Endophytic Strain Bacillus subtilis 26D Increases Levels of Phytohormones and Repairs Growth of Potato Plants after Colorado Potato Beetle Damage
Abstract
1. Introduction
2. Results
2.1. Level of Phytohormones in the Culture Medium of B. subtilis 26D
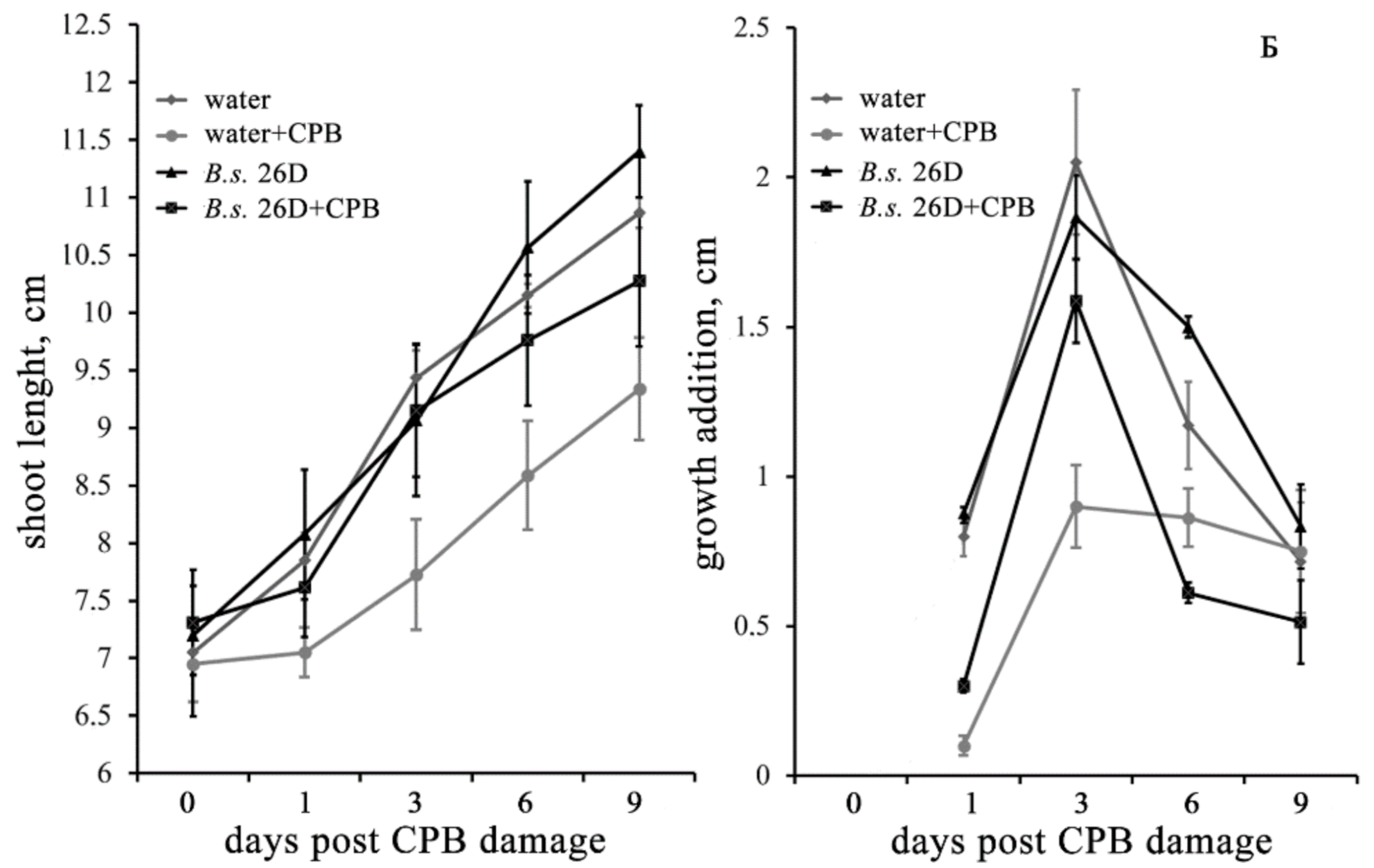
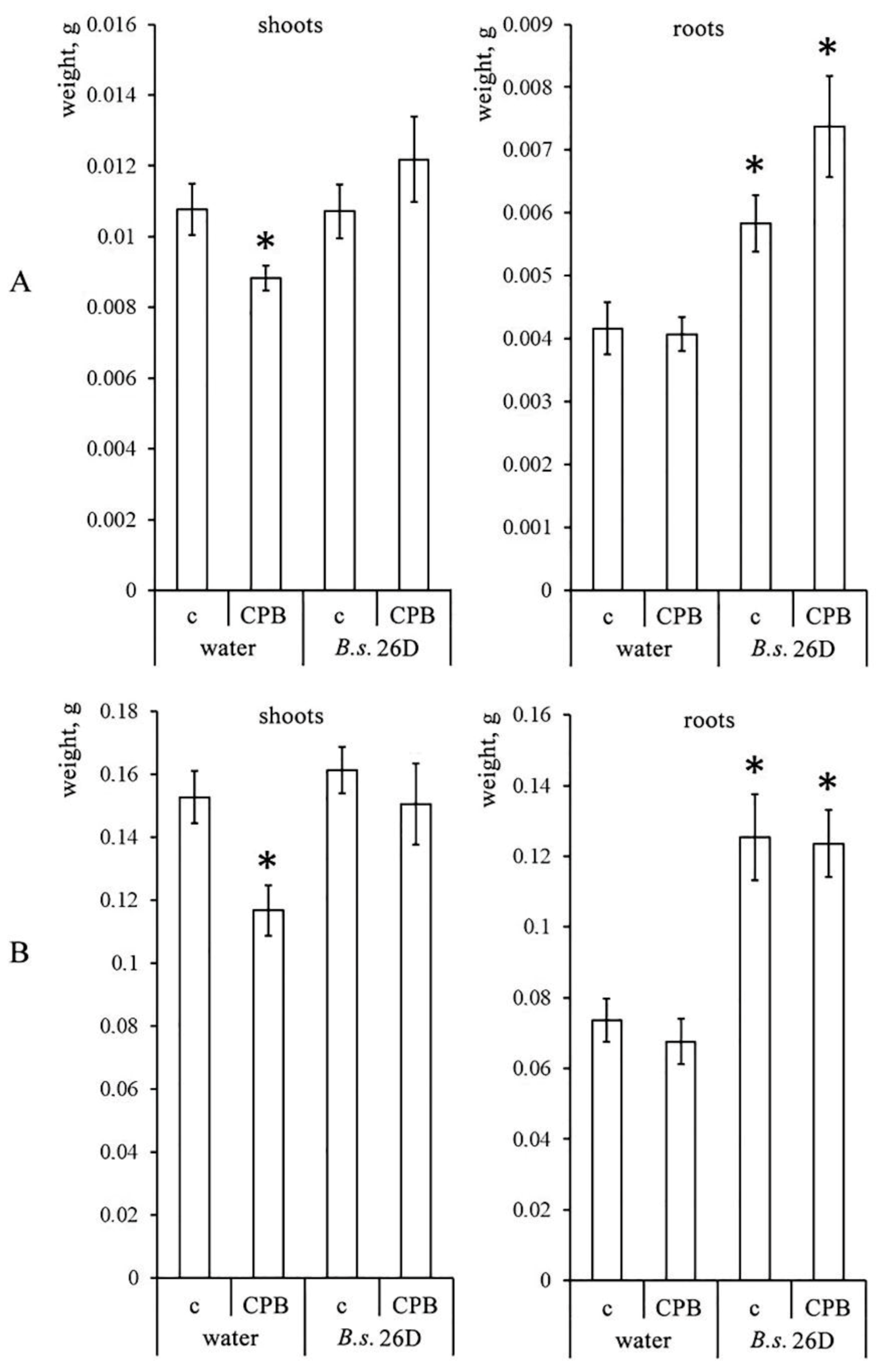
2.2. Influence of B. subtilis 26D and CPB on the Growth Characteristics of Potato Plants
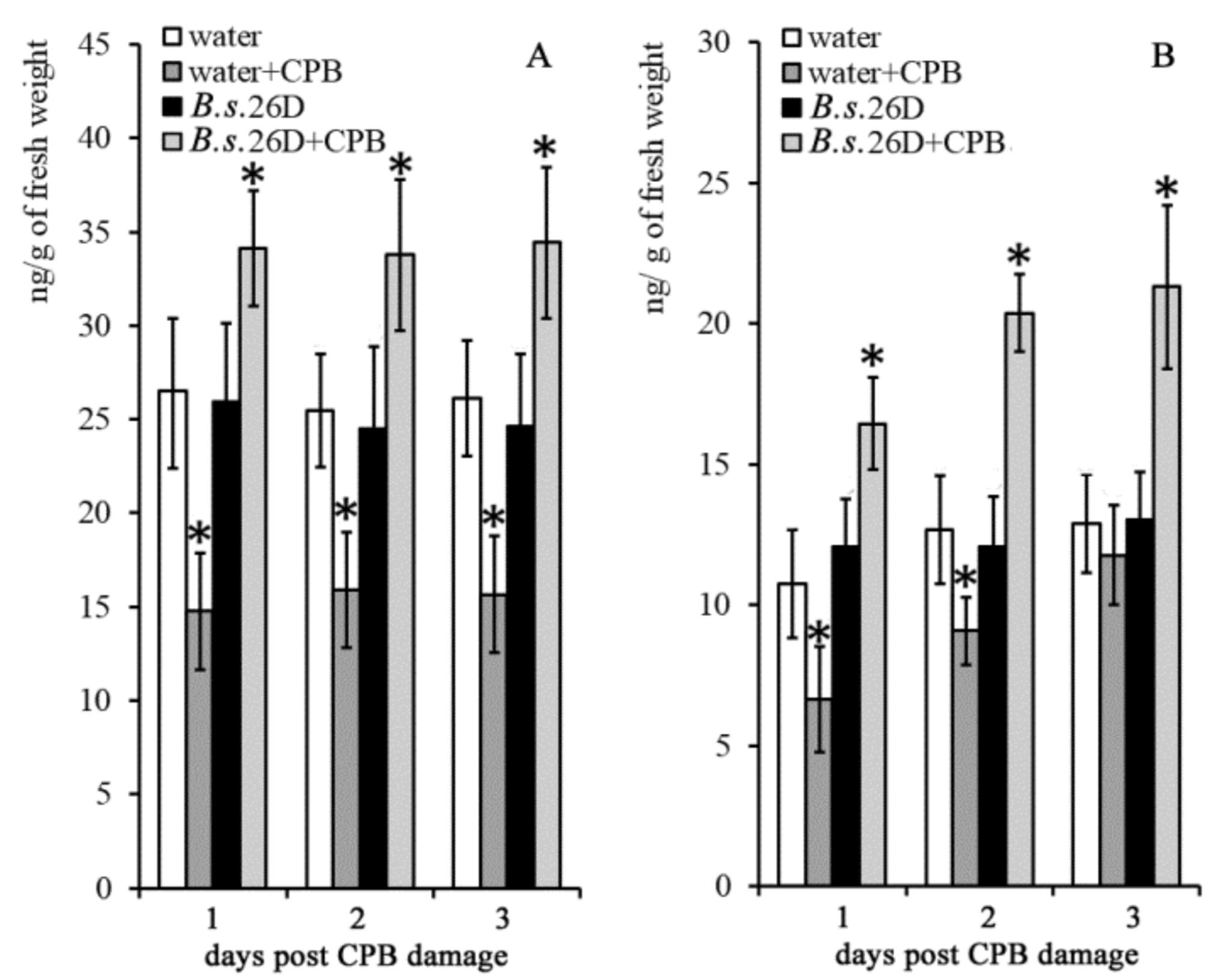
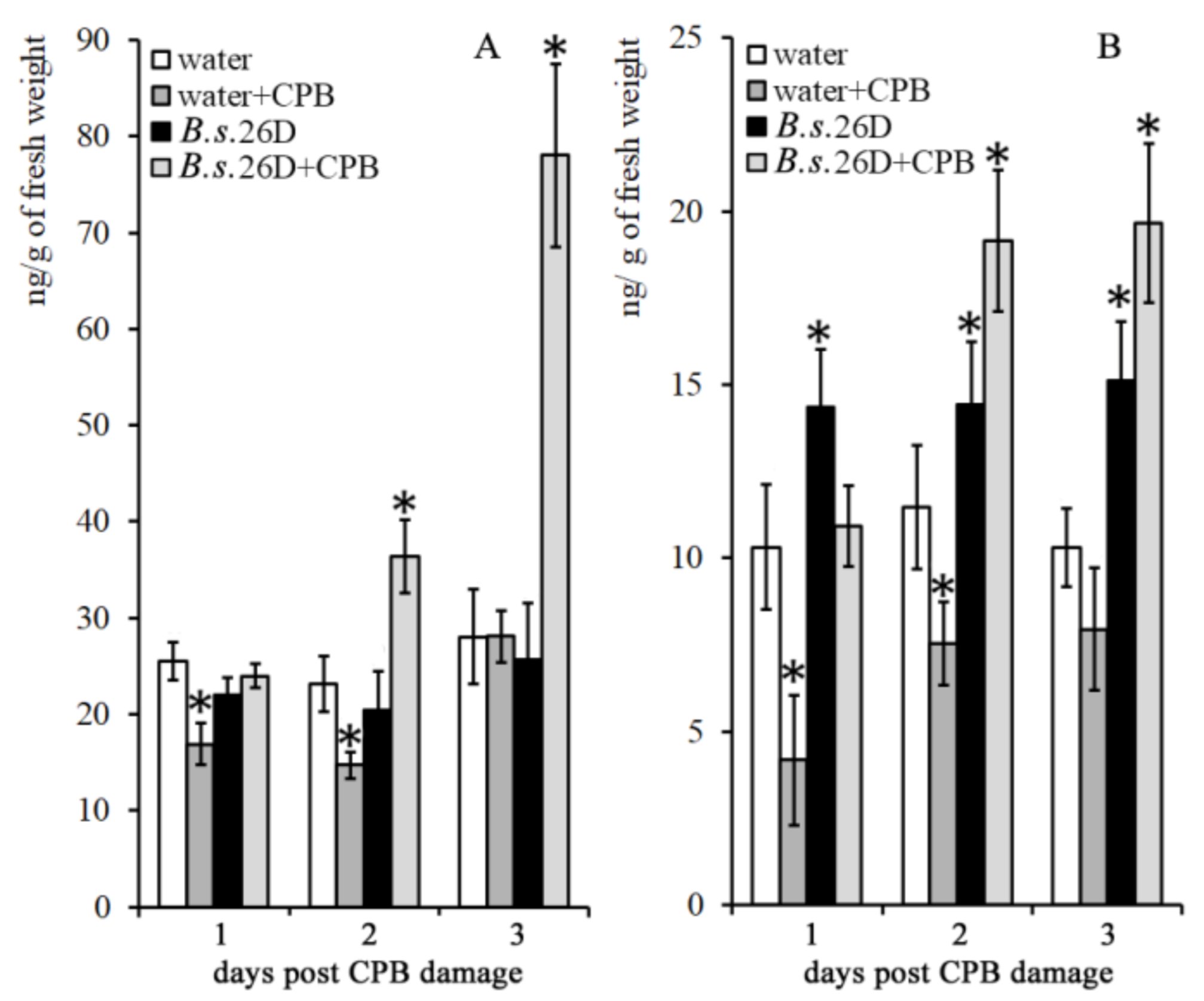
2.3. Influence of B. subtilis 26D and CPBs on the Content of Phytohormones in Potato Plants
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant, Microbe and Insect Material
5.2. Plant Damaging
5.3. Plant Growth Parameters
5.4. Phytohormones Assay
5.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Clements, J.; Olson, J.M.; Sanchez-Sedillo, B.; Bradford, B.; Groves, R.L. Changes in emergence phenology, fatty acid composition, and xenobiotic metabolizing enzyme expression is associated with increased insecticide resistance in the Colorado potato beetle. Arch. Insect Biochem. Physiol. 2020, 103, e21630. [Google Scholar] [CrossRef] [PubMed]
- Isah, T. Stress and defense responses in plant secondary metabolites production. Biol. Res. 2019, 52, 39. [Google Scholar] [CrossRef] [PubMed]
