The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance
Abstract
:1. Introduction
2. Defense Response of GLS to Fungal Diseases
3. Defense Responses of GLS to Bacterial Diseases
4. Defense Response of GLS to Pests
5. Defense Response of GLS to Insects and Aphids
6. Conclusions and Future Research Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Ahmad, M.; Ali, Q.; Hafeez, M.M.; Malik, A. Improvement for Biotic and Abiotic Stress Tolerance in Crop Plants. Biol. Clin. Sci. Res. J. 2021, 2021, e004. [Google Scholar]
- Kliebenstein, D.J.; Osbourn, A. Making New Molecules-evolution of Pathways for Novel Metabolites in Plants. Curr. Opin. Plant Biol. 2012, 15, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Rejeb, I.B.; Pastor, V.; Mauch-Mani, B. Plant Responses to Simultaneous Biotic and Abiotic Stress: Molecular Mechanisms. Plants 2014, 3, 458–475. [Google Scholar] [CrossRef] [PubMed]
- Pastorczyk, M.; Bednarek, P. The Function of Glucosinolates and Related Metabolites in Plant Innate Immunity. Adv. Bot. Res. 2016, 80, 171–198. [Google Scholar]
- Moller, B.L. Functional Diversifications of Cyanogenic Glucosides. Curr. Opin. Plant Biol. 2010, 13, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Baldwin, I.T. New Insights into Plant Responses to the Attack from Insect Herbivores. Annu. Rev. Genet. 2010, 44, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Bouranis, D.L.; Malagoli, M.; Avice, J.C.; Bloem, E. Advances in Plant Sulfur Research. Plants 2020, 9, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, A.; Abreu, A.C.; Ferreira, C.; Saavedra, M.J.; Simoes, L.C.; Simoes, M. Antibacterial Activity and Mode of Action of Selected Glucosinolate Hydrolysis Products Against Bacterial Pathogens. J. Food Sci. Technol. 2015, 52, 4737–4748. [Google Scholar] [CrossRef] [Green Version]
- Jeandroz, S.; Lamotte, O. Editorial: Plant Responses to Biotic and Abiotic Stresses: Lessons from Cell Signaling. Front. Plant Sci. 2017, 8, 1772. [Google Scholar] [CrossRef] [Green Version]
- Gupta, A.; Hisano, H.; Hojo, Y.; Matsuura, T.; Ikeda, Y.; Mori, I.C.; Senthil-Kumar, M. Global Profiling of Phytohormone Dynamics during Combined Drought and Pathogen Stress in Arabidopsis Thaliana Reveals ABA and JA as Major Regulators. Sci. Rep. 2017, 7, 4017. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, S.T.; Coaker, G.; Day, B.; Staskawicz, B.J. Host-microbe Interactions: Shaping the Evolution of the Plant Immune Response. Cell 2006, 124, 803–814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beran, F.; Kollner, T.G.; Gershenzon, J.; Tholl, D. Chemical Convergence between Plants and Insects: Biosynthetic Origins and Functions of Common Secondary Metabolites. New Phytol. 2019, 223, 52–67. [Google Scholar] [CrossRef] [Green Version]
- Ishida, M.; Hara, M.; Fukino, N.; Kakizaki, T.; Morimitsu, Y. Glucosinolate Metabolism, Functionality and Breeding for the Improvement of Brassicaceae Vegetables. Breed Sci. 2014, 64, 48–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blazevic, I.; Montaut, S.; Burcul, F.; Olsen, C.E.; Burow, M.; Rollin, P.; Agerbirk, N. Glucosinolate Structural Diversity, Identification, Chemical Synthesis and Metabolism in Plants. Phytochemistry 2020, 169, 112100. [Google Scholar] [CrossRef] [PubMed]
- Fahey, J.W.; Zalcmann, A.T.; Talalay, P. The Chemical Diversity and Distribution of Glucosinolates and Isothiocyanates among Plants. Phytochemistry 2001, 56, 5–51. [Google Scholar] [CrossRef]
