Heat Stress Impact on Yield and Composition of Quinoa Straw under Mediterranean Field Conditions
Abstract
:1. Introduction
2. Results
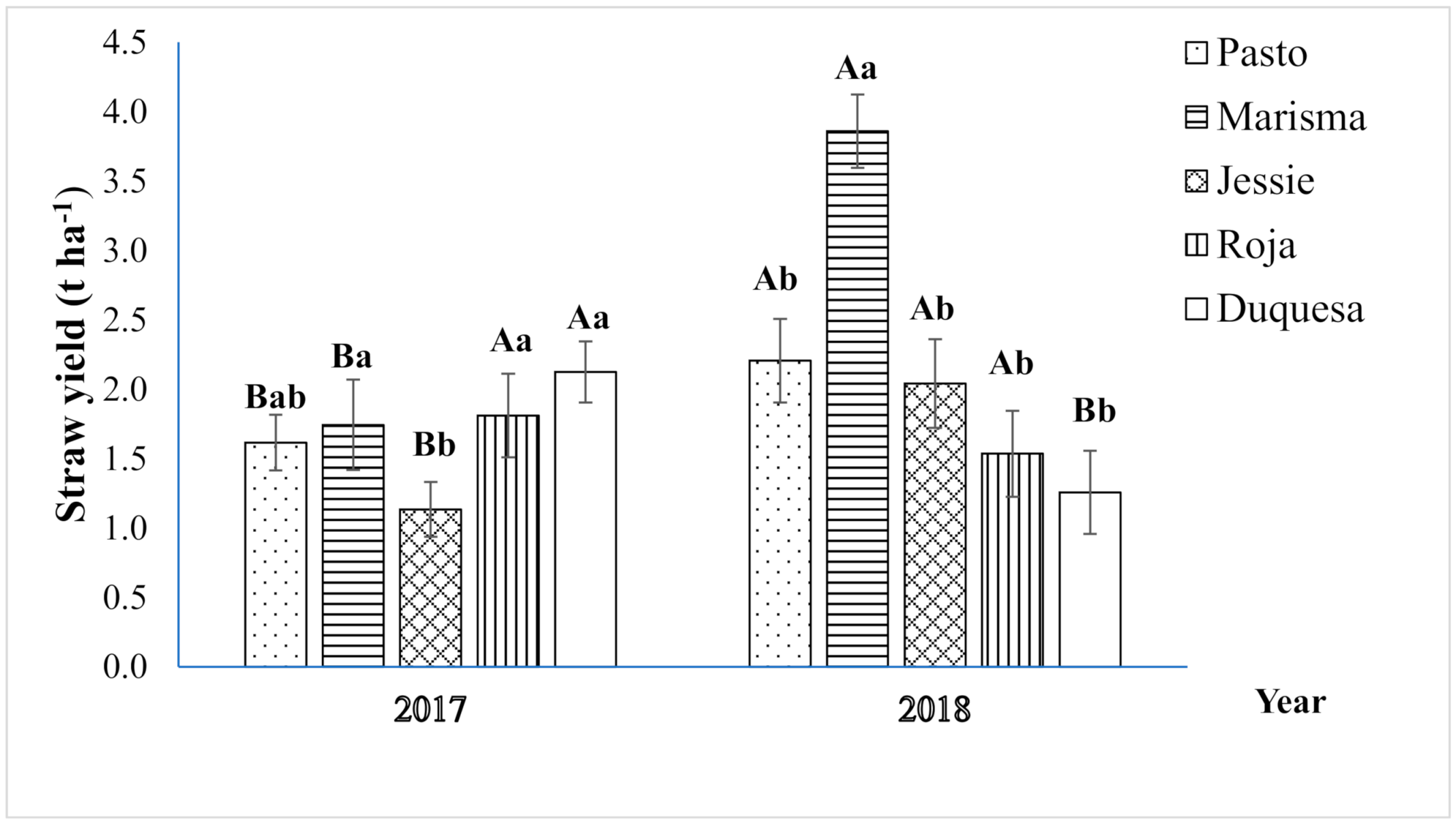
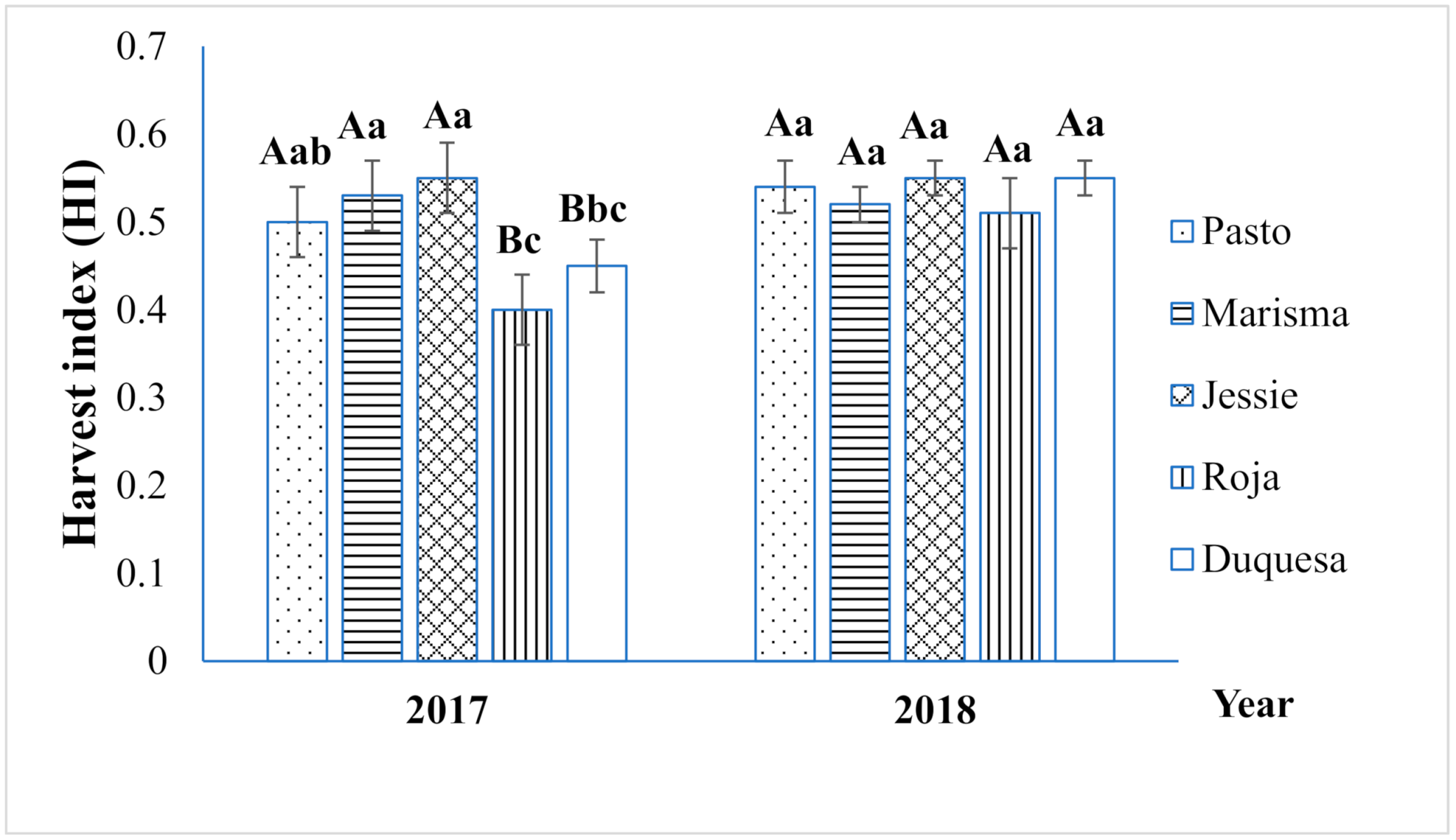
2.1. Straw Yield and Biomass Partitioning
2.2. Crude Protein (CP), Crude Fibre (CF), and Ash Content
2.3. Fibre Composition
2.4. Mineral Composition
2.5. Relative Feed Value
3. Discussion
4. Materials and Methods
4.1. Location, Climate and Soil Characteristics of the Experimental Site
4.2. Layouts of Experiments, Plant Material and Crop Management
4.3. Analysis and Measurements
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godde, C.M.; Mason-D’Croz, D.; Mayberry, D.; Thornton, P.; Herrero, M. Impacts of Climate Change on the Livestock Food Supply Chain, a Review of the Evidence. Glob. Food Sec. 2021, 28, 100488. [Google Scholar] [CrossRef] [PubMed]
- Naylor, A.; Ford, J.; Pearce, T.; Van Alstine, J. Conceptualizing Climate Vulnerability in Complex Adaptive Systems. One Earth 2020, 2, 444–454. [Google Scholar] [CrossRef]
- Shayanmehr, S.; Rastegari Henneberry, S.; Sabouhi Sabouni, M.; Shahnoushi Foroushani, N. Climate Change and Sustainability of Crop Yield in Dry Regions Food Insecurity. Sustainability 2020, 12, 9890. [Google Scholar] [CrossRef]
- Abumhadi, N.; Todorovska, E.; Assenov, B.; Tsonev, S.; Vulcheva, D.; Vulchev, D.; Atanasova, L.; Savova, S.; Atanassov, A.; Keith, W. Agricultural Research in 21 St Century: Challenges Facing the Food Security under the Impacts of Climate Change. Bulg. J. Agric. Sci. 2012, 18, 801–818. [Google Scholar]
- Wu, J.-Z.; Zhang, J.; Ge, Z.-M.; Xing, L.-W.; Han, S.-Q.; Shen, C.; Kong, F.-T. Impact of climate change on maize yield in China from 1979 to 2016. Integr. Agric. 2021, 20, 289–299. [Google Scholar] [CrossRef]
- Bazile, D.; Jacobsen, S.-E.; Verniau, A. The Global Expansion of Quinoa: Trends and Limits. Front. Plant Sci. 2016, 7, 622. [Google Scholar] [CrossRef] [Green Version]
- Blakstad, M.M.; Smith, E.R. Climate Change Worsens Global Inequity in Maternal Nutrition. Lancet Planet. Health. E 2020, 4, e547–e548. [Google Scholar] [CrossRef]
- Farooq, M.; Bramley, H.; Palta, J.A.; Siddique, K.H.M. Heat Stress in Wheat during Reproductive and Grain-Filling Phases. Crit. Rev. Plant Sci. 2011, 30, 491–507. [Google Scholar] [CrossRef]
- Halford, N.G. New Insights on the Effects of Heat Stress on Crops. J. Exp. Bot. 2009, 60, 4215–4216. [Google Scholar] [CrossRef] [Green Version]
- Scocco, P.; Piermarteri, K.; Malfatti, A.; Tardella, F.M.; Catorci, A. Increase of Drought Stress Negatively Affects the Sustainability of Extensive Sheep Farming in Sub-Mediterranean Climate. J. Arid Environ. 2016, 128, 50–58. [Google Scholar] [CrossRef]