- Kadoić-Balaško, M.; Mikac, K.M.; Bažok, R.; Lemic, D. Modern techniques in Colorado Potato Beetle (Leptinotarsa decemlineata Say) control and resistance management: History review and future perspectives. Insects 2020, 11, 581. [Google Scholar] [CrossRef] [PubMed]
- Tamošiūnė, I.; Stanienė, G.; Haimi, P.; Stanys, V.; Rugienius, R.; Baniulis, D. Endophytic Bacillus and Pseudomonas spp. modulate apple shoot growth, cellular redox balance, and protein expression under in vitro conditions. Front. Plant Sci. 2018, 9, 889. [Google Scholar] [CrossRef]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef]
- Costarelli, A.; Bianchet, C.; Ederli, L.; Salerno, G.; Piersanti, S.; Rebora, M.; Pasqualini, S. Salicylic acid induced by herbivore feeding antagonizes jasmonic acid mediated plant defenses against insect attack. Plant Signal. Behav. 2020, 15, 1704517. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Mekureyaw, M.F.; Pandey, C.; Roitsch, T. Role of cytokinins for interactions of plants with microbial pathogens and pest insects. Front. Plant Sci. 2020, 10, 1777. [Google Scholar] [CrossRef]
- Arkhipova, T.N.; Veselov, S.U.; Melentiev, A.I.; Kudoyarova, G.R. Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 2005, 272, 201–215. [Google Scholar] [CrossRef]
- Pérez-Flores, P.; Valencia-Cantero, E.; Altamirano-Hernández, J.; Pelagio-Flores, R.; López-Bucio, J.; García-Juárez, P.; Macías-Rodríguez, L. Bacillus methylotrophicus M4-96 isolated from maize (Zea mays) rhizoplane increases growth and auxin content in Arabidopsis thaliana via emission of volatiles. Protoplasma 2017, 254, 2201. [Google Scholar] [CrossRef]
- Idris, E.E.; Iglesias, E.J.; Talon, M.; Borriss, R. Tryptophan-dependent production of indole-3-acetic acid (IAA) affects level of plant growth promotion by Bacillus amyloliquefaciens FZB42. Mol. Plant-Microbe Interact. 2007, 20, 619–626. [Google Scholar] [CrossRef]
- Shao, J.; Li, S.; Zhang, N.; Cui, X.; Zhou, X.; Zhang, G.; Shen, Q.; Zhang, R. Analysis and cloning of the synthetic pathway of the phytohormone indole-3-acetic acid in the plant-beneficial Bacillus amyloliquefaciens SQR9. Microb. Cell Factories 2015, 14, 130. [Google Scholar] [CrossRef]
- Park, Y.G.; Mun, B.G.; Kang, S.M.; Hussain, A.; Shahzad, R.; Seo, C.W.; Kim, A.Y.; Lee, S.U.; Oh, K.Y.; Lee, D.Y.; et al. Bacillus aryabhattai SRB02 tolerates oxidative and nitrosative stress and promotes the growth of soybean by modulating the production of phytohormones. PLoS ONE 2017, 12, e0173203. [Google Scholar] [CrossRef]
