Dehydroabietic Acid Is a Novel Survivin Inhibitor for Gastric Cancer
Abstract
:1. Introduction
2. Results
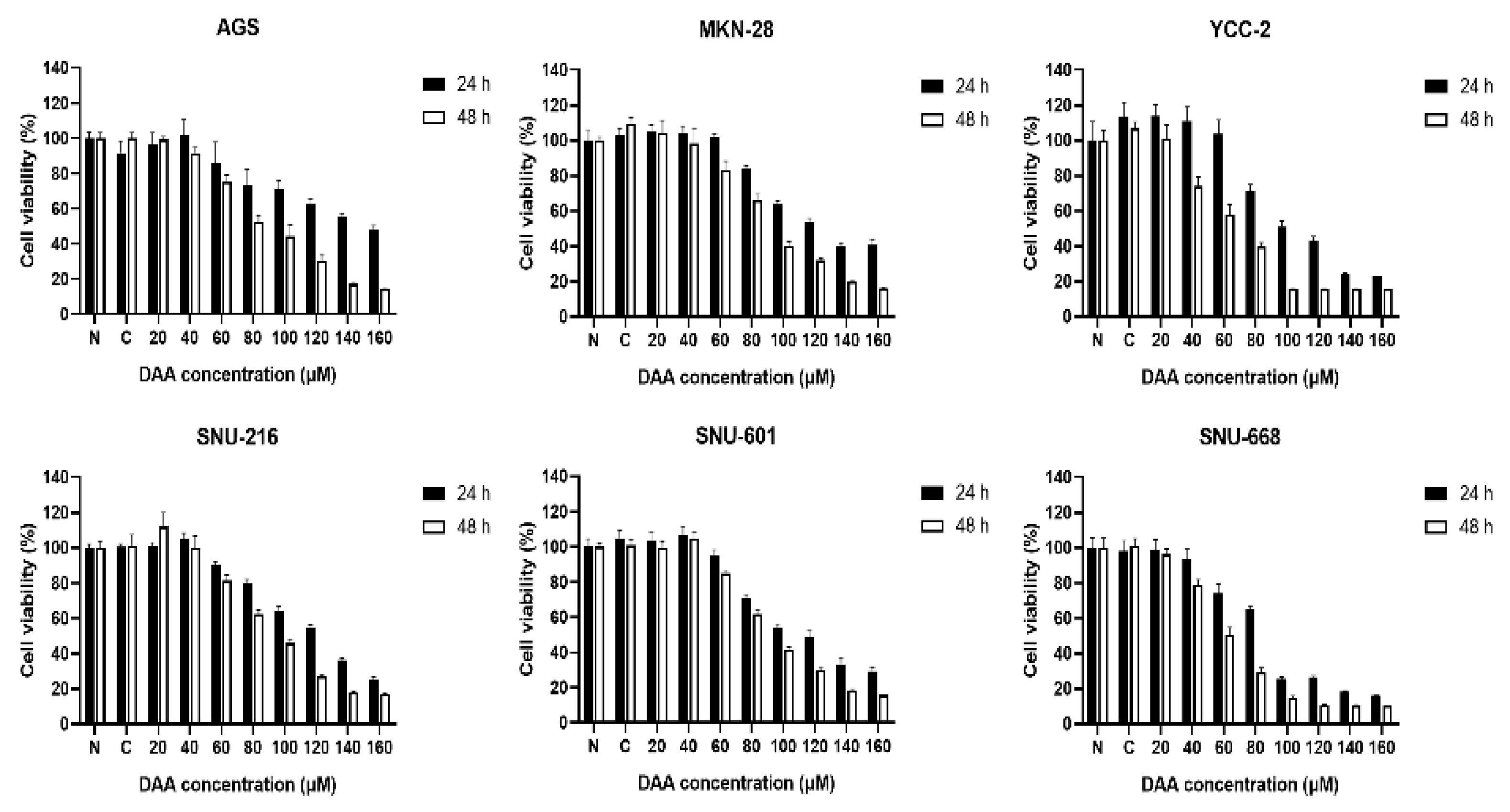
2.1. DAA Significantly Reduced the Growth Rate of Gastric Cancer Cells
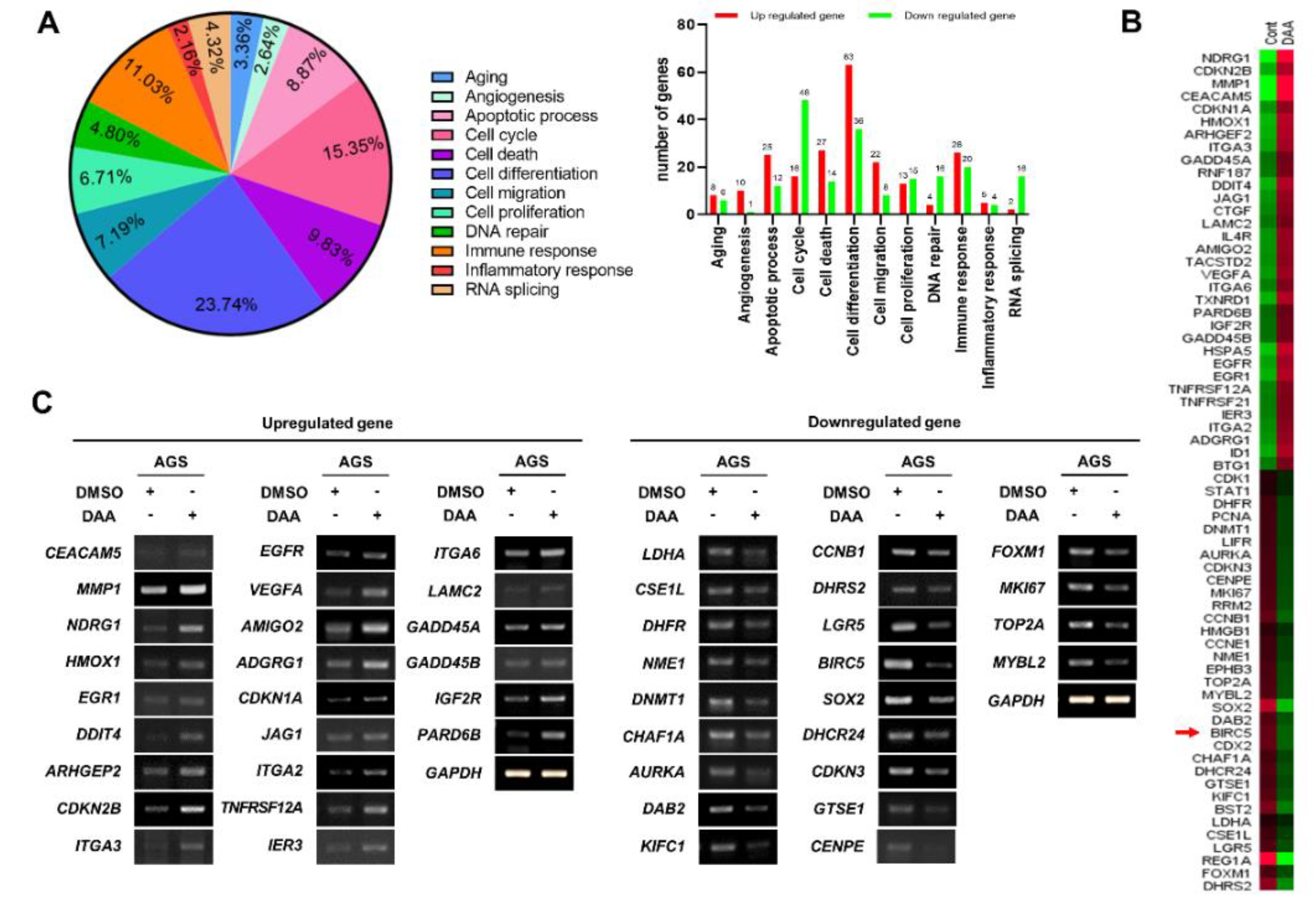
2.2. DAA Treatment in AGS Cells Modulated Gene Expression Related to Cell Proliferation
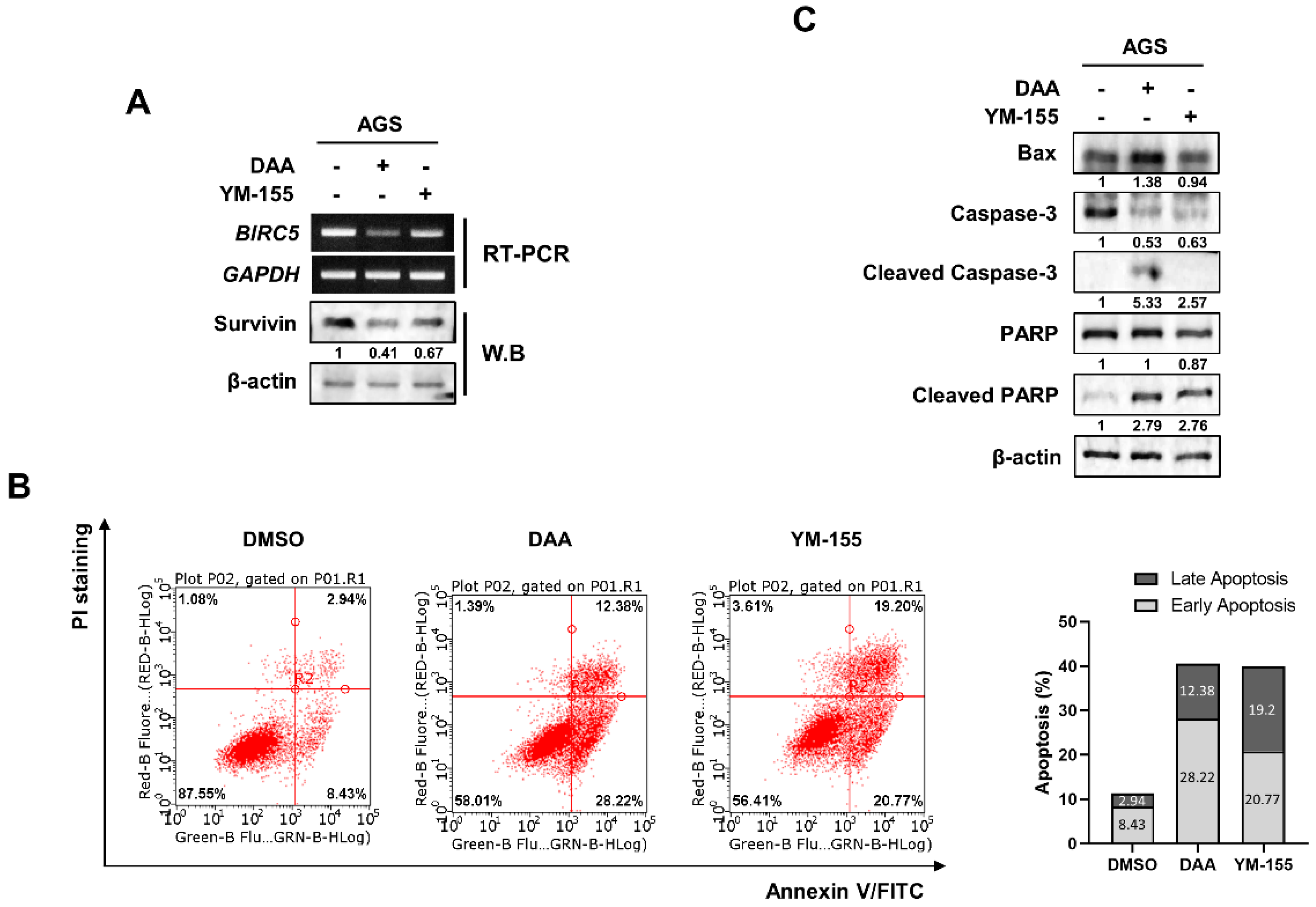
2.3. DAA Treatment Induced Downregulation of mRNA and Protein Survivin Expression
2.4. DAA Exhibited a Better Inhibitory Effect on Survivin Than YM-155
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Cell Culture
4.3. Cell Proliferation Assay
4.4. Library Preparation and RNA Sequencing
4.5. Construct of Survivin Overexpression Plasmids
4.6. Annexin V-FITC/PI Apoptosis Assay
4.7. RNA Isolation, cDNA Synthesis, and Polymerase Chain Reaction (PCR)
4.8. Western Blotting
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orditura, M.; Galizia, G.; Sforza, V.; Gambardella, V.; Fabozzi, A.; Laterza, M.M.; Andreozzi, F.; Ventriglia, J.; Savastano, B.; Mabilia, A.; et al. Treatment of gastric cancer. World J. Gastroenterol. 2014, 20, 1635–1649. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Wu, Y.; Yang, J.; Yang, D.; Fang, X. Progress in the treatment of advanced gastric cancer. Tumour Biol. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Crisci, S.; Amitrano, F.; Saggese, M.; Muto, T.; Sarno, S.; Mele, S.; Vitale, P.; Ronga, G.; Berretta, M.; Di Francia, R. Overview of Current Targeted Anti-Cancer Drugs for Therapy in Onco-Hematology. Medicina 2019, 55, 414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerbe, P.; Bohlmann, J. Plant diterpene synthases: Exploring modularity and metabolic diversity for bioengineering. Trends Biotechnol. 