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R.; Alpert, P.; Artale, V.; Li, L.; Luterbacher, J.; May, W.; Trigo, R.; Tsimplis, M.; et al. The Mediterranean Climate: An Overview of the Main Characteristics and Issues. Dev. Earth Environ. Sci. 2006, 4, 1–26. [Google Scholar] [CrossRef]
- Joffre, R.; Rambal, S. Mediterranean Ecosystems. In Encyclopedia of Life Sciences; Mcmillan Publishers, Ltd.: New York, NY, USA, 2001; pp. 1–7. [Google Scholar] [CrossRef]
- Jacobsen, S.-E.; Jensen, C.R.; Liu, F. Improving Crop Production in the Arid Mediterranean Climate. Field Crop. Res. 2012, 128, 34–47. [Google Scholar] [CrossRef]
- Lam, D. How the World Survived the Population Bomb: Lessons From 50 Years of Extraordinary Demographic History. Demography 2011, 48, 1231–1262. [Google Scholar] [CrossRef] [Green Version]
- Filik, G. Biodegradability of Quinoa Stalks: The Potential of Quinoa Stalks as a Forage Source or as Biomass for Energy Production. Fuel 2020, 266, 117064. [Google Scholar] [CrossRef]
- Jacobsen, S.E.; Mujica, A.; Jensen, C.R. The Resistance of Quinoa (Chenopodium quinoa Willd.) to Adverse Abiotic Factors. Food Rev. Int. 2003, 19, 99–109. [Google Scholar] [CrossRef]
- Asher, A.; Galili, S.; Whitney, T.; Rubinovich, L. The Potential of Quinoa Chenopodium Quinoa. Cultivation in Israel as a Dual-Purpose Crop for Grain Production and Livestock Feed. Sci. Hortic. 2020, 272, 109534. [Google Scholar] [CrossRef]
- Ruales, J.; Nair, B.M. Nutritional Quality of the Protein in Quinoa (Chenopodium quinoa, Willd.) Seeds. Plant Foods Hum. Nutr. 1992, 42, 1–11. [Google Scholar] [CrossRef]
- Hinojosa, L.; González, J.A.; Barrios-Masias, F.H.; Fuentes, F.; Murphy, K.M. Plants Quinoa Abiotic Stress Responses: A Review. Plants 2018, 7, 106. [Google Scholar] [CrossRef] [Green Version]
- Razzaghi, F.; Ahmadi, S.H.; Jacobsen, S.-E.; Jensen, C.R.; Andersen, M.N. Effects of Salinity and Soil-Drying on Radiation Use Efficiency, Water Productivity and Yield of Quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2012, 198, 173–184. [Google Scholar] [CrossRef]
- Rezzouk, F.Z.; Shahid, M.A.; Elouafi, I.A.; Zhou, B.; Araus, J.L.; Serret, M.D. Agronomic Performance of Irrigated Quinoa in Desert Areas: Comparing Different Approaches for Early Assessment of Salinity Stress. Agric. Water Manag. 2020, 240, 106205. [Google Scholar] [CrossRef]
- Iqbal, S.; Basra, S.M.A.; Afzal, I.; Wahid, A. Exploring Potential of Well Adapted Quinoa Lines for Salt Tolerance. Int. J. Agric. Biol. 2017, 19, 933–940. [Google Scholar] [CrossRef]
