The Impacts of Woolly Cupgrass on the Antioxidative System and Growth of a Maize Hybrid
Abstract
:1. Introduction
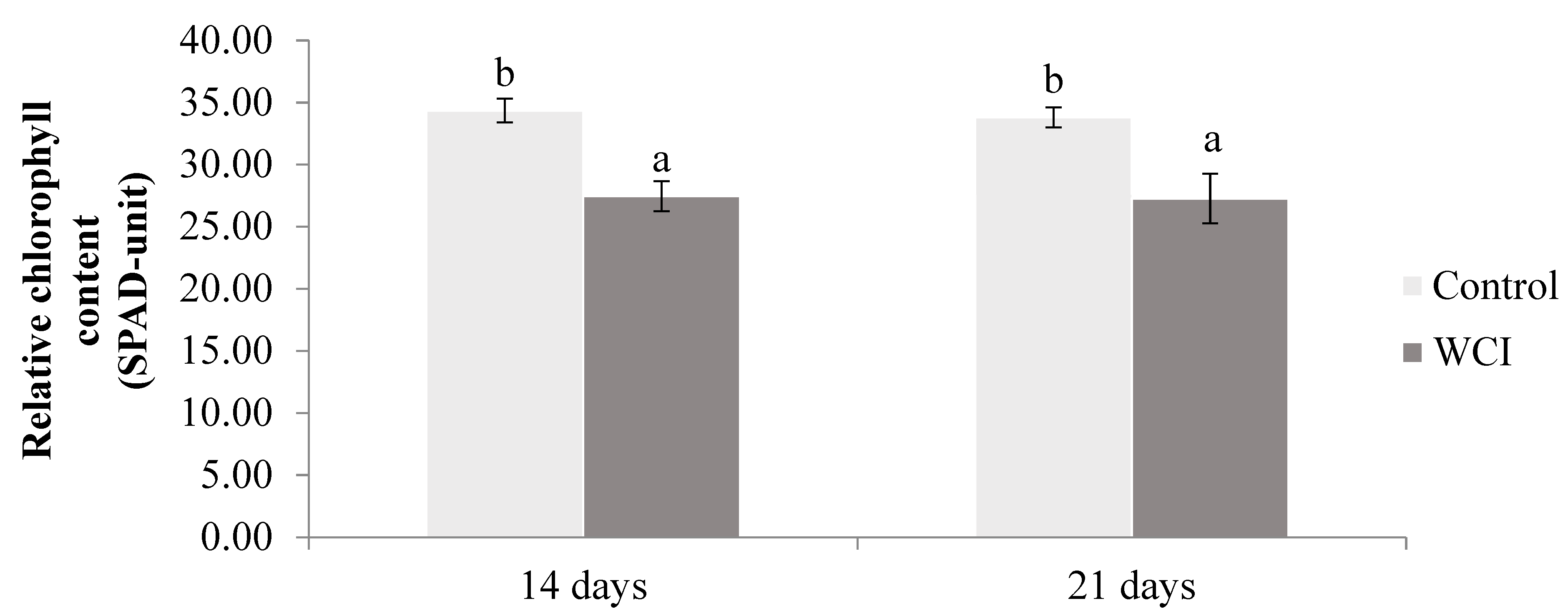
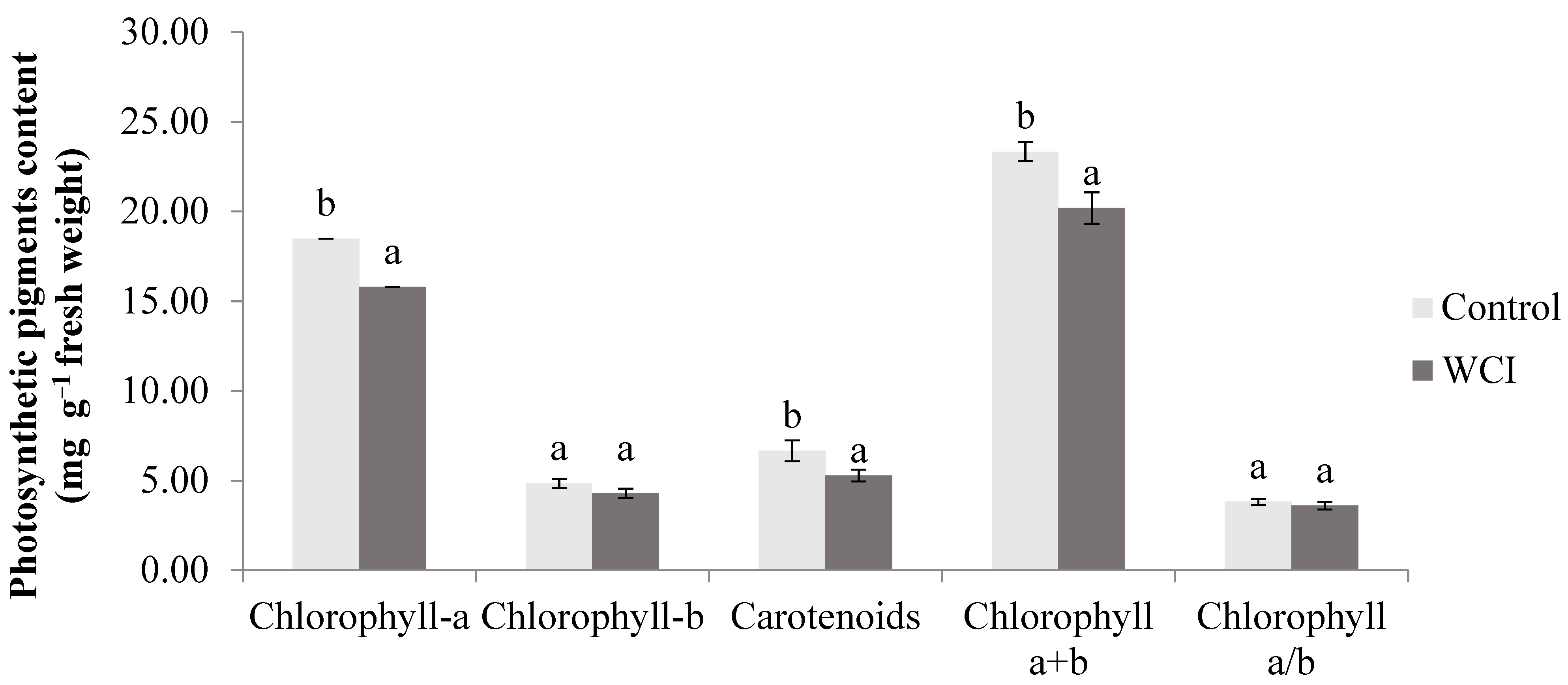
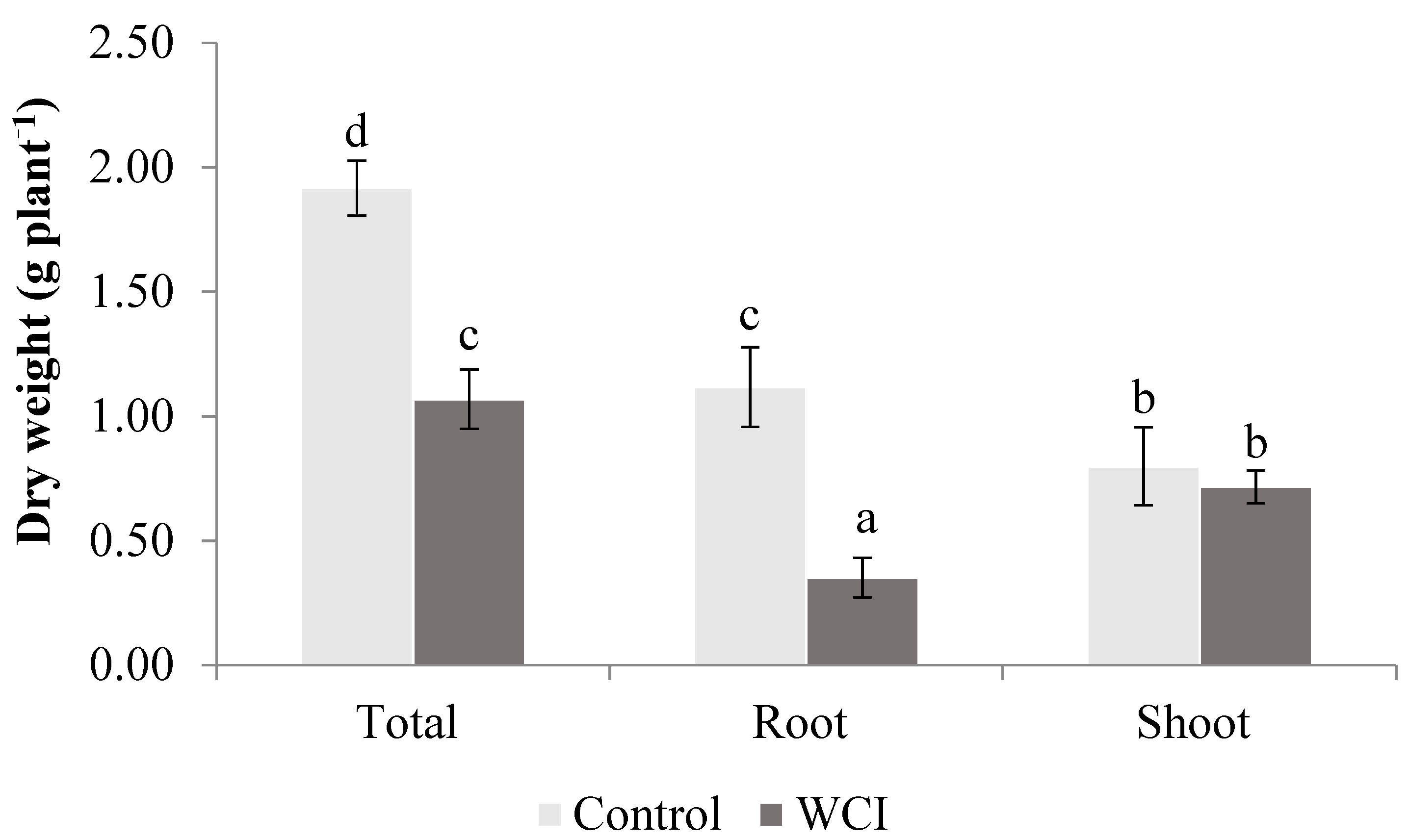
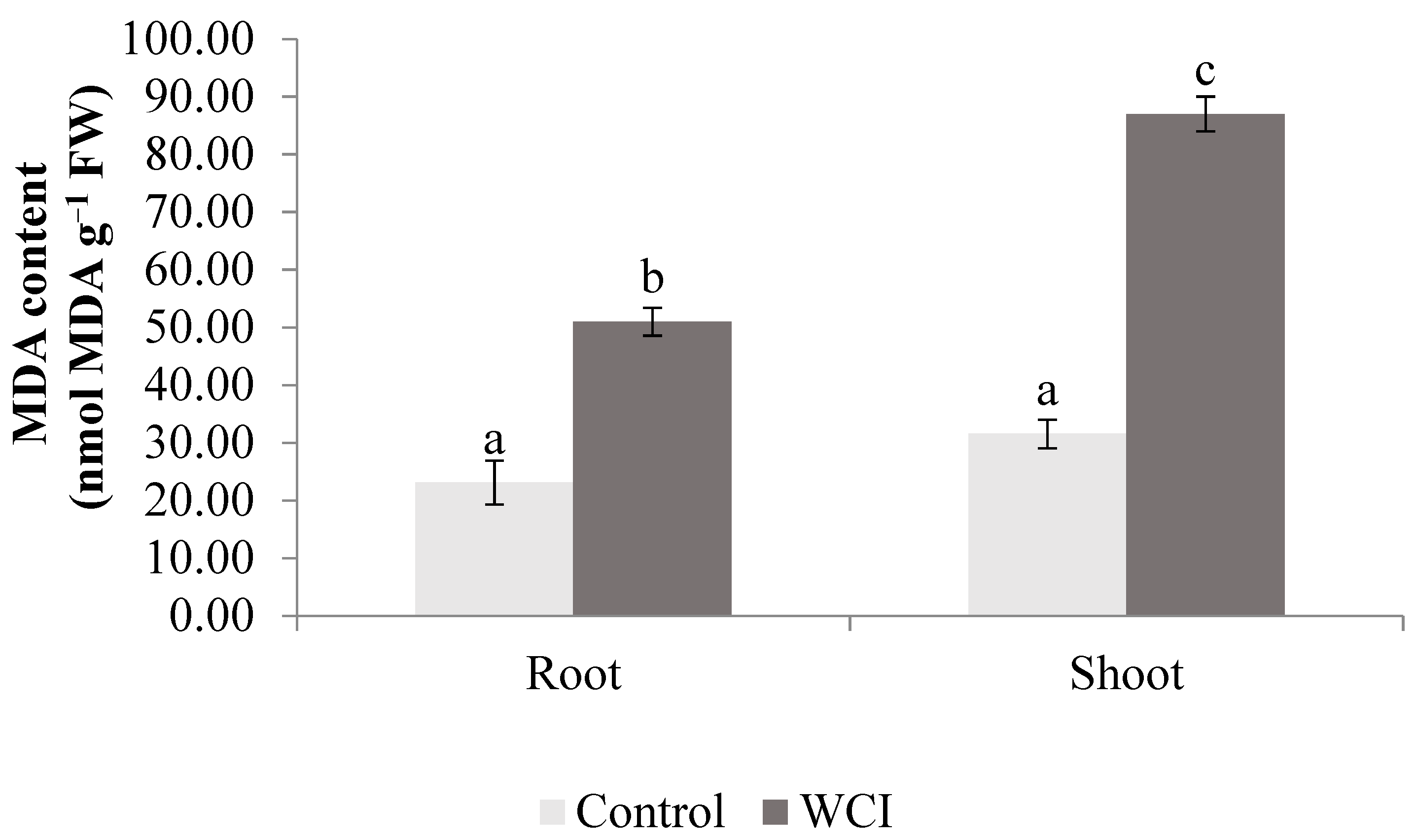
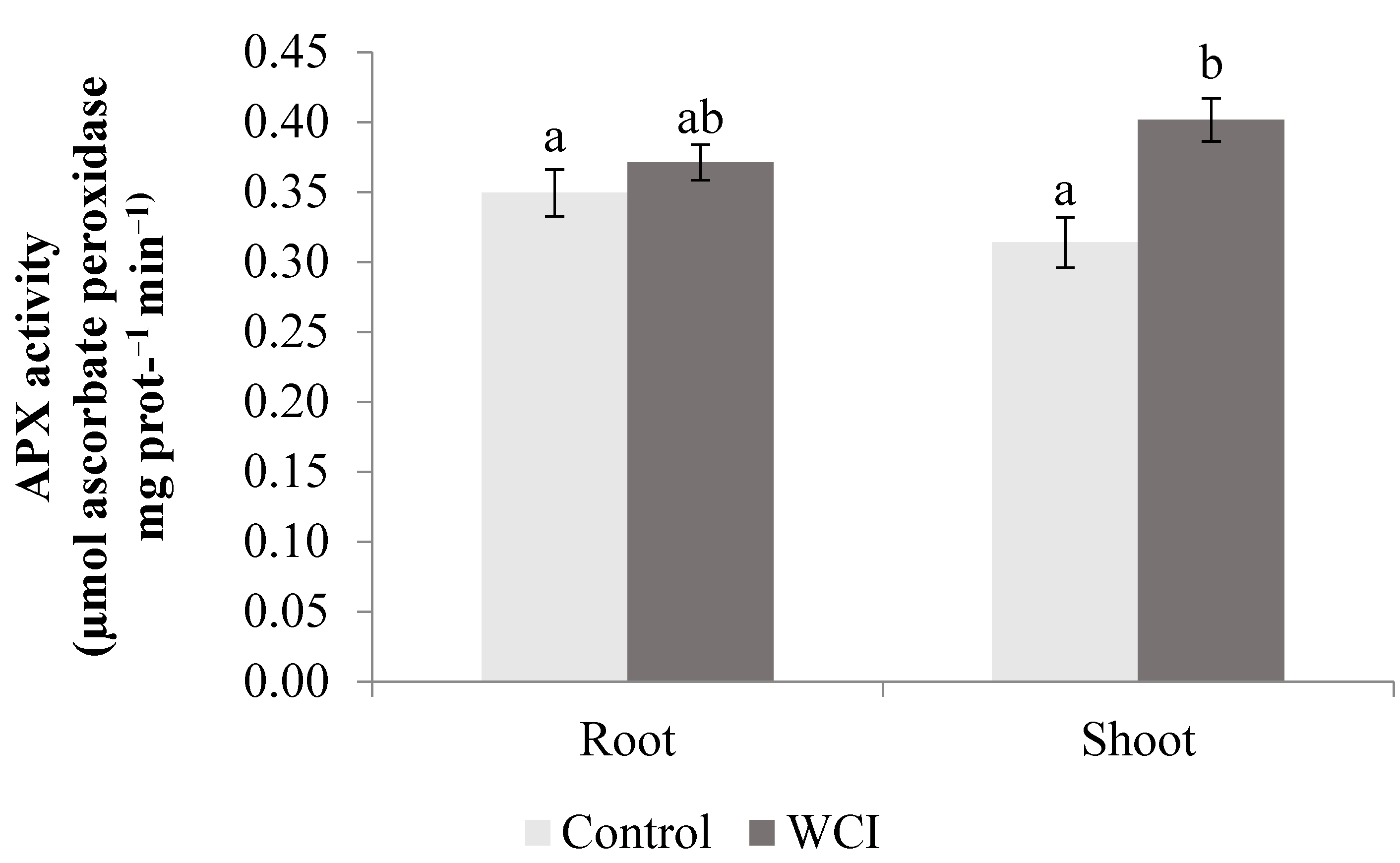
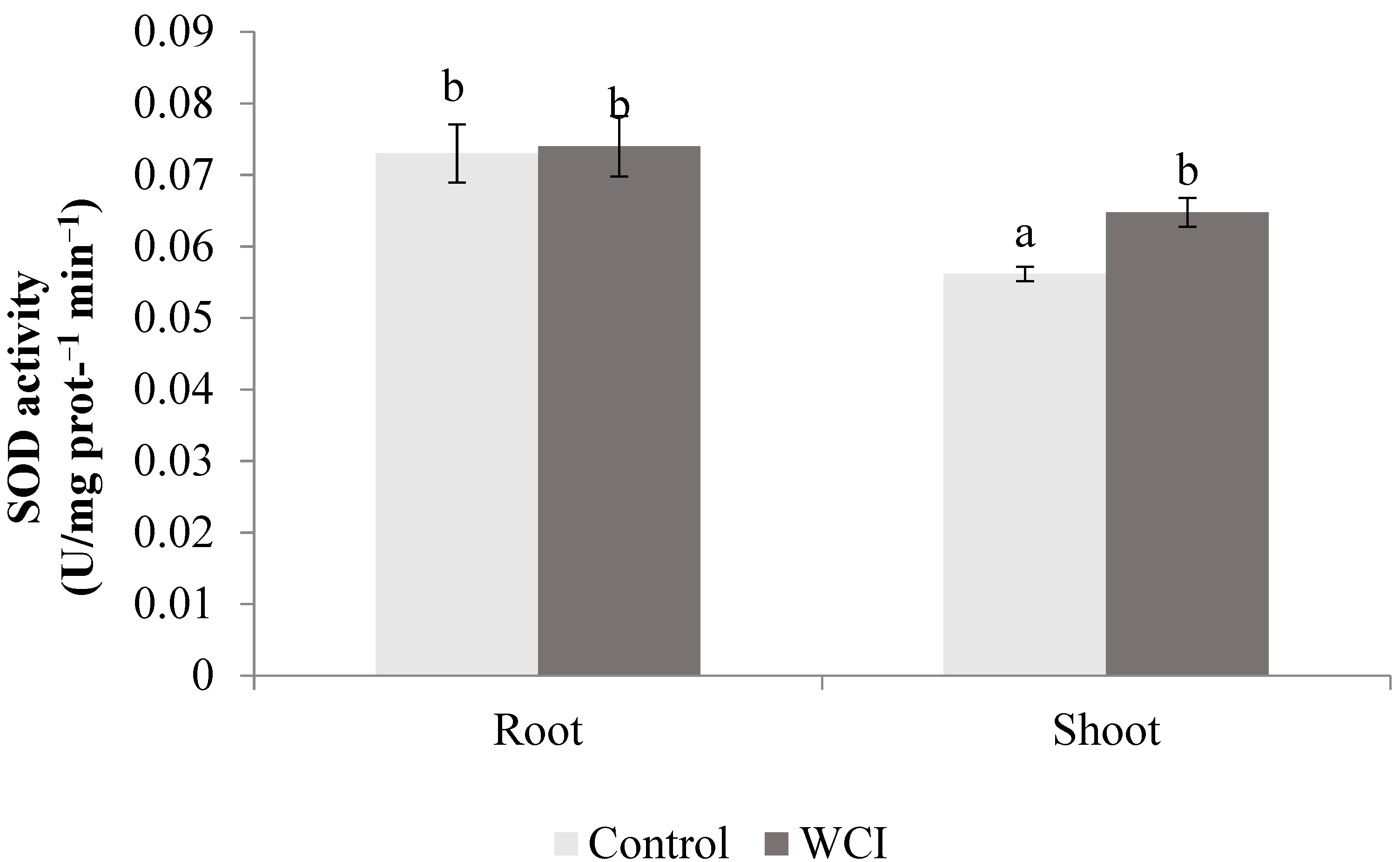
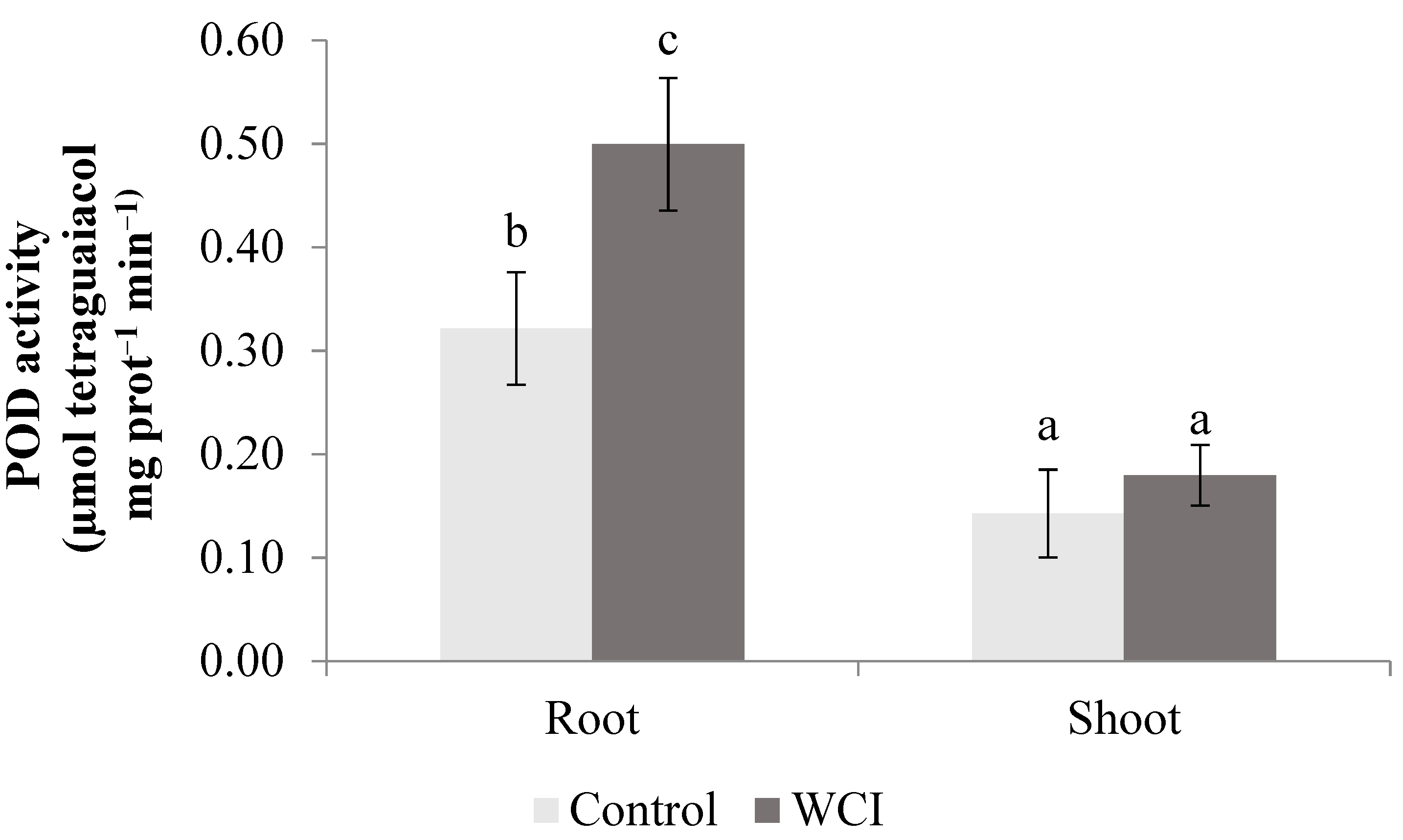
2. Results
3. Discussion
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
4.2. Quantification of the Photosynthetic Pigments
- chlorophyll-a = (11.65 × a664−2.69 × b647)
- chlorophyll-b = (20.81 × b647−4.53 × a664)
- carotenoids = (1000 × car480−1.28 × a664−56.7 × b647)
- total chlorophyll content = chlorophyll-a + chlorophyll-b