- Halkier, B.A.; Gershenzon, J. Biology and Biochemistry of Glucosinolates. Annu. Rev. Plant Biol. 2006, 57, 303–333. [Google Scholar] [CrossRef] [Green Version]
- Clarke, D.B. Glucosinolates, Structures and Analysis in Food. Anal. Methods 2010, 2, 4. [Google Scholar] [CrossRef]
- Agerbirk, N.; Olsen, C.E. Glucosinolate Structures in Evolution. Phytochemistry 2012, 77, 16–45. [Google Scholar] [CrossRef] [PubMed]
- Sonderby, I.E.; Geu-Flores, F.; Halkier, B.A. Biosynthesis of Glucosinolates—Gene Discovery and Beyond. Trends Plant Sci. 2010, 15, 283–290. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Li, L.; Fang, Z.; Yang, L.; Zhuang, M.; Zhang, Y.; Lv, H. Transcriptome Reveals the Gene Expression Patterns of Sulforaphane Metabolism in Broccoli Florets. PLoS ONE 2019, 14, e0213902. [Google Scholar] [CrossRef]
- Andini, S.; Dekker, P.; Gruppen, H.; Araya-Cloutier, C.; Vincken, J.P. Modulation of Glucosinolate Composition in Brassicaceae Seeds by Germination and Fungal Elicitation. J. Agric. Food Chem. 2019, 67, 12770–12779. [Google Scholar] [CrossRef] [PubMed]
- Frerigmann, H.; Bottcher, C.; Baatout, D.; Gigolashvili, T. Glucosinolates are Produced in Trichomes of Arabidopsis Thaliana. Front. Plant Sci. 2012, 3, 242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, I.M.; Park, B.; Dang, Y.M.; Kim, S.Y.; Seo, H.Y. Simultaneous Direct Determination of 15 Glucosinolates in Eight Brassica Species by UHPLC-Q-Orbitrap-MS. Food Chem. 2019, 282, 127–133. [Google Scholar] [CrossRef]
- Bekaert, M.; Edger, P.P.; Hudson, C.M.; Pires, J.C.; Conant, G.C. Metabolic and Evolutionary Costs of Herbivory Defense: Systems Biology of Glucosinolate Synthesis. New Phytol. 2012, 196, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Kliebenstein, D.J.; Kroymann, J.; Mitchell-Olds, T. The Glucosinolate-myrosinase System in an Ecological and Evolutionary Context. Curr. Opin. Plant Biol. 2005, 8, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, R.J.; van Dam, N.M.; van Loon, J.J. Role of Glucosinolates in Insect-plant Relationships and Multitrophic Interactions. Annu. Rev. Entomol. 2009, 54, 57–83. [Google Scholar] [CrossRef]
- Barth, C.; Jander, G. Arabidopsis Myrosinases TGG1 and TGG2 have Redundant Function in Glucosinolate Breakdown and Insect Defense. Plant J. 2006, 46, 549–562. [Google Scholar] [CrossRef]
- Burow, M.; Halkier, B.A. How does a Plant Orchestrate Defense in Time and Space? Using Glucosinolates in Arabidopsis as Case Study. Curr. Opin. Plant Biol. 2017, 38, 142–147. [Google Scholar] [CrossRef]
- Koroleva, O.A.; Davies, A.; Deeken, R.; Thorpe, M.R.; Tomos, A.D.; Hedrich, R. Identification of a New Glucosinolate-Rich Cell Type in Arabidopsis Flower Stalk. Plant Physiol. 2000, 124, 599–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husebye, H.; Chadchawan, S.; Winge, P.; Thangstad, O.P.; Bones, A.M. Guard Cell- and Phloem Idioblast-specific Expression of Thioglucoside Glucohydrolase 1 (myrosinase) in Arabidopsis. Plant Physiol. 2002, 128, 1180–1188. [Google Scholar] [CrossRef] [Green Version]
- Thangstad, O.P.; Gilde, B.; Chadchawan, S.; Seem, M.; Bones, A.M. Cell Specific, Cross-species Expression of Myrosinases in Brassica Napus, Arabidopsis Thaliana and Nicotiana Tabacum. Plant Mol. Biol. 2004, 54, 597–611. [Google Scholar] [CrossRef] [PubMed]