- Adolf, V.I.; Jacobsen, S.E.; Shabala, S. Salt Tolerance Mechanisms in Quinoa (Chenopodium quinoa Willd.). Environ. Exp. Bot. 2013, 92, 43–54. [Google Scholar] [CrossRef]
- Ahmadi, S.H.; Solgi, S.; Sepaskhah, A.R. Quinoa: A Super or Pseudo-Super Crop? Evidences from Evapotranspiration, Root Growth, Crop Coefficients, and Water Productivity in a Hot and Semi-Arid Area under Three Planting Densities. Agric. Water Manag. 2019, 225, 105784. [Google Scholar] [CrossRef]
- Präger, A.; Munz, S.; Nkebiwe, P.; Mast, B.; Graeff-Hönninger, S. Yield and Quality Characteristics of Different Quinoa (Chenopodium quinoa Willd.) Cultivars Grown under Field Conditions in Southwestern Germany. Agronomy 2018, 8, 197. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Ren, Y.; Jiang, F.; Liang, J.; Du, S. kui. Pretreatment of Quinoa Straw with 1-Butyl-3-Methylimidazolium Chloride and Physiochemical Characterization of Biomass. Renew. Energy 2020, 146, 1364–1371. [Google Scholar] [CrossRef]
- She, D.; Sun, R.C.; Jones, G.L. Chemical Modification of Straw as Novel Materials for Industries. In Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels; Elsevier: Amsterdam, The Netherlands, 2010; pp. 209–217. [Google Scholar] [CrossRef]
- Wang, K.; Sun, R.C. Biorefinery Straw for Bioethanol. In Cereal Straw as a Resource for Sustainable Biomaterials and Biofuels; Elsevier: Oxford, UK, 2011; pp. 267–287. [Google Scholar] [CrossRef]
- Alandia, G.; Rodriguez, J.P.; Jacobsen, S.-E.; Bazile, D.; Condori, B. Global Expansion of Quinoa and Challenges for the Andean Region. Glob. Food Sec. 2020, 26, 100429. [Google Scholar] [CrossRef]
- Tovar, J.C.; Quillatupa, C.; Callen, S.T.; Elizabeth Castillo, S.; Pearson, P.; Shamin, A.; Schuhl, H.; Fahlgren, N.; Gehan, M.A. Heating Quinoa Shoots Results in Yield Loss by Inhibiting Fruit Production and Delaying Maturity. Plant J. 2019, 102, 1058–1073. [Google Scholar] [CrossRef] [Green Version]
- Bertero, H.D. Environmental Control of Development. In State of the Art Report on Quinoa around the World in 2013; Bazile, D., Bertero, D., Nieto, C., Eds.; FAO & CIRAD: Rome, Italy, 2015; pp. 120–130. [Google Scholar]
- Hinojosa, L.; Matanguihan, J.B.; Murphy, K.M. Effect of High Temperature on Pollen Morphology, Plant Growth and Seed Yield in Quinoa (Chenopodium quinoa Willd.). J. Agron. Crop Sci. 2019, 205, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Akter, N.; Rafiqul Islam, M. Heat Stress Effects and Management in Wheat. A Review. Agron. Sustain. Dev. 2017, 37, 1–17. [Google Scholar] [CrossRef]
- Feng, B.; Liu, P.; Li, G.; Dong, S.T.; Wang, F.H.; Kong, L.A.; Zhang, J.W. Effect of Heat Stress on the Photosynthetic Characteristics in Flag Leaves at the Grain-Filling Stage of Different Heat-Resistant Winter Wheat Varieties. J. Agron. Crop Sci. 2014, 200, 143–155. [Google Scholar] [CrossRef]
