Oxidation Cycle with Intact Leaves and Isolated Chloroplasts of C3 Plants. Plant Physiol. 1978, 62, 954–967. [Google Scholar] [CrossRef] [Green Version]
- Cegelski, L.; Schaefer, J. Glycine Metabolism in Intact Leaves by in Vivo 13C and 15N Labeling. J. Biol. Chem. 2005, 280, 39238–39245. [Google Scholar] [CrossRef] [Green Version]
- Arrivault, S.; Guenther, M.; Fry, S.C.; Fuenfgeld, M.M.; Veyel, D.; Mettler-Altmann, T.; Stitt, M.; Lunn, J.E. Synthesis and Use of Stable-Isotope-Labeled Internal Standards for Quantification of Phosphorylated Metabolites by LC–MS/MS. Biochemistry 2015, 87, 6896–6904. [Google Scholar] [CrossRef] [PubMed]
- Fernie, A.R.; Bauwe, H. Photorespiration: Methods and Protocols; Humana Press: Totowa, NJ, USA, 2017. [Google Scholar]
- Arrivault, S.; Guenther, M.; Ivakov, A.; Feil, R.; Vosloh, D.; van Dongen, J.T.; Sulpice, R.; Stitt, M. Use of reverse-phase liquid chromatography, linked to tandem mass spectrometry, to profile the Calvin cycle and other metabolic intermediates in Arabidopsis rosettes at different carbon dioxide concentrations. Plant J. 2009, 59, 826–839. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A. Is there a metabolic requirement for photorespiratory enzyme activities in heterotrophic tissues? Mol. Plant 2014, 7, 248–251. [Google Scholar] [CrossRef] [Green Version]
- Mintz-Oron, S.; Meir, S.; Malitsky, S.; Ruppin, E.; Aharoni, A.; Shlomi, T. Reconstruction of Arabidopsis metabolic network models accounting for subcellular compartmentalization and tissue-specificity. Proc. Natl. Acad. Sci. USA 2011, 109, 339–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lernmark, U.; Henricson, D.; Wigge, B.; Gardestrom, P. Glycine oxidation in mitochondria isolated from light grown and etiolated plant tissue. Physiol. Plant 1991, 82, 339–344. [Google Scholar] [CrossRef]
- Jacoby, R.P.; Millar, A.H.; Taylor, N.L. Application of selected reaction monitoring mass spectrometry to field-grown crop plants to allow dissection of the molecular mechanisms of abiotic stress tolerance. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Igamberdiev, A.U.; Bykova, N.V. Involvement of cyanide-resistant and rotenone-insensitive pathways of mitochondrial electron transport during oxidation of glycine in higher plants. FEBS Lett. 1997, 412, 265–269. [Google Scholar] [CrossRef]
- Trufelli, H.; Palma, P.; Famiglini, G. An overview of matrix effects in liquid chromatography–mass spectrometry. Mass Spectrom. Rev. 2011, 30, 491–509. [Google Scholar] [CrossRef]
- Tohge, T.; Mettler, T.; Arrivault, S.; Carroll, A.J. From models to crop species: Caveats and solutions for translational metabolomics. Front. Plant Sci. 2011, 2, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- King, R.; Bonfiglio, R.; Fernandez, C. Mechanistic investigation of ionization suppression in electrospray ionization. J. Am. Soc. Mass Spectrom. 2000, 11, 942–950. [Google Scholar] [CrossRef] [Green Version]
- Bonfiglio, R.; King, R.C.; Olah, T.V. The effects of sample preparation methods on the variability of the electrospray ionization response for model drug compounds. Rapid Commun. Mass Spectrom. 1999, 13, 1175–1185. [Google Scholar] [CrossRef]