- van Dam, N.M.; Tytgat, T.O.G.; Kirkegaard, J.A. Root and Shoot Glucosinolates: A Comparison of Their Diversity, Function and Interactions in Natural and Managed Ecosystems. Phytochem. Rev. 2008, 8, 171–186. [Google Scholar] [CrossRef] [Green Version]
- Bjorkman, M.; Klingen, I.; Birch, A.N.; Bones, A.M.; Bruce, T.J.; Johansen, T.J.; Meadow, R.; Molmann, J.; Seljasen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in Plant Protection and Human Health—Influences of Climate, Environment and Agronomic Practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef]
- Blažević, I.; Radonić, A.; Mastelić, J.; Zekić, M.; Skočibušić, M.; Maravić, A. Glucosinolates, Glycosidically Bound Volatiles and Antimicrobial Activity of Aurinia sinuata (Brassicaceae). Food Chem. 2010, 121, 1020–1028. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The Enzymic and Chemically Induced Decomposition of Glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Ober, J.A.; Kliebenstein, D.J. The Gene Controlling the Quantitative Trait Locus EPITHIOSPECIFIER MODIFIER1 Alters Glucosinolate Hydrolysis and Insect Resistance in Arabidopsis. Plant Cell 2006, 18, 1524–1536. [Google Scholar] [CrossRef] [Green Version]
- Burow, M.; Bergner, A.; Gershenzon, J.; Wittstock, U. Glucosinolate Hydrolysis in Lepidium sativum—Identification of the Thiocyanate-forming Protein. Plant Mol. Biol. 2007, 63, 49–61. [Google Scholar] [CrossRef] [PubMed]
- Esteve, M. Mechanisms Underlying Biological Effects of Cruciferous Glucosinolate-Derived Isothiocyanates/Indoles: A Focus on Metabolic Syndrome. Front. Nutr. 2020, 7, 111. [Google Scholar] [CrossRef]
- Bongoni, R.; Verkerk, R.; Steenbekkers, B.; Dekker, M.; Stieger, M. Evaluation of Different Cooking Conditions on Broccoli (Brassica oleracea var. italica) to Improve the Nutritional Value and Consumer Acceptance. Plant Foods Hum. Nutr. 2014, 69, 228–234. [Google Scholar] [CrossRef]
- Novotny, C.; Schulzova, V.; Krmela, A.; Hajslova, J.; Svobodova, K.; Koudela, M. Ascorbic Acid and Glucosinolate Levels in New Czech Cabbage Cultivars: Effect of Production System and Fungal Infection. Molecules 2018, 23, 8. [Google Scholar] [CrossRef] [Green Version]
- Rhee, J.H.; Choi, S.; Lee, J.E.; Hur, O.S.; Assefa, A.D. Glucosinolate Content in Brassica Genetic Resources and Their Distribution Pattern within and between Inner, Middle, and Outer Leaves. Plants 2020, 9, 1421. [Google Scholar] [CrossRef]
- Keck, A.S.; Finley, J.W. Cruciferous Vegetables: Cancer Protective Mechanisms of Glucosinolate Hydrolysis Products and Selenium. Integr. Cancer Ther. 2004, 3, 5–12. [Google Scholar] [CrossRef]
- Dinkova-Kostova, A.T.; Kostov, R.V. Glucosinolates and Isothiocyanates in Health and Disease. Trends Mol. Med. 2012, 18, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, D.; Lehmann, C.; Florian, S.; Barknowitz, G.; Haack, M.; Mewis, I.; Wiesner, M.; Schreiner, M.; Glatt, H.; Brigelius-Flohe, R.; et al. Glucosinolates from Pak Choi and Broccoli Induce Enzymes and Inhibit Inflammation and Colon Cancer Differently. Food Funct. 2014, 5, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.Y.; Fu, T.M.; Chen, H.; Ishikawa, T.; Chiang, S.Y.; Katon, J.; et al. Reactivation of PTEN Tumor Suppressor for Cancer Treatment through Inhibition of a MYC-WWP1 Inhibitory Pathway. Science 2019, 364, 6441. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Mao, Q.; Cao, M.; Xie, L. Cruciferous Vegetables Intake and Risk of Prostate Cancer: A Meta-analysis. Int. J. Urol. 2012, 19, 134–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Q.J.; Yang, Y.; Vogtmann, E.; Wang, J.; Han, L.H.; Li, H.L.; Xiang, Y.B. Cruciferous Vegetables Intake and the Risk of Colorectal Cancer: A Meta-analysis of Observational Studies. Ann. Oncol. 2013, 24, 1079–1087. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lv, K. Cruciferous Vegetables Intake is Inversely Associated with Risk of Breast Cancer: A Meta-analysis. Breast 2013, 22, 309–313. [Google Scholar] [CrossRef]