- Mahrookashani, A.; Siebert, S.; Hüging, H.; Ewert, F. Independent and Combined Effects of High Temperature and Drought Stress around Anthesis on Wheat. J. Agron. Crop Sci. 2017, 203, 453–463. [Google Scholar] [CrossRef]
- Matías, J.; Cruz, V.; Rodríguez, M.R.; Calvo, P. Heat stress lowers yields, alters nutrient uptake, and changes seed quality in quinoa grown under Mediterranean field conditions. J. Agron. Crop Sci. 2021, accepted, in press. [Google Scholar] [CrossRef]
- Lavini, A.; Pulvento, C.; d’Andria, R.; Riccardi, M.; Choukr-Allah, R.; Belhabib, O.; Yazar, A.; İncekaya, Ç.; Metin Sezen, S.; Qadir, M.; et al. Quinoa’s Potential in the Mediterranean Region. J. Agron. Crop Sci. 2014, 200, 344–360. [Google Scholar] [CrossRef]
- Hammer, G.L.; Broad, I.J. Genotype and Environment Effects on Dynamics of Harvest Index during Grain Filling in Sorghum. Agron. J. 2003, 95, 199–206. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.; Bean, B.; Brown, B.; Bruening, W.; Edwards, J.; Flowers, M.; Karow, R.; Lee, C.; Morgan, G.; Ottman, M.; et al. Harvest Index and Straw Yield of Five Classes of Wheat. Biomass Bioenerg. 2016, 85, 223–227. [Google Scholar] [CrossRef] [Green Version]
- Hay, R.K.M. Harvest Index: A Review of Its Use in Plant Breeding and Crop Physiology. Ann. Appl. Biol. 1995, 126, 197–216. [Google Scholar] [CrossRef]
- Unkovich, M.; Baldock, J.; Forbes, M. Variability in Harvest Index of Grain Crops and Potential Significance for Carbon Accounting: Examples from Australian Agriculture. Adv. Agron. 2010, 105, 173–219. [Google Scholar] [CrossRef]
- Ali, S.; Chattha, M.U.; Hassan, M.U.; Khan, I.; Chattha, M.B.; Iqbal, B.; Rehman, M.; Nawaz, M.; Amin, M.Z. Growth, Biomass Production, and Yield Potential of Quinoa (Chenopodium quinoa Willd.) as Affected by Planting Techniques Under Irrigated Conditions. Int. J. Plant Prod. 2020, 14, 427–441. [Google Scholar] [CrossRef]
- De Santis, G.; Ronga, D.; Caradonia, F.; Ambrosio, T.D.; Troisi, J.; Rascio, A.; Fragasso, M.; Pecchioni, N.; Rinaldi, M. Evaluation of Two Groups of Quinoa Chenopodium Quinoa Willd.. Accessions with Different Seed Colours for Adaptation to the Mediterranean Environment. Crop Pasture Sci. 2018, 69, 1264–1275. [Google Scholar] [CrossRef]
- Assefa, Y.; Purcell, L.C.; Salmeron, M.; Naeve, S.; Casteel, S.N.; Kovács, P.; Archontoulis, S.; Licht, M.; Below, F.; Kandel, H.; et al. Assessing Variation in US Soybean Seed Composition Protein and Oil. Front. Plant Sci. 2019, 10, 298. [Google Scholar] [CrossRef]