- chlorophyll a/b = chlorophyll-a/chlorophyll-b
4.3. Morphological Parameters
4.4. Determination of Lipid Peroxidation
4.5. Antioxidant Enzyme Assays
4.6. Statistical Analysis
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hejda, M.; Pysek, P.; Jarosik, V. Impact of invasive plants on the species richness, diversity and composition of invaded communities. J. Ecol. 2009, 97, 393–403. [Google Scholar] [CrossRef]
- Ziska, L.H.; McConnell, L.L. Climate change, carbon dioxide, and pest biology: Monitor, mitigate, manage. J. Agric. Food Chem. 2015, 64, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Weber, E.; Gut, D. A survey of weeds that are increasingly spreading in Europe. Agron. Sustain. Dev. 2005, 25, 109–121. [Google Scholar] [CrossRef] [Green Version]
- Follak, S.; Schleicher, C.; Schwarz, M.; Essl, F. Major emerging alien plants in Austrian crop fields. Weed Res. 2017, 57, 406–416. [Google Scholar] [CrossRef]
- EPPO. EPPO Observation List of Invasive. 2019; Alien Plants. Available online: http://www.eppo.int/INVASIVE_PLANTS/ias_lists.htm#A1A2Lists (accessed on 18 January 2021).
- Fuhrer, J. Agroecosystem responses to combinations of elevated CO2, ozone and global climate change. Agric. Ecosyst. Environ. 2003, 97, 1–20. [Google Scholar] [CrossRef]
- Diez, J.M.; D’Antonio, C.M.; Dukes, J.D.; Grosholz, E.D.; Olden, J.D.; Sorte, C.J.B.; Blumenthal, D.M.; Bradley, B.A.; Early, R.; Ibáñez, I.; et al. Will extreme climatic effects facilitate biological