- Römisch-Margl, W.; Schramek, N.; Radykewicz, T.; Ettenhuber, C.; Eylert, E.; Huber, C.; Römisch-Margl, L.; Schwarz, C.; Dobner, M.; Demmel, N.; et al. 13CO2 as a universal metabolic tracer in isotopologue perturbation experiments. Phytochemistry 2007, 68, 2273–2289. [Google Scholar] [CrossRef]
- Eisenreich, W.; Bacher, A. Advances of high-resolution NMR techniques in the structural and metabolic analysis of plant biochemistry. Phytochemistry 2007, 68, 2799–2815. [Google Scholar] [CrossRef] [PubMed]
- Heise, R.; Arrivault, S.; Szecowka, M.; Tohge, T.; Nunes-Nesi, A.; Stitt, M.; Nikoloski, Z.; Fernie, A.R. Flux profiling of photosynthetic carbon metabolism in intact plants. Nat. Protoc. 2014, 9, 1803–1824. [Google Scholar] [CrossRef] [PubMed]
- Ma, F.; Jazmin, L.J.; Young, J.D.; Allen, D.K. Isotopically nonstationary 13C flux analysis of changes in Arabidopsis thaliana leaf metabolism due to high light acclimation. Proc. Natl. Acad. Sci. USA 2014, 111, 16967–16972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szecowka, M.; Heise, R.; Tohge, T.; Nunes-Nesi, A.; Vosloh, D.; Huege, J.; Feil, R.; Lunn, J.; Nikoloski, Z.; Stitt, M.; et al. Metabolic Fluxes in an Illuminated Arabidopsis Rosette. Plant Cell 2013, 25, 694–714. [Google Scholar] [CrossRef] [Green Version]
- Adebiyi, A.O.; Jazmin, L.J.; Young, J.D. 13C flux analysis of cyanobacterial metabolism. Photosyn. Res. 2014, 126, 19–32. [Google Scholar] [CrossRef]
- Cheah, Y.E.; Young, J.D. Isotopically nonstationary metabolic flux analysis (INST-MFA): Putting theory into practice. Curr. Opin. Biotechnol. 2018, 54, 80–87. [Google Scholar] [CrossRef]
- Clark, L.C.; Wolf, R.; Granger, D.; Taylor, Z. Continuous recording of blood oxygen tensions by polarography. J. Appl. Physiol. 1953, 6, 189–193. [Google Scholar] [CrossRef]
- Severinghaus, J.W.; Astrup, P.B. History of blood gas analysis. IV. Leland Clark’s oxygen electrode. J. Clin. Monit. 1986, 2, 125–139. [Google Scholar] [CrossRef]
- Bittig, H.C.; Körtzinger, A.; Neill, C.; van Ooijen, E.; Plant, J.N.; Hahn, J.; Johnson, K.S.; Yang, B.; Emerson, S.R. Oxygen Optode Sensors: Principle, Characterization, Calibration, and Application in the Ocean. Front. Mar. Sci. 2018, 4, 429. [Google Scholar] [CrossRef]
- Fischer, M.; Falke, D.; Pawlik, T.; Sawers, R.G. Oxygen-dependent control of respiratory nitrate reduction in mycelium of Streptomyces coelicolor A3(2). J. Bacteriol. 2014, 196, 4152–4162. [Google Scholar] [CrossRef] [Green Version]
- Flitsch, D.; Ladner, T.; Lukacs, M.; Büchs, J. Easy to use and reliable technique for online dissolved oxygen tension measurement in shake flasks using infrared fluorescent oxygen-sensitive nanoparticles. Microb. Cell Fact. 2016, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helm, I.; Karina, G.; Jalukse, L.; Pagano, T.; Leito, I. Comparative validation of amperometric and optical analyzers of dissolved oxygen: A case study. Environ. Monit. Assess 2018, 190, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bentzon-Tilia, M.; Severin, I.; Hansen, L.H.; Riemann, L. Genomics and Ecophysiology of Heterotrophic Nitrogen-Fixing Bacteria Isolated from Estuarine Surface Water. mBio 2015, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortner, A.; Huber, D.; Haske, O. Laccase mediated oxidation of industrial lignins: Is oxygen limiting? Process Biochem. 2015, 50, 1277–1283. [Google Scholar] [CrossRef]