- Santoyo, G.; Moreno-Hagelsieb, G.; Orozco-Mosqueda, M.C.; Glick, B.R. Plant growth-promoting bacterial endophytes. Microbiol Res. 2016, 183, 92–99. [Google Scholar] [CrossRef]
- Bouizgarne, B. Bacteria for plant growth promotion and disease management. In Bacteria in Agrobiology: Disease Management; Maheshwari, D.K., Ed.; Springer: Berlin/Heidelberg, Germany, 2012; Chapter 2; pp. 15–46. [Google Scholar] [CrossRef]
- Sorokan, A.; Cherepanova, E.; Burkhanova, G.; Veselova, S.; Rumyantsev, S.; Alekseev, V.; Mardanshin, I.; Sarvarova, E.; Khairullin, R.; Benkovskaya, G.; et al. Endophytic Bacillus spp. as a prospective biological tool for control of viral diseases and non-vector Leptinotarsa decemlineata Say. in Solanum tuberosum L. Front. Microbiol. 2020, 11, 569457. [Google Scholar] [CrossRef]
- Sorokan, A.; Benkovskaya, G.; Burkhanova, G.; Blagova, D.; Maksimov, I. Endophytic strain Bacillus subtilis 26DCryChS producing Cry1Ia toxin from Bacillus thuringiensis promotes multifaceted potato defense against Phytophthora infestans (Mont.) de Bary and pest Leptinotarsa decemlineata Say. Plants 2020, 9, 1115. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Hashem, A.; Abd Allah, E.F. Bacillus: A Biological Tool for Crop Improvement through Bio-Molecular Changes in Adverse Environments. Front. Physiol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Domínguez-Arrizabalaga, M.; Villanueva, M.; Escriche, B.; Ancín-Azpilicueta, C.; Caballero, P. Insecticidal activity of Bacillus thuringiensis proteins against Coleopteran pests. Toxins 2020, 12, 430. [Google Scholar] [CrossRef]
- Dehghanian, S.Z.; Abdollahi, M.; Charehgani, H.; Niazi, A. Combined effect of salicylic acid and Pseudomonas fluorescens CHA0 on the expression of PR1 gene and control of Meloidogyne javanica in tomato. Biol. Control. 2020, 141, 104134. [Google Scholar] [CrossRef]
- Saleem, M.; Meckes, N.; Pervaiz, Z.H.; Traw, M.B. Microbial interactions in the phyllosphere increase plant performance under herbivore biotic stress. Front. Microbiol. 2017, 8, 41. [Google Scholar] [CrossRef]
- Melentyev, A.I.; Kudoyarova, G.R.; Veselov, S.Y.; Arkhipova, T.N.; Gilvanova, E.A.; Usanov, N.G.; Kuzmina, L.Y.; Simoyan, M.V. Strain Bacillus Subtilis ib-22 as Producer of Cytokinins. RU Patent No 2178970, 10 February 2002. Available online: https://patents.google.com/patent/RU2178970C2/en (accessed on 29 April 2021).