- Gibson, L.R.; Mullen, R.E. Soybean Seed Composition under High Day and Night Growth Temperatures. J. Am. Oil Chem. Soc. 1996, 73, 733–737. [Google Scholar] [CrossRef]
- Thomas, J.M.G.; Boote, K.J.; Allen, L.H.; Gallo-Meagher, M.; Davis, J.M. Elevated Temperature and Carbon Dioxide Effects on Soybean Seed Composition and Transcript Abundance. Crop Sci. 2003, 43, 1548–1557. [Google Scholar] [CrossRef]
- Wolf, R.B.; Cavins, J.F.; Kleiman, R.; Black, L.T. Effect of Temperature on Soybean Seed Constituents: Oil, Protein, Moisture, Fatty Acids, Amino Acids and Sugars. J. Am. Oil Chem. Soc. 1982, 59, 230–232. [Google Scholar] [CrossRef]
- Habermann, E.; Dias de Oliveira, E.A.; Contin, D.R.; Delvecchio, G.; Viciedo, D.O.; de Moraes, M.A.; de Mello Prado, R.; de Pinho Costa, K.A.; Braga, M.R.; Martinez, C.A. Warming and Water Deficit Impact Leaf Photosynthesis and Decrease Forage Quality and Digestibility of a C4 Tropical Grass. Physiol. Plant. 2019, 165, 383–402. [Google Scholar] [CrossRef] [PubMed]
- López, S.; Davies, D.R.; Giráldez, F.J.; Dhanoa, M.S.; Dijkstra, J.; France, J. Assessment of Nutritive Value of Cereal and Legume Straws Based on Chemical Composition and in Vitro Digestibility. J. Sci. Food Agric. 2005, 85, 1550–1557. [Google Scholar] [CrossRef]
- Liu, L.; Ye, X.P.; Womac, A.R.; Sokhansanj, S. Variability of Biomass Chemical Composition and Rapid Analysis Using FT-NIR Techniques. Carbohydr. Polym. 2010, 81, 820–829. [Google Scholar] [CrossRef]
- Sorieul, M.; Dickson, A.; Hill, S.J.; Pearson, H. Plant Fibre: Molecular Structure and Biomechanical Properties, of a Complex Living Material, Influencing Its Deconstruction towards a Biobased Composite. Materials 2016, 9, 618. [Google Scholar] [CrossRef]
- Le Gall, H.; Philippe, F.; Domon, J.M.; Gillet, F.; Pelloux, J.; Rayon, C. Cell Wall Metabolism in Response to Abiotic Stress. Plants 2015, 4, 112–166. [Google Scholar] [CrossRef]
- Houston, K.; Tucker, M.R.; Chowdhury, J.; Shirley, N.; Little, A. The Plant Cell Wall: A Complex and Dynamic Structure as Revealed by the Responses of Genes under Stress Conditions. Front. Plant Sci. 2016, 10, 984. [Google Scholar] [CrossRef] [Green Version]
- Lima, R.B.; Dos Santos, T.B.; Vieira, L.G.E.; Ferrarese, M.D.L.L.; Ferrarese-Filho, O.; Donatti, L.; Boeger, M.R.T.; Petkowicz, C.L.D.O. Heat Stress Causes Alterations in the Cell-Wall Polymers and Anatomy of Coffee Leaves Coffea arabica L. Carbohydr. Polym. 2013, 93, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Collins, S.R.; Wellner, N.; Martinez Bordonado, I.; Harper, A.L.; Miller, C.N.; Bancroft, I.; Waldron, K.W. Variation in the Chemical Composition of Wheat Straw: The Role of Tissue Ratio and Composition. Biotechnol. Biofuels 2014, 7, 121. [Google Scholar] [CrossRef]