- Lucarini, E.; Micheli, L.; Trallori, E.; Citi, V.; Martelli, A.; Testai, L.; de Nicola, G.R.; Iori, R.; Calderone, V.; Ghelardini, C.; et al. Effect of Glucoraphanin and Sulforaphane against Chemotherapy-induced Neuropathic Pain: Kv7 Potassium Channels Modulation by H2S Release In Vivo. Phytother. Res. 2018, 32, 2226–2234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbaoui, B.; Lucas, C.R.; Riedl, K.M.; Clinton, S.K.; Mortazavi, A. Cruciferous Vegetables, Isothiocyanates, and Bladder Cancer Prevention. Mol. Nutr. Food Res. 2018, 62, e1800079. [Google Scholar] [CrossRef] [PubMed]
- Kellingray, L.; Le Gall, G.; Doleman, J.F.; Narbad, A.; Mithen, R.F. Effects of In Vitro Metabolism of a Broccoli Leachate, Glucosinolates and S-methylcysteine Sulphoxide on the Human Faecal Microbiome. Eur. J. Nutr. 2020, 1–14. [Google Scholar]
- Maina, S.; Misinzo, G.; Bakari, G.; Kim, H.Y. Human, Animal and Plant Health Benefits of Glucosinolates and Strategies for Enhanced Bioactivity: A Systematic Review. Molecules 2020, 25, 16. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Talalay, P. Persuasive Evidence that Quinone Reductase Type 1 (DT diaphorase) Protects Cells against the Toxicity of Electrophiles and Reactive Forms of Oxygen. Free Radic. Biol. Med. 2000, 29, 231–240. [Google Scholar] [CrossRef]
- Rask, L.; Andréasson, E.; Ekbom, B.; Eriksson, S.; Bo, P.; Meijer, J. Myrosinase: Gene Family Evolution and Herbivore Defense in Brassicaceae. Plant Mol. Biol. 2000, 42, 93. [Google Scholar] [CrossRef]
- Kos, M.; Houshyani, B.; Wietsma, R.; Kabouw, P.; Vet, L.; Loon, J.; Dicke, M. Effects of Glucosinolates on a Generalist and Specialist Leaf-chewing Herbivore and an Associated Parasitoid. Phytochemistry 2012, 77, 162–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tongjin, L.; Xiaohui, Z.; Haohui, Y.; Niels, A.; Yang, Q. Aromatic Glucosinolate Biosynthesis Pathway in Barbarea Vulgaris and Its Response to Plutella Xylostella Infestation. Front. Plant Sci. 2016, 7, 83. [Google Scholar]
- Dubuis, P.-H.; Marazzi, C.; Städler, E.; Mauch, F. Sulphur Deficiency Causes a Reduction in Antimicrobial Potential and Leads to Increased Disease Susceptibility of Oilseed Rape. J. Phytopathol. 2005, 153, 27–36. [Google Scholar] [CrossRef] [Green Version]
- Rausch, T.; Wachter, A. Sulfur Metabolism: A Versatile Platform for Launching Defence Operations. Trends Plant Sci. 2005, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Mocniak, L.E.; Elkin, K.; Bollinger, J.M., Jr. Lifetimes of the Aglycone Substrates of Specifier Proteins, the Autonomous Iron Enzymes That Dictate the Products of the Glucosinolate-Myrosinase Defense System in Brassica Plants. Biochemistry 2020, 59, 2432–2441. [Google Scholar] [CrossRef]
- Santamaria, M.E.; Garcia, A.; Arnaiz, A.; Rosa-Diaz, I.; Romero-Hernandez, G.; Diaz, I.; Martinez, M. Comparative Transcriptomics Reveals Hidden Issues in the Plant Response to Arthropod Herbivores. J. Integr. Plant Biol. 2021, 63, 312–326. [Google Scholar] [CrossRef]