2015, 33, 419–428. [Google Scholar] [CrossRef] [PubMed]
- de Sousa, I.P.; Sousa Teixeira, M.V.; Jacometti Cardoso Furtado, N.A. An Overview of Biotransformation and Toxicity of Diterpenes. Molecules 2018, 23, 1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, W.J.; Kang, H.G.; Kim, S.J. Dehydroabietic acid inhibits the gastric cancer cell growth via induced apoptosis and cell cycle arrest. Mol. Cell Toxicol. 2021, 17, 133–139. [Google Scholar] [CrossRef]
- Golstein, P.; Kroemer, G. Cell death by necrosis: Towards a molecular definition. Trends Biochem. Sci. 2007, 32, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Kolb, J.P.; Oguin, T.H., 3rd; Oberst, A.; Martinez, J. Programmed Cell Death and Inflammation: Winter Is Coming. Trends Immunol. 2017, 38, 705–718. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Szondy, Z.; Sarang, Z.; Kiss, B.; Garabuczi, E.; Koroskenyi, K. Anti-inflammatory Mechanisms Triggered by Apoptotic Cells during Their Clearance. Front. Immunol. 2017, 8, 909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, H.; Suri, P.; Gupta, J.C.; Talwar, G.P.; Dubey, S. Survivin: A unique target for tumor therapy. Cancer Cell Int. 2016, 16, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mita, A.C.; Mita, M.M.; Nawrocki, S.T.; Giles, F.J. Survivin: Key regulator of mitosis and apoptosis and novel target for cancer therapeutics. Clin. Cancer Res. 2008, 14, 5000–5005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hensley, P.; Mishra, M.; Kyprianou, N. Targeting caspases in cancer therapeutics. Biol. Chem. 2013, 394, 831–843. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhu, Z.; Sun, Z.; Sun, X.; Wang, Z.; Xu, H. Survivin gene expression increases gastric cancer cell lymphatic metastasis by upregulating vascular endothelial growth factor-C expression levels. Mol. Med. Rep. 2014, 9, 600–606. [Google Scholar] [CrossRef] [Green Version]
- Li, F.Z.; Aljahdali, I.; Ling, X. Cancer therapeutics using survivin BIRC5 as a target: What can we do after over two decades of study? J. Exp. Clin. Cancer Res. 2019, 38. [Google Scholar] [CrossRef] [Green Version]
- Nie, S.; Yang, G.; Lu, H. Current Molecular Targeted Agents for Advanced Gastric Cancer. Oncol. Targets Ther. 2020, 13, 4075–4088. [Google Scholar] [CrossRef]
- Mirzaei, S.A.; Dinmohammadi, F.; Alizadeh, A.; Elahian, F. Inflammatory pathway interactions and cancer multidrug resistance regulation. Life Sci. 2019, 235, 116825. [Google Scholar] [CrossRef]
- Yang, Y.L.; Li, X.M. The IAP family: Endogenous caspase inhibitors with multiple biological activities. Cell Res. 2000, 10, 169–177. [Google Scholar] [CrossRef]
- Duffy, M.J.; O’Donovan, N.; Brennan, D.J.; Gallagher, W.M.; Ryan, B.M. Survivin: A promising tumor biomarker. Cancer Lett. 2007, 249, 49–60. [Google Scholar] [CrossRef]
- Lord, C.J.; Ashworth, A. The DNA damage response and cancer therapy. Nature 2012, 481, 287–294. [Google Scholar] [CrossRef]