- Platače, R.; Adamovičs, A. The Evaluation of Ash Content in Grass Biomass Used for Energy Production. WIT Trans. Ecol. Environ. 2014, 190, 1057–1065. [Google Scholar] [CrossRef] [Green Version]
- Reguera, M.; Conesa, C.M.; Gil-Gómez, A.; Haros, C.M.; Pérez-Casas, M.Á.; Briones-Labarca, V.; Bolaños, L.; Bonilla, I.; Álvarez, R.; Pinto, K.; et al. The Impact of Different Agroecological Conditions on the Nutritional Composition of Quinoa Seeds. Peer J. 2018, 14, e4442. [Google Scholar] [CrossRef]
- Murphy, K.M.; Reeves, P.G.; Jones, S.S. Relationship between Yield and Mineral Nutrient Concentrations in Historical and Modern Spring Wheat Cultivars. Euphytica 2008, 163, 381–390. [Google Scholar] [CrossRef]
- Patakas, A.; Zioziou, E.; Nikolaou, N.; Radoglou, K.; Noitsakis, B. The role of organic solute and ion accumulation in osmotic adjustment in drought-stressed grapevines. Plant Sci. 2002, 163, 361–367. [Google Scholar] [CrossRef]
- Argentel-Martínez, L.; Garatuza-Payan, J.; Yepez, E.A.; Arredondo, T.; De los Santos-Villalobos, S. Water regime and osmotic adjustment under warming conditions on wheat in the Yaqui Valley, Mexico. Peer J. 2019, 7, e7029. [Google Scholar] [CrossRef]
- Pearce, G.R.; Beard, J.; Hilliard, E.P. Variability in the Chemical Composition of Cereal Straws and in Vitro Digestibility with and without Sodium Hydroxide Treatment. Aust. J. Exp. Agric. 1979, 19, 350–353. [Google Scholar] [CrossRef]
- Linn, J.G.; Martin, N.P. Forage Quality Analyses and Interpretation. Vet. Clin. North Am. Food Anim. Pract. 1991, 7, 509–523. [Google Scholar] [CrossRef]
- Moore, J.E.; Burns, J.C.; Fisher, D.S. Multiple Regression Equations for Predicting Relative Feed Value of Grass Hays. In Proceedings of the Proc. American Forage and Grassld, Vancouver, BC, Canada, 13–15 June 1996; Williams, M.J., Ed.; pp. 135–139. [Google Scholar]
- Wheeler, T.; Von Braun, J. Climate Change Impacts on Global Food Security. Science 2013, 341, 508–513. [Google Scholar] [CrossRef]
- Mondal, S.; Singh, R.P.; Crossa, J.; Huerta-Espino, J.; Sharma, I.; Chatrath, R.; Singh, G.P.; Sohu, V.S.; Mavi, G.S.; Sukaru, V.S.P.; et al. Earliness in Wheat: A Key to Adaptation under Terminal and Continual High Temperature Stress in South Asia. F. Crop. Res. 2013, 151, 19–26. [Google Scholar] [CrossRef]
- Association of Official Agricultural Chemist (AOAC). Official Methods of Analysis of AOAC International, 17th ed.; Horwitz, W., Ed.; AOAC: Arlington, TX, USA, 2000. [Google Scholar]
- MAPA, Ministerio de Agricultura, Pesca y Alimentación. Métodos Oficiales de Análisis; Tomo III; MAPA: Madrid, Spain, 1995; pp. 205–285.