- Calmes, B.; N’Guyen, G.; Dumur, J.; Brisach, C.A.; Campion, C.; Iacomi, B.; Pigné, S.; Dias, E.; Macherel, D.; Guillemette, T.; et al. Glucosinolate-derived Isothiocyanates Impact Mitochondrial Function in Fungal Cells and Elicit an Oxidative Stress Response Necessary for Growth Recovery. Front. Plant Sci. 2015, 6, 414. [Google Scholar] [CrossRef] [Green Version]
- Pereira, F.; Rosa, E.; Fahey, J.W.; Stephenson, K.K.; Carvalho, R.; Aires, A. Influence of Temperature and Ontogeny on the Levels of Glucosinolates in Broccoli (Brassica oleracea Var. italica) Sprouts and Their Effect on the Induction of Mammalian Phase 2 Enzymes. J. Agric. Food Chem. 2002, 50, 6239–6244. [Google Scholar] [CrossRef]
- LoPez-Berenguer, C.; MartiNez-Ballesta, M.D.; Moreno, D.; Carvajal, M.; Garcia-Viguera, C. Growing Hardier Crops for Better Health: Salinity Tolerance and the Nutritional Value of Broccoli. J. Agric. Food Chem. 2009, 57, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Wang, X.; Guo, R.; Wang, Q. Effect of Salt Stress on Phenolic Compounds, Glucosinolates, Myrosinase and Antioxidant Activity in Radish Sprouts. Food Chem. 2010, 121, 1014–1019. [Google Scholar] [CrossRef]
- Safavi Fard, N.; Heidari Sharif Abad, H.; Shirani Rad, A.H.; Majidi Heravan, E.; Daneshian, J. Effect of Drought Stress on Qualitative Characteristics of Canola Cultivars in Winter Cultivation. Ind. Crop. Prod. 2018, 114, 87–92. [Google Scholar] [CrossRef]
- Eom, S.H.; Baek, S.A.; Kim, J.K.; Hyun, T.K. Transcriptome Analysis in Chinese Cabbage (Brassica rapa ssp. pekinensis) Provides the Role of Glucosinolate Metabolism in Response to Drought Stress. Molecules 2018, 23, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huseby, S.; Koprivova, A.; Lee, B.R.; Saha, S.; Mithen, R.; Wold, A.B.; Bengtsson, G.B.; Kopriva, S. Diurnal and Light Regulation of Sulphur Assimilation and Glucosinolate Biosynthesis in Arabidopsis. J. Exp. Bot. 2013, 64, 1039–1048. [Google Scholar] [CrossRef] [Green Version]
- del Carmen Martinez-Ballesta, M.; Moreno, D.A.; Carvajal, M. The Physiological Importance of Glucosinolates on Plant Response to Abiotic Stress in Brassica. Int. J. Mol. Sci. 2013, 14, 11607–11625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vale, A.P.; Santos, J.; Brito, N.V.; Fernandes, D.; Rosa, E.; Oliveira, M.B. Evaluating the Impact of Sprouting Conditions on the Glucosinolate Content of Brassica Oleracea Sprouts. Phytochemistry 2015, 115, 252–260. [Google Scholar] [CrossRef] [PubMed]
- Xuan, W.; Beeckman, T.; Xu, G. Plant Nitrogen nutrition: Sensing and Signaling. Curr. Opin. Plant Biol. 2017, 39, 57–65. [Google Scholar] [CrossRef]
- Sanchez-Pujante, P.J.; Borja-Martinez, M.; Pedreno, M.A.; Almagro, L. Biosynthesis and Bioactivity of Glucosinolates and Their Production in Plant In Vitro Cultures. Planta 2017, 246, 19–32. [Google Scholar] [CrossRef]
- Park, S.; Rim, S.J.; Jo, M.; Lee, M.G.; Kim, C.E. Comorbidity of Alcohol Use and Other Psychiatric Disorders and Suicide Mortality: Data from the South Korean National Health Insurance Cohort, 2002 to 2013. Alcohol. Clin. Exp. Res. 2019, 43, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Korbas, M.; Jajor, E.; Budka, A. Clubroot (Plasmodiophora Brassicae)—A Threat for Oilseed Rape. J. Plant Prot. Res. 2009, 49, 4. [Google Scholar] [CrossRef]