- Bloom, A.J. Photorespiration and nitrate assimilation: A major intersection between plant carbon and nitrogen. Photosyn. Res. 2014, 123, 117–128. [Google Scholar] [CrossRef] [Green Version]
- Bloom, A.J.; Burger, M.; Asensio, J.S.; Cousins, A.B. Carbon dioxide enrichment inhibits nitrate assimilation in wheat and Arabidopsis. Science 2010, 328, 899–903. [Google Scholar] [CrossRef] [Green Version]
- Aranjuelo, I.; Cabrerizo, P.M.; Arrese, C. Pea plant responsiveness under elevated [CO2] is conditioned by the N source (N2 fixation versus NO3− fertilization). Environ. Exp. Bot. 2013, 95, 34–40. [Google Scholar] [CrossRef] [Green Version]
- Rachmilevitch, S.; Cousins, A.B.; Bloom, A.J. Nitrate assimilation in plant shoots depends on photorespiration. Proc. Natl. Acad. Sci. USA 2004, 101, 11506–11510. [Google Scholar] [CrossRef] [Green Version]
- Lekshmy, S.; Jain, V.; Khetarpal, S.; Pandey, R. Inhibition of nitrate uptake and assimilation in wheat seedlings grown under elevated CO2. Indian J. Plant Physiol. 2013, 18, 23–29. [Google Scholar] [CrossRef]
- Pleijel, H.; Uddling, J. Yield vs. quality trade-offs for wheat in response to carbon dioxide and ozone. Glob. Chang. Biol. 2011, 18, 596–605. [Google Scholar] [CrossRef]
- Bloom, A.J.; Asensio JS, R.; Randall, L.; Rachmilevitch, S.; Cousins, A.B.; Carlisle, E.A. CO2 enrichment inhibits shoot nitrate assimilation in C3 but not C4 plants and slows growth under nitrate in C3 plants. Ecology 2012, 93, 355–367. [Google Scholar] [CrossRef]
- Carlisle, E.; Myers, S.S.; Raboy, V.; Bloom, A.J. The effects of inorganic nitrogen form and CO2 concentration on wheat yield and nutrient accumulation and distribution. Front. Plant Sci. 2012, 3, 195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloom, A.J.; Burger, M.; Kimball, B.A.; Pinter, P.J., Jr. Nitrate assimilation is inhibited by elevated CO2 in field-grown wheat. Nat. Clim. Chang. 2014, 4, 477–480. [Google Scholar] [CrossRef]
- Carlisle, E.; Yarnes, C.; Toney, M.D.; Bloom, A.J. Nitrate reductase 15N discrimination in Arabidopsis thaliana, Zea mays, Aspergillus niger, Pichea angusta, and Escherichia coli. Front. Plant Sci. 2014, 5, 317. [Google Scholar] [CrossRef] [Green Version]
- Warburg, O. Assimilatory quotient and photochemical yield. Am. J. Bot. 1948, 35, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Bloom, A.J.; Caldwell, R.M.; Finazzo, J.; Warner, R.L. Oxygen and carbon dioxide fluxes from barley shoots depend on nitrate assimilation. Plant Physiol. 1989, 91, 352–356. [Google Scholar] [CrossRef] [Green Version]
- Cen, Y.P.; Turpin, D.H.; Layzell, D.B. Whole-plant gas exchange and reductive biosynthesis in white lupin. Plant Physiol. 2001, 126, 1555–1565. [Google Scholar] [CrossRef] [Green Version]
- Matt, P.; Geiger, M.; Walch-Liu, P.; Engels, C. Elevated carbon dioxide increases nitrate uptake and nitrate reductase activity when tobacco is growing on nitrate, but increases ammonium uptake and inhibits nitrate reductase activity when tobacco is growing on ammonium nitrate. Plant Cell Environ. 2011, 24, 1119–1137. [Google Scholar] [CrossRef]