| Variety | CP1 (%) | CF2 (%) | Ash (%) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | |
| Pasto | 11.7 | 7.6 | 9.7 | 29.8 | 32.5 | 31.1 | 15.1 | 15.7 ab | 15.4 abc |
| Marisma | 13.5 | 8.1 | 10.8 | 27.8 | 32.3 | 30.0 | 16.7 | 14.6 ab | 15.6 ab |
| Jessie | 12.7 | 10.5 | 11.6 | 24.7 | 30.1 | 27.4 | 17.4 | 15.9 a | 16.6 a |
| Roja | 13.2 | 6.9 | 10.0 | 30.3 | 39.2 | 34.7 | 13.0 | 12.6 b | 12.8 c |
| Duquesa | 13.1 | 8.4 | 10.8 | 27.6 | 35.0 | 31.3 | 14.4 | 13.0 ab | 13.7 bc |
| Mean | 12.8 A | 8.4 B | 10.6 | 28.0 B | 33.8 A | 30.9 | 15.3 | 14.3 | 14.8 |
| HSD | 4.0 | 5.3 | 4.4 | 6.5 | 16.2 | 7.8 | 4.6 | 3.2 | 2.6 |
| Significance | |||||||||
| Year (Y) | ** | * | n.s. | ||||||
| Variety (V) | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | n.s. | * | ** |
| Y × V | n.s. | n.s. | n.s. | ||||||
| Variety | NDF1 (%) | ADF2 (%) | ADL3 (%) | HEM4 (%) | CEL5 (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | |
| Pasto | 47.2 | 52.2 | 49.7 | 34.2 | 38.0 | 36.1 ab | 5.1 ab | 6.0 | 5.5 ab | 13.0 ab | 14.2 | 13.6 | 29.2 a | 32.0 | 30.6 ab |
| Marisma | 41.4 | 53.7 | 46.7 | 30.3 | 37.9 | 34.1 ab | 4.5 b | 6.1 | 5.3 b | 11.2 b | 15.8 | 13.5 | 25.8 ab | 31.8 | 28.8 ab |
| Jessie | 41.8 | 51.7 | 47.6 | 26.6 | 36.6 | 31.6 b | 6.0 a | 6.6 | 6.3 a | 15.2 a | 15.0 | 15.1 | 20.6 b | 30.0 | 25.3 b |
| Roja | 48.3 | 63.6 | 46.7 | 34.0 | 47.8 | 40.9 a | 5.9 a | 7.0 | 6.5 a | 14.4 a | 15.8 | 15.1 | 28.1 ab | 40.8 | 34.5 a |
| Duquesa | 42.1 | 55.7 | 48.9 | 28.8 | 42.1 | 35.5 ab | 4.6 ab | 7.0 | 5.8 ab | 13.3 ab | 13.6 | 13.5 | 24.2 ab | 35.1 | 29.7 ab |
| Mean | 44.1 B | 55.4 A | 49.8 | 30.8 B | 40.5 A | 35.6 | 5.2 B | 6.5 A | 5.9 | 13.4 | 14.9 | 14.2 | 25.6 B | 34.0 A | 29.8 |
| HSD | 11.4 | 19.5 | 10.0 | 9.3 | 16.2 | 8.3 | 1.4 | 1.4 | 0.9 | 3.1 | 4.2 | 2.5 | 8.3 | 15.1 | 7.7 |
| Significance | |||||||||||||||
| Year (Y) | ** | ** | ** | n.s. | ** | ||||||||||
| Variety (V) | n.s. | n.s. | n.s. | n.s. | n.s. | * | * | n.s. | ** | * | n.s. | n.s. | * | n.s. | * |
| Y × V | n.s. | n.s. | n.s. | n.s. | n.s. | ||||||||||
| Variety | N (%) | P (%) | K (%) | Ca (%) | Mg (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | |
| Pasto | 1.9 | 1.2 | 1.5 | 0.23 | 0.15 b | 0.19 ab | 4.3 b | 5.1 | 4.7 ab | 1.6 a | 1.5 a | 1.6 a | 0.62 | 0.68 | 0.65 |
| Marisma | 2.2 | 1.3 | 1.7 | 0.28 | 0.15 b | 0.22 ab | 4.6 ab | 5.0 | 4.8 ab | 1.8 a | 1.1 abc | 1.5 ab | 0.82 | 0.69 | 0.76 |