invasions? Frontiers 2012, 10, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Partosfalvi, P.; Madarász, J.; Dancza, I. Occurrence of Eriochloa villosa (Thunb.) Kunth in Hungary. Növényvédelem 2008, 44, 297–304. [Google Scholar]
- Simard, M.J.; Nurse, R.E.; Darbyshire, S.J. Emergence pattern and seed production of a Canadian woolly cupgrass (Erichloa villosa) population in legume forage. Can. J. Plant Sci. 2015, 95, 539–548. [Google Scholar] [CrossRef] [Green Version]
- Oerke, E.C. Crop losses to pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Owen, M.D.K. Woolly Cupgrass Biology and Management. Proc. Crop Prod. Conf. Iowa State. Univ., Ames, IA. 2. 1990; pp. 61–72. Available online: https://core.ac.uk/download/pdf/212818331.pdf (accessed on 18 January 2021).
- Bello, I.A.; Hatterman-Valenti, H.; Owen, M.D.K. Factors affecting germination and seed production of Eriochloa villosa. Weed Sci. 2000, 48, 749–754. Available online: https://shibbolethsp.jstor.org/start?entityID=https%3A%2F%2Fidp.unideb.hu%2Fsimplesaml%2Fsaml2%2Fidp%2Fmetadata.php&dest=https://www.jstor.org/stable/23433050&site=jstor (accessed on 20 January 2021). [CrossRef]
- Allison, K.; Darbyshire, S. Identification of Eriochloa villosa. Seed Technol. 2001, 23, 169–172. Available online: https://shibbolethsp.jstor.org/start?entityID=https%3A%2F%2Fidp.unideb.hu%2Fsimplesaml%2Fsaml2%2Fidp%2Fmetadata.php&dest=https://www.jstor.org/stable/23433050&site=jstor (accessed on 21 January 2021).