- Passama, L.; Gojon, A.; Robin, P.; Salsac, L. In situ nitrate reductase activity as an indicator of nitrate availability. Plant Soil 1987, 102, 145–148. [Google Scholar] [CrossRef]
- Kaiser, W.M.; Kandlbinder, A.; Stoimenova, M.; Glaab, J. Discrepancy between nitrate reduction rates in intact leaves and nitrate reductase activity in leaf extracts: What limits nitrate reduction in situ? Planta 2000, 210, 801–807. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, J.Q.; Crawford, N.M. Identification and characterization of a chlorate-resistant mutant of Arabidopsis thaliana with mutations in both nitrate reductase structural genes NIA1 and NIA2. Mol. Gen. Genet. 1993, 239, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Warner, R.L.; Kleinhofs, A. Nitrate utilization by nitrate reductase-deficient barley mutants. Plant Physiol. 1981, 67, 740–743. [Google Scholar] [CrossRef] [PubMed]
- Eichelberger, K.D.; Lambert, R.J.; Below, F.E. Divergent phenotypic recurrent selection for nitrate reductase activity in maize. II. Efficient use of fertilizer nitrogen. Crop Sci. 1989, 29, 1398–1402. [Google Scholar] [CrossRef]
- Andrews, M.; Condron, L.M.; Kemp, P.D.; Topping, J.F. Elevated CO₂ effects on nitrogen assimilation and growth of C₃ vascular plants are similar regardless of N-form assimilated. J. Exp. Bot. 2019, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Andrews, M.; Condron, L.M.; Kemp, P.D. Will rising atmospheric CO2 concentration inhibit nitrate assimilation in shoots but enhance it in roots of C3 plants? Physiol. Plant 2020, 170, 40–45. [Google Scholar] [CrossRef]
- Obata, T.; Florian, A.; Timm, S.; Bauwe, H.; Fernie, A.R. On the metabolic interactions of (photo)respiration. J. Exp. Bot. 2016, 67, 3003–3014. [Google Scholar] [CrossRef] [Green Version]
- Scheibe, R. Malate valves to balance cellular energy supply. Physiol. Plant 2004, 120, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Voss, I.; Sunil, B.; Scheibe, R.; Raghavendra, A.S. Emerging concept for the role of photorespiration as an important part of abiotic stress response. Plant Biol. 2013, 15, 713–722. [Google Scholar] [CrossRef]
- Taniguchi, M.; Miyake, H. Redox-shuttling between chloroplast and cytosol: Integration of intra-chloroplast and extra-chloroplast metabolism. Curr. Opin. Plant Biol. 2012, 15, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Backhausen, J.E.; Emmerlich, A.; Holtgrefe, S.; Horton, P.; Nast, G.; Rogers, J.J.; Müller-Röber, B.; Scheibe, R. Transgenic potato plants with altered expression levels of chloroplast NADP-malate dehydrogenase: Interactions between photosynthetic electron transport and malate metabolism in leaves and in isolated intact chloroplasts. Planta 1998, 207, 105–114. [Google Scholar] [CrossRef]
- Bloom, A.J. The increasing importance of distinguishing among plant nitrogen sources. Curr. Opin. Plant. Biol. 2015, 25, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Dutilleul, C.; Lelarge, C.; Prioul, J.-L.; De Paepe, R.; Foyer, C.H.; Noctor, G. Mitochondria-driven changes in leaf NAD status exert a crucial influence on the control of nitrate assimilation and the integration of carbon and nitrogen metabolism. Plant Physiol. 2005, 139, 64–78. [Google Scholar] [CrossRef] [Green Version]