| Jessie | 2.0 | 1.7 | 1.9 | 0.23 | 0.25 a | 0.24 a | 6.1 a | 5.8 | 6.0 a | 1.1 b | 1.2 ab | 1.2 bc | 0.63 | 0.77 | 0.70 |
| Roja | 2.1 | 1.2 | 1.6 | 0.25 | 0.09 b | 0.17 b | 4.6 ab | 4.1 | 4.4 b | 0.9 b | 0.6 c | 0.8 d | 0.60 | 0.45 | 0.53 |
| Duquesa | 2.1 | 1.3 | 1.7 | 0.22 | 0.12 b | 0.17 b | 5.1 ab | 4.9 | 5.0 ab | 1.1 b | 0.9 bc | 1.0 cd | 0.63 | 0.55 | 0.59 |
| Mean | 2.0 A | 1.3 B | 1.7 | 0.24 A | 0.15 B | 0.20 | 5.0 | 5.0 | 5.0 | 1.3 A | 1.1 B | 1.2 | 0.66 | 0.63 | 0.64 |
| HSD | 0.6 | 1.0 | 0.5 | 0.10 | 0.09 | 0.06 | 1.7 | 2.6 | 1.4 | 0.4 | 0.5 | 0.3 | 0.38 | 0.34 | 0.26 |
| Significance | |||||||||||||||
| Year (Y) | ** | *** | n.s. | * | n.s. | ||||||||||
| Variety (V) | n.s. | n.s. | n.s. | n.s. | ** | * | * | n.s. | * | *** | ** | *** | n.s. | n.s. | n.s. |
| Y × V | n.s. | ** | n.s. | ** | n.s. | ||||||||||
| Variety | DDM1 | DMI2 | RFV3 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 2017 | 2018 | Mean | 2017 | 2018 | Mean | 2017 | 2018 | Mean | |
| Pasto | 2.6 | 2.3 | 2.4 | 62.3 | 59.3 | 60.8 ab | 124.2 | 106.3 | 115.2 ab |
| Marisma | 2.9 | 2.3 | 2.6 | 65.3 | 59.4 | 62.3 ab | 147.5 | 104.1 | 125.8 ab |
| Jessie | 2.9 | 2.3 | 2.6 | 68.2 | 60.3 | 64.3 a | 152.7 | 109.3 | 131.0 a |
| Roja | 2.5 | 1.9 | 2.2 | 62.4 | 51.7 | 57.1 b | 120.9 | 77.9 | 99.4 b |
| Duquesa | 2.9 | 2.2 | 2.5 | 66.5 | 56.1 | 61.3 ab | 147.1 | 94.9 | 121.0 ab |
| Mean | 2.7 A | 2.2 B | 2.5 | 64.9 A | 57.4 B | 61.1 | 138.5 A | 98.5 B | 118.5 |
| HSD | 0.7 | 0.7 | 0.5 | 7.2 | 12.7 | 6.5 | 49.6 | 51.4 | 31.5 |
| Significance | |||||||||
| Year (Y) | ** | ** | ** | ||||||
| Variety (V) | n.s. | n.s. | n.s. | n.s. | n.s. | * | n.s. | n.s. | * |
| Y × V | n.s. | n.s. | n.s. | ||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matías, J.; Cruz, V.; Reguera, M. Heat Stress Impact on Yield and Composition of Quinoa Straw under Mediterranean Field Conditions. Plants 2021, 10, 955. https://doi.org/10.3390/plants10050955
Matías J, Cruz V, Reguera M. Heat Stress Impact on Yield and Composition of Quinoa Straw under Mediterranean Field Conditions. Plants. 2021; 10(5):955. https://doi.org/10.3390/plants10050955
Chicago/Turabian StyleMatías, Javier, Verónica Cruz, and María Reguera. 2021. "Heat Stress Impact on Yield and Composition of Quinoa Straw under Mediterranean Field Conditions" Plants 10, no. 5: 955. https://doi.org/10.3390/plants10050955