- Li, J.; Yuan, J. Caspases in apoptosis and beyond. Oncogene 2008, 27, 6194–6206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shlomovitz, I.; Speir, M.; Gerlic, M. Flipping the dogma—Phosphatidylserine in non-apoptotic cell death. Cell Commun. Signal. 2019, 17, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gim, J.; Won, S.; Park, T. LPEseq: Local-Pooled-Error Test for RNA Sequencing Experiments with a Small Number of Replicates. PLoS ONE 2016, 11, e0159182. [Google Scholar] [CrossRef] [PubMed]

| Gene Symbol | Accession Number | Gene Name | Fold Change | q-Value |
|---|---|---|---|---|
| Cell cycle | ||||
| CDKN2B | NM_004936 | cyclin-dependent kinase inhibitor 2B | 2.484 | 0.0190 |
| CDKN1A | NM_001220777 | cyclin-dependent kinase inhibitor 1A | 2.095 | 0.0977 |
| GADD45A | NM_001924 | growth arrest and DNA damage inducible alpha | 1.701 | 0.3246 |
| PARD6B | NM_032521 | par-6 family cell polarity regulator beta | 1.621 | 0.4235 |
| Cell proliferation | ||||
| HMOX1 | NM_002133 | heme oxygenase 1 | 2.661 | 0.0094 |
| ARHGEF2 | NM_004723 | Rho/Rac guanine nucleotide exchange factor 2 | 2.493 | 0.0184 |
| EGFR | NM_005228 | epidermal growth factor receptor | 2.278 | 0.0426 |
| VEGFA | NM_001171625 | vascular endothelial growth factor A | 2.175 | 0.0969 |
| JAG1 | NM_000214 | jagged 1 | 2.094 | 0.0871 |
| ITGA2 | NM_002203 | integrin subunit alpha 2 | 1.966 | 0.1823 |
| BTG1 | NM_001731 | B-cell translocation gene 1, anti-proliferative | 1.781 | 0.2930 |
| Apoptosis | ||||