- Khan, A.L.; Halo, B.A.; Elyassi, A.; Ali, S.; Al-Hosni, K.; Hussain, J. Indole acetic acid and ACC deaminase from endophytic bacteria improves the growth of Solanum lycopersicum. Elect. J. Biotechnol. 2016, 21, 58–64. [Google Scholar] [CrossRef]
- Karnwal, A. Isolation and identification of plant growth promoting rhizobacteria from maize (Zea mays L.) rhizosphere and their plant growth promoting effect on rice (Oryza sativa L.). J. Plant. Protect. Res. 2017, 57, 144–151. [Google Scholar] [CrossRef]
- Machado, R.A.; Ferrieri, A.P.; Robert, C.A.; Glauser, G.; Kallenbach, M.; Baldwin, I.T.; Erb, M. Leaf-herbivore attack reduces carbon reserves and regrowth from the roots via jasmonate and auxin signaling. New Phytol. 2013, 200, 1234–1246. [Google Scholar] [CrossRef]
- Yan, S.; Jiao, C.; McLamore, E.S.; Wang, N.; Yao, H.; Shen, Y. Insect herbivory of leaves affects the auxin flux along root apices in Arabidopsis thaliana. J. Plant. Growth Regul. 2017, 36, 846–854. [Google Scholar] [CrossRef]
- Spaepen, S.; Bossuyt, S.; Engelen, K.; Marchal, K.; Vanderleyden, J. Phenotypical and molecular responses of Arabidopsis thaliana roots as a result of inoculation with the auxin-producing bacterium Azospirillum brasilense. New Phytol. 2014, 201, 850–861. [Google Scholar] [CrossRef]
- Kieber, J.J.; Schaller, E.G. Cytokinin signaling in plant development. Development 2018, 145, dev149344. [Google Scholar] [CrossRef]
- Schäfer, M.; Meza-Canales, I.D.; Navarro-Quezada, A.; Brütting, C.; Vanková, R.; Baldwin, I.T.; Meldau, S. Cytokinin levels and signaling respond to wounding and the perception of herbivore elicitors in Nicotiana attenuata. J. Integr. Plant. Biol. 2015, 57, 198–212. [Google Scholar] [CrossRef]
- Dervinis, C.; Frost, C.J.; Lawrence, S.D.; Novak, N.G.; Davis, J.M. Cytokinin primes plant responses to wounding and reduces insect performance. J. Plant. Growth Regul. 2010, 29, 289–297. [Google Scholar] [CrossRef]
- Giron, D.; Huguet, E.; Stone, G.N.; Body, M. Insect-induced effects on plants and possible effectors used by galling and leaf-mining insects to manipulate their host-plant. J. Insect Physiol. 2016, 84, 70–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Chen, M.; Zhang, Y.; Gao, Q.; Noman, A.; Wang, Q.; Li, H.; Chen, L.; Zhou, P.; Lu, J.; et al. OsMKK3, a stress-responsive protein kinase, positively regulates rice resistance to Nilaparvata lugens via phytohormone dynamics. Int. J. Mol. Sci. 2019, 12, 3023. [Google Scholar] [CrossRef] [PubMed]
- Schmelz, E.A.; Engelberth, J.; Alborn, H.T.; Tumlinson, J.H., 3rd; Teal, P.E. Phytohormone-based activity mapping of insect herbivore-produced elicitors. Proc. Natl. Acad. Sci. USA 2009, 106, 653–657. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.R.; Veselov, D.S.; Sharipova, G.V.; Akhiyarova, R.G.; Dodd, I.C.; Veselov, S.Y. Water relations and growth of original barley plants and its ABA-deficient mutants at increased air temperature. Russ. J. Plant. Physiol. 2014, 61, 188–193. [Google Scholar] [CrossRef]