- Darbyshire, S.J.; Wilson, C.E.; Allison, K. The Biology of invasive alien plants in Canada. 1. Eriochloa villosa (Thunb.) Kunth. Can. J. Plant Sci. 2003, 83, 987–999. [Google Scholar] [CrossRef]
- Follak, S.; Schwarz, M.; Essl, F. First record of Eriochloa villosa (Thunb.) Kunth in Austria and notes on its distribution and agricultural impact in Central Europe. BioInvasions Rec. 2020, 9, 8–16. [Google Scholar] [CrossRef]
- Khan, M.; Haq, N. Weed control in maize (Zea mays L.) with pre and post-emergence herbicides. Pak. J. Weed Sci. Res. 2004, 10, 39–46. [Google Scholar]
- Rice, E.L. Allelopathy; Academy Press: New York, NY, USA, 1984; p. 422. [Google Scholar]
- Huang, C.Z.; Xu, L.; Sun, J.J.; Zhang, Z.H.; Fu, M.L.; Teng, H.Y.; Yi, K.K. Allelochemical p-hydroxybenzoic acid inhibits root growth via regulating ROS accumulation in cucumber (Cucumis sativus L.). J. Integr. Agric. 2020, 19, 518–527. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defence mechanisms in plants under stressful conditions. J. Bot. 2012, 217, 1–26. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.K.; Zhu, D.F.; Zhang, Y.P.; Chen, H.Z.; Xiang, J.; Lin, X.Q. Low pH-induced changes of antioxidant enzyme and ATPase activities in the roots of rice (Oryza sativa L.) seedlings. PLoS ONE 2015, 10, e0116971. [Google Scholar] [CrossRef] [Green Version]
- Liu, E.U.; Liu, C.P. Effects of simulated acid rain on the antioxidative system in Cinnamomum philippinense seedlings. Water Air Soil Pollut. 2011, 215, 127–135. [Google Scholar] [CrossRef]
- Chen, J.; Wang, W.H.; Liu, T.W.; Wu, F.H.; Zheng, H.L. Photosynthetic and antioxidant responses of Liquidambar formosana and Schima superba seedlings to sulfuric-rich and nitric-rich simulated acid rain. Plant Physiol. Biochem. 2013, 64, 41–51. [Google Scholar] [CrossRef] [PubMed]
- Nimisha Amist, N.; Li, Z.R.; Bai, L.Y. Allelopathy in sustainable weeds management. Allelopath. J. 2009, 2, 109–138. [Google Scholar] [CrossRef]
- Farhoudi, R.; Lee, D.J. Allelopathic effects of barley extract (Hordeum vulgare) on sucrose synthase activity, lipid peroxidation and antioxidant enzymatic activities of Hordeum spontoneum and Avena ludoviciana. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2013, 83, 447–452. [Google Scholar] [CrossRef]
- Farooq, M.; Hussain, T.; Cheema, A.W.Z.A. Differential response of maize and mung bean to tobacco allelopathy. Exp. Agric. 2014, 50, 611–624. [Google Scholar] [CrossRef]
- Karkanis, A.; Alexiou, A.; Katsaros, C.; Petropoulos, S. Allelopathic activity of spearmint (Mentha spicata L.) and peppermint (Mentha × piperita L.) reduces yield, growth, and photosynthetic rate in a succeeding crop of maize (Zea mays L.). Agronomy 2019, 9, 461. [Google Scholar] [CrossRef] [Green Version]
- Oyerinde, R.O.; Otusanya, O.O.; Akpor, O.B. Allelopathic effect of Tithonia diversifolia on the germination, growth and chlorophyll contents of maize (Zea mays L.). Sci. Res. Essay 2009, 4, 1553–1558. Available online: https://academicjournals.org/journal/SRE/article-full-text-pdf/C029F6B20170 (accessed on 22 January 2021).
- Abdul-Raoof, K.M.A.; Siddiqui, M.B. Evaluation of allelopathic impact of aqueous extract of root and aerial root of Tinospora cordifolia (Willd.) Miers on some weed plants. An. Univ. Oradea Fasc. Biol. 2012, 19, 29–34. [Google Scholar]
- Elisante, F.; Tarimo, M.T.; Ndakidemi, P.A. Allelopathic effect of seed and leaf aqueous extracts of Datura stramonium on leaf chlorophyll content, shoot and root elongation of Cenchrus ciliaris and Neonotonia wightii. Am. J. Plant Sci. 2013, 4, 2332–2339. [Google Scholar] [CrossRef] [Green Version]
- Kabir, A.K.M.S.; Karim, S.M.R.; Begum, M.; Juraimi, A.S. Allelopathic potential of rice varieties against spinach (Spinacia oleracea). Int. J. Agric. Biol. 2010, 12, 809–815. [Google Scholar]
- Motamedi, M.; Karimmojeni, H.; Sini, F.G. Allelopathic effect of Carthamus tinctorius on weeds and crops. Planta Daninha 2020, 38, 1–8. [Google Scholar] [CrossRef]
- Nádasy, E.; Pásztor, G.; Béres, I.; Szilágyi, G. Allelopathic effects of Abutilon theophrasti, Asclepias syriaca and Panicum ruderale on maize. In Proceedings of the German Conference on Weed Biology and Weed Control, Braunschweig, Germany, 27 February–1 March 2018; Volume 458, pp. 453–457. [Google Scholar]
- Zohaib, A.; Anjum, S.A.; Jabbar, A.; Tabassum, T.; Abbas, T.; Nazir, U. Allelopathic effect of leguminous weeds on rate, synchronization and time of germination, and biomass partitionong in rice. Planta Daninha 2017, 35, e017160380. [Google Scholar] [CrossRef]
- Rehmatullah, N.; Khan, A.R.; Khan, M.J.; Anwar, M.; Gull, P.; Fatima, M.; Shah, W.A. Allelopathic effect of different concentration of leave extract of Lawsonia inermis L., seed germination of Steria italica, Pennisetum americanum and Lectuca sativa. Int. J. Pharmacogn. Phytochem. Res. 2016, 8, 1278–1285. Available online: http://impactfactor.org/PDF/IJPPR/8/IJPPR,Vol8,Issue8,Article5.pdf (accessed on 22 January 2021).