| HSPA5 | NM_005347 | heat shock protein family A (HSP70) member 5 | 2.806 | 0.0138 |
| AMIGO2 | NM_181847 | adhesion molecule with Ig-like domain 2 | 2.116 | 0.1138 |
| IER3 | NM_003897_4 | immediate early response 3 | 1.838 | 0.2725 |
| IGF2R | NM_000876 | insulin-like growth factor 2 receptor | 1.665 | 0.4105 |
| DNA replication | ||||
| CTGF | NM_001901 | connective tissue growth factor | 1.936 | 0.1439 |
| Gene Symbol | Accession Number | Gene Name | Fold Change | q-Value |
|---|---|---|---|---|
| Cell cycle | ||||
| CDK1 | NM_001170406 | cyclin-dependent kinase 1 | 0.657 | 0.3084 |
| CCNE1 | NM_001238 | cyclin E1 | 0.559 | 0.1132 |
| AURKA | NM_198434 | aurora kinase A | 0.537 | 0.0878 |
| CDKN3 | NM_001130851 | cyclin-dependent kinase inhibitor 3 | 0.527 | 0.0754 |
| GTSE1 | NM_016426 | G2 and S-phase expressed protein 1 | 0.524 | 0.0695 |
| CENPE | NM_001813 | centromere protein E | 0.524 | 0.0696 |
| MKI67 | NM_001145966 | marker of proliferation Ki-67 | 0.512 | 0.0813 |
| KIFC1 | NM_002263 | kinesin family member C1 | 0.486 | 0.0359 |
| CCNB1 | NM_031966 | cyclin B1 | 0.451 | 0.0168 |
| Cell proliferation | ||||
| STAT1 | NM_007315 | signal transducer and activator of transcription 1 | 0.657 | 0.3363 |
| NME1 | NM_198175 | NME/NM23 nucleoside diphosphate kinase 1 | 0.559 | 0.1130 |
| LIFR | NM_001127671 | leukemia inhibitory factor receptor alpha | 0.547 | 0.0905 |
| FOXM1 | NM_001243088 | forkhead box M1 | 0.521 | 0.0652 |
| DHCR24 | NM_014762 | 24-dehydrocholesterol reductase | 0.496 | 0.0756 |
| CDX2 | NM_001265 | caudal type homeobox 2 | 0.444 | 0.0173 |