- Schäfer, M.; Fischer, C.; Baldwin, I.T.; Meldau, S. Grasshopper oral secretions increase salicylic acid and abscisic acid levels in wounded leaves of Arabidopsis thaliana. Plant. Signal. Behav. 2011, 6, 1256–1258. [Google Scholar] [CrossRef]
- Vos, I.A.; Verhage, A.; Schuurink, R.C.; Watt, L.G.; Pieterse, C.M.J.; Van Wees, S.C.M. Onset of herbivore-induced resistance in systemic tissue primed for jasmonate-dependent defenses is activated by abscisic acid. Front. Plant. Sci. 2013, 4, 539. [Google Scholar] [CrossRef]
- Erb, M.; Flors, V.; Karlen, D.; de Lange, E.; Planchamp, C.; D’Alessandro, M.; Turlings, T.C.; Ton, J. Signal signature of aboveground-induced resistance upon belowground herbivory in maize. Plant. J. 2009, 59, 292–302. [Google Scholar] [CrossRef]
- Available online: http://ibg.anrb.ru/wp-content/uploads/2019/04/Katalog-endofit.doc (accessed on 29 April 2021).
- Yokota, T.; Murofushi, N. Extraction, purification and identification. In Hormonal Regulation of Development; MacMillan, J., Ed.; Springer: New York, NY, USA, 1980; p. 113. [Google Scholar] [CrossRef]
- Vysotskaya, L.; Veselov, S.; Kudoyarova, G. Effect on shoot water relations, and cytokinin and abscisic acid levels of inducing expression of a gene coding for isopentenyltransferase in roots of transgenic tobacco plants. J. Exp. Bot. 2010, 61, 3709–3717. [Google Scholar] [CrossRef][Green Version]
- Veselov, S.Y.; Timergalina, L.N.; Akhiyarova, G.R.; Kudoyarova, G.R.; Korobova, A.V.; Ivanov, I.; Arkhipova, T.N.; Prinsen, E. Study of cytokinin transport from shoots to roots of wheat plants is informed by a novel method of differential localization of free cytokinin bases or their ribosylated forms by means of their specific fixation. Protoplasma 2018, 255, 1581–1594. [Google Scholar] [CrossRef]
| Phytohormones Level, ng/mL of Culture Medium | ||||
|---|---|---|---|---|
| IAA | ABA | Zeatin | Zeatin-Riboside | |
| B. subtilis 26D | 83.6 ± 3.9 | 0 | 103.8 ± 12.9 | 46.2 ± 6.0 |
| Sterile LB | 0 | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sorokan, A.; Veselova, S.; Benkovskaya, G.; Maksimov, I. Endophytic Strain Bacillus subtilis 26D Increases Levels of Phytohormones and Repairs Growth of Potato Plants after Colorado Potato Beetle Damage. Plants 2021, 10, 923. https://doi.org/10.3390/plants10050923
Sorokan A, Veselova S, Benkovskaya G, Maksimov I. Endophytic Strain Bacillus subtilis 26D Increases Levels of Phytohormones and Repairs Growth of Potato Plants after Colorado Potato Beetle Damage. Plants. 2021; 10(5):923. https://doi.org/10.3390/plants10050923
Chicago/Turabian StyleSorokan, Antonina, Svetlana Veselova, Galina Benkovskaya, and Igor Maksimov. 2021. "Endophytic Strain Bacillus subtilis 26D Increases Levels of Phytohormones and Repairs Growth of Potato Plants after Colorado Potato Beetle Damage" Plants 10, no. 5: 923. https://doi.org/10.3390/plants10050923
APA StyleSorokan, A., Veselova, S., Benkovskaya, G., & Maksimov, I. (2021). Endophytic Strain Bacillus subtilis 26D Increases Levels of Phytohormones and Repairs Growth of Potato Plants after Colorado Potato Beetle Damage. Plants, 10(5), 923. https://doi.org/10.3390/plants10050923