- Ullah, N.; Haq, I.U.; Safdar, N.; Mirza, B. Physiological and biochemical mechanisms of allelopathy mediated by the allelochemical extracts of Phytolacca latbenia (Moq.) H. Walter. Toxicol. Ind. Health 2015, 31, 931–937. [Google Scholar] [CrossRef]
- Ma, H.; Chen, Y.; Chen, J.; Zhang, Y.; Zhang, T.; He, H. Comparison of allelopathic efects of two typical invasive plants: Mikania micrantha and Ipomoea cairica in Hainan island. Sci. Rep. Nat. Res. 2020, 10, 11332. [Google Scholar] [CrossRef]
- Hatata, M.M.; Salama, M.; El-Darier, S.M. Allelopathic effect and oxidative stress induced by aqueous extract of Achillea santolina L. shoot on Triticum aestivum L. plant. Egypt. J. Exp. Biol. 2009, 5, 131–141. [Google Scholar]
- Gindri, D.M.; Coelho, C.M.M.; Uarrota, V.G. Physiological and biochemical effects of Lantana camara L. allelochemicals on the seed germination of Avena sativa L. Pesqui. Agropecuária Trop. 2020, 50, 1–11. [Google Scholar] [CrossRef]
- Ding, H.; Imran, A.; Ali, A.; Abdul-rehman, K.; Cheng, Z. Bioassay evaluation of the potential allelopathic effects of garlic (Allium sativum L.) root exudates on lettuce and cucumber. Pak. J. Bot. 2018, 50, 371–380. [Google Scholar]
- Kapoor, D.; Rinzim; Tiwari, A.; Sehgal, A.; Landi, M.; Brestic, M.; Sharma, A. Exploiting the allelopathic potential of aqueous leaf extracts of Artemisia absinthium and Psidium guajava against Parthenium hysterophorus, a widespread weed in India. Plants 2019, 8, 552. [Google Scholar] [CrossRef] [Green Version]
- El-Shora, H.M.; El-Gawad, A.M.A. Evaluation of Allelopathic Potential of Rumex dentatus Root Extract and Allelochemicals on Cicer Arietinum. J. Stress Physiol. Biochem. 2014, 10, 167–180. Available online: http://www.jspb.ru/issues/2014/N1/JSPB_2014_1_167-180.pdf (accessed on 22 January 2021).
- Madany, M.M.Y.; Saleh, A.M. Phytotoxicity of Euphorbia helioscopia L. on Triticum aestivum L. and Pisum sativum L. Ann. Agric. Sci. 2015, 60, 141–151. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Saglam, A.; Saruhan, N.; Terzi, R.; Kadioglu, A. The relations between antioxidant enzymes and chlorophyll fluorescence parameters in common bean cultivars differing in sensitivity to drought stress. Russ. J. Plant Physiol. 2011, 58, 60–68. [Google Scholar] [CrossRef]
- Abu-Romman, S. Differential allelopathic expression of different plant parts of Achillea Biebersteinii. Acta Biol. Hung. 2016, 67, 159–168. [Google Scholar] [CrossRef] [Green Version]
- Bouhaouel, I.; Gfeller, A.; Boudabbous, K.; Fauconnier, M.L.; Amara, H.S.; du Jardin, P. Physiological and biochemical parameters: New tools to screen barley root exudate allelopathic potential (Hordeum vulgare L. subsp. vulgare). Acta Physiol. Plant. 2018, 40, 38. [Google Scholar] [CrossRef]
- Moran, R.; Porath, D. Chlorophyll Determination in intact tissues using N,N-dimethylformamide. Plant Physiol. 1980, 65, 478–479. [Google Scholar] [CrossRef] [Green Version]