| DHRS2 | NM_005794 | dehydrogenase/reductase (SDR family) member 2 | 0.356 | 0.0023 |
| SOX2 | NM_003106 | SRY-box 2 | 0.264 | 0.0001 |
| REG1A | NM_002909 | regenerating family member 1 alpha | 0.144 | 0.0001 |
| Apoptosis | ||||
| HMGB1 | NM_002128 | high mobility group box 1 | 0.621 | 0.2869 |
| BIRC5 | NM_001168 | baculoviral IAP repeat containing 5 | 0.449 | 0.0162 |
| DNA replication | ||||
| PCNA | NM_182649 | proliferating cell nuclear antigen | 0.55 | 0.0937 |
| CHAF1A | NM_005483 | chromatin assembly factor 1 subunit A | 0.538 | 0.0852 |
| TOP2A | NM_001067 | topoisomerase (DNA) II alpha | 0.511 | 0.0885 |
| RRM2 | NM_001165931 | ribonucleotide reductase regulatory subunit M2 | 0.507 | 0.0482 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, W.-J.; Kim, W.; Bae, J.-M.; Gim, J.; Kim, S.-J. Dehydroabietic Acid Is a Novel Survivin Inhibitor for Gastric Cancer. Plants 2021, 10, 1047. https://doi.org/10.3390/plants10061047
Kim W-J, Kim W, Bae J-M, Gim J, Kim S-J. Dehydroabietic Acid Is a Novel Survivin Inhibitor for Gastric Cancer. Plants. 2021; 10(6):1047. https://doi.org/10.3390/plants10061047
Chicago/Turabian StyleKim, Won-Jin, Woong Kim, Jang-Mi Bae, Jungsoo Gim, and Seok-Jun Kim. 2021. "Dehydroabietic Acid Is a Novel Survivin Inhibitor for Gastric Cancer" Plants 10, no. 6: 1047. https://doi.org/10.3390/plants10061047
APA StyleKim, W. -J., Kim, W., Bae, J. -M., Gim, J., & Kim, S. -J. (2021). Dehydroabietic Acid Is a Novel Survivin Inhibitor for Gastric Cancer. Plants, 10(6), 1047. https://doi.org/10.3390/plants10061047