- Schneidereit, J.; Häusler, R.E.; Fiene, G.; Kaiser, W.M.; Weber AP, M. Antisense repression reveals a crucial role of the plastidic 2-oxoglutarate/malate translocator DiT1 at the interface between carbon and nitrogen metabolism. Plant J. 2005, 45, 206–224. [Google Scholar] [CrossRef]
- Cossins, E.A. The fascinating world of folate and one-carbon metabolism. Can. J. Bot. 2000, 78, 691–708. [Google Scholar]
- Hanson, A.D.; Roje, S. One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 2001, 52, 119–137. [Google Scholar] [CrossRef]
- Crider, K.S.; Yang, T.P.; Berry, R.J.; Bailey, L.B. Folate and DNA methylation: A review of molecular mechanisms and the evidence for folate’s role. Adv. Nutr. 2012, 3, 21–38. [Google Scholar] [CrossRef] [Green Version]
- Gorelova, V.; Ambach, L.; Rébeillé, F.; Stove, C.; Van Der Straeten, D. Folates in Plants: Research Advances and Progress in Crop Biofortification. Front. Chem. 2017, 5, 21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abadie, C.; Tcherkez, G. Plant sulphur metabolism is stimulated by photorespiration. Commun. Biol. 2019, 2, 1–7. [Google Scholar] [CrossRef]
- Tcherkez, G.; Tea, I. 32S/34S isotope fractionation in plant sulphur metabolism. New Phytol. 2013, 200, 44–53. [Google Scholar] [CrossRef]
- Amrani, A.; Kamyshny, A.; Lev, O.; Aizenshtat, Z. Sulfur Stable Isotope Distribution of Polysulfide Anions in an (NH4)2Sn Aqueous Solution. Inorg. Chem. 2006, 45, 1427–1429. [Google Scholar] [CrossRef]
- Rausch, T.; Wachter, A. Sulfur metabolism: A versatile platform for launching defence operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, G.L. The nature of the active methyl donor formed enzymatically from L-methionine and adenosine triphosphate1,2. J. Am. Chem. Soc. 1952, 74, 2942–2943. [Google Scholar] [CrossRef]
- Timm, S.; Bauwe, H. The variety of photorespiratory phenotypes—Employing the current status for future research directions on photorespiration. Plant Biol. 2013, 15, 737–747. [Google Scholar] [CrossRef]
- Eisenhut, M.; Ruth, W.; Haimovich, M.; Bauwe, H.; Kaplan, A.; Hagemann, M. The photorespiratory glycolate metabolism is essential for cyanobacteria and might have been conveyed endosymbiontically to plants. Proc. Natl. Acad. Sci. USA 2008, 105, 17199–17204. [Google Scholar] [CrossRef] [Green Version]
- Eisenhut, M.; Bräutigam, A.; Timm, S.; Florian, A.; Tohge, T. Photorespiration is crucial for dynamic response of photosynthetic metabolism and stomatal movement to altered CO2 availability. Mol. Plant 2017, 10, 47–61. [Google Scholar] [CrossRef] [Green Version]
- Ziotti, A.; Silva, B.P.; Neto, M. Photorespiration is crucial for salinity acclimation in castor bean. Environ. Exp. Bot. 2019, 167, 103845. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, X.; Bloom, A. Photorespiration: The Futile Cycle? Plants 2021, 10, 908. https://doi.org/10.3390/plants10050908
Shi X, Bloom A. Photorespiration: The Futile Cycle? Plants. 2021; 10(5):908. https://doi.org/10.3390/plants10050908
Chicago/Turabian StyleShi, Xiaoxiao, and Arnold Bloom. 2021. "Photorespiration: The Futile Cycle?" Plants 10, no. 5: 908. https://doi.org/10.3390/plants10050908
APA StyleShi, X., & Bloom, A. (2021). Photorespiration: The Futile Cycle? Plants, 10(5), 908. https://doi.org/10.3390/plants10050908