- Wellburn, R.A. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvent with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Pukacka, S.; Ratajczak, E. Production and scavenging of reactive oxygen species in Fagus sylvatica seeds during storage at varied temperature and humidity. J. Plant Physiol. 2005, 162, 873–885. [Google Scholar] [CrossRef] [PubMed]
- Mishra, N.P.; Mishra, R.K.; Singhal, G.S. Changes in the activities of anti-oxidant enzymes during exposure of intact wheat leaves to strong visible light at different temperatures in the presence of protein synthesis inhibitors. Plant Physiol. 1993, 102, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zeislin, N.; Ben-Zaken, R. Peroxidases, phenylalanine ammonia-lyase and lignification in peduncles of rose flowers. Plant Physiol. Biochem. 1991, 29, 147–151. [Google Scholar]
- Giannopolities, C.H.; Ries, S.K. Superoxide dismutase I. Occurrence in higher plant. Plant Physiol. 1977, 59, 309–314. Available online: https://shibbolethsp.jstor.org/start?entityID=https%3A%2F%2Fidp.unideb.hu%2Fsimplesaml%2Fsaml2%2Fidp%2Fmetadata.php&dest=https://www.jstor.org/stable/4264724&site=jstor (accessed on 22 January 2021). [CrossRef]
- Beyer, W.F.; Fridovich, I. Assaying for superoxide dismutase activity: Some large consequences of minor changes in conditions. Anal. Biochem. 1987, 161, 559–566. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Kim, T.K. T test as a parametric statistic. Korean J. Anesthesiol. 2015, 68, 540–546. [Google Scholar] [CrossRef] [Green Version]
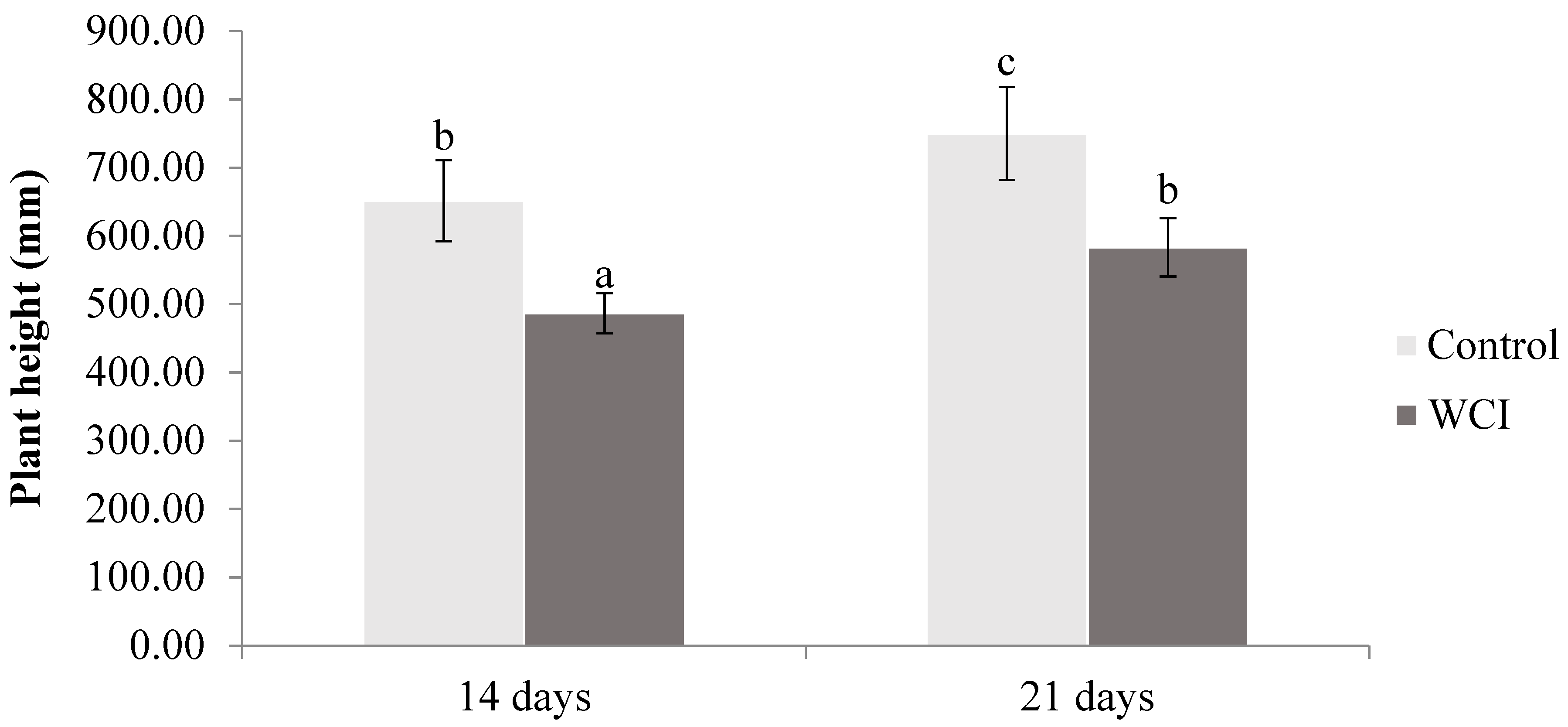

| Treatments | ||
|---|---|---|
| Root Parameters | Control | WCI |
| Total length | 757.17 ± 41.22 b | 478.49 ± 79.62 a |
| Volume | 1.54 ± 0.07 b | 0.76 ± 0.11 a |
| Diameter | 0.49 ± 0.01 a | 0.46 ± 0.03 a |
| Surface | 117.58 ± 12.05 b | 67.24 ± 13.39 a |
| <0.2 mm | 382.67 ± 30.58 b | 283.89 ± 55.53 a |
| 0.2–0.4 mm | 118.95 ± 20.75 b | 53.77 ± 8.15 a |
| 0.4–0.6 mm | 27.91 ± 4.85 b | 11.21 ± 2.61 a |
| 0.6–0.8 mm | 61.81 ± 1.19 b | 42.19 ± 3.45 a |
| 0.8–1 mm | 84.18 ± 6.18 b | 41.10 ± 5.10 a |
| >1.0 mm | 94.79 ± 7.48 b | 46.23 ± 10.14 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szilágyi, A.; Radócz, L.; Hájos, M.T.; Juhász, C.; Kovács, B.; Kovács, G.; Budayné Bódi, E.; Radványi, C.; Moloi, M.J.; Szőke, L. The Impacts of Woolly Cupgrass on the Antioxidative System and Growth of a Maize Hybrid. Plants 2021, 10, 982. https://doi.org/10.3390/plants10050982
Szilágyi A, Radócz L, Hájos MT, Juhász C, Kovács B, Kovács G, Budayné Bódi E, Radványi C, Moloi MJ, Szőke L. The Impacts of Woolly Cupgrass on the Antioxidative System and Growth of a Maize Hybrid. Plants. 2021; 10(5):982. https://doi.org/10.3390/plants10050982
Chicago/Turabian StyleSzilágyi, Arnold, László Radócz, Mária Takácsné Hájos, Csaba Juhász, Béla Kovács, Gabriella Kovács, Erika Budayné Bódi, Csaba Radványi, Makoena Joyce Moloi, and Lóránt Szőke. 2021. "The Impacts of Woolly Cupgrass on the Antioxidative System and Growth of a Maize Hybrid" Plants 10, no. 5: 982. https://doi.org/10.3390/plants10050982