- Yu, F.; Zhang, W.; Wang, S.; Wang, H.; Yu, L.; Zeng, X.; Fei, Z.; Li, J. Genome Sequence of Fusarium Oxysporum f. sp. Conglutinans, the Etiological Agent of Cabbage Fusarium Wilt. Mol. Plant Microbe Interact. 2021, 34, 210–213. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.; Hockenhull, J.; Munk, L. Seedling and Adult Plant Resistance to Downy Mildew (Peronospora parasitica) in Cauliflower (Brassica oleracea convar. botrytis var. botrytis). Plant Pathol. 1999, 48, 604–612. [Google Scholar] [CrossRef]
- Mahalingam, T.; Chen, W.; Rajapakse, C.S.; Somachandra, K.P.; Attanayake, R.N. Genetic Diversity and Recombination in the Plant Pathogen Sclerotinia Sclerotiorum Detected in Sri Lanka. Pathogens 2020, 9, 4. [Google Scholar] [CrossRef] [PubMed]
- Samal, B.; Chatterjee, S. New Insight into Bacterial Social Communication in Natural Host: Evidence for Interplay of Heterogeneous and Unison Quorum Response. PLoS Genet. 2019, 15, e1008395. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.-H.; Yang, Q.; Ma, R.-C. Erwinia Carotovora ssp. Carotovora Infection Induced “Defense Lignin” Accumulation and Lignin Biosynthetic Gene Expression in Chinese Cabbage (Brassica rapa L. ssp. pekinensis). J. Integr. Plant Biol. 2007, 49, 993–1002. [Google Scholar] [CrossRef]
- Takikawa, Y.; Takahashi, F. Bacterial Leaf Spot and Blight of Crucifer Plants (Brassicaceae) Caused by Pseudomonas Syringae pv. Maculicola and P. cannabina pv. alisalensis. J. Gen. Plant Pathol. 2014, 80, 466–474. [Google Scholar] [CrossRef]
- Gong, J.; Ju, H.K.; Kim, I.H.; Seo, E.Y.; Cho, I.S.; Hu, W.X.; Han, J.Y.; Kim, J.K.; Choi, S.R.; Lim, Y.P.; et al. Sequence Variations Among 17 New Radish Isolates of Turnip mosaic virus Showing Differential Pathogenicity and Infectivity in Nicotiana benthamiana, Brassica rapa, and Raphanus sativus. Phytopathology 2019, 109, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Iamba, K.; Malapa, S. Efficacy of Selected Plant Extracts against Diamondback Moth (Plutella xylostella L.) on Round Cabbage In Situ. J. Entomol. Zool. Stud. 2020, 8, 1240–1247. [Google Scholar]
- Gabryś, B.; Pawluk, M. Acceptability of Different Species of Brassicaceae as Hosts for the Cabbage Aphid. In Proceedings of the 10th International Symposium on Insect-Plant Relationships; Springer: Dordrecht, The Netherlands, 1999; pp. 105–109. [Google Scholar]
- Griese, E.; Pineda, A.; Pashalidou, F.G.; Iradi, E.P.; Fatouros, N.E. Plant Responses to Butterfly Oviposition Partly Explain Preference–Performance Relationships on Different Brassicaceous Species. Oecologia 2020, 192, 293–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tierens, M.J.; Thomma, B.; Brouwer, M.; Schmidt, J.; Kistner, K.; Porzel, A.; Mauch-Mani, B.; Broekaert, C. Study of the Role of Antimicrobial Glucosinolate-Derived Isothiocyanates in Resistance of Arabidopsis to Microbial Pathogens. Plant Physiol. 2001, 125, 1688–1699. [Google Scholar] [CrossRef] [Green Version]
- Sellam, A.; Dongo, A.; Guillemette, T.; Hudhomme, P.; Simoneau, P. Transcriptional Responses to Exposure to the Brassicaceous Defence Metabolites Camalexin and Allyl-isothiocyanate in the Necrotrophic Fungus Alternaria Brassicicola. Mol. Plant Pathol. 2007, 8, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Humphry, M.; Bednarek, P.; Kemmerling, B.; Koh, S.; Stein, M.; Gobel, U.; Stuber, K.; Pislewska-Bednarek, M.; Loraine, A.; Schulze-Lefert, P.; et al. A Regulon Conserved in Monocot and Dicot Plants Defines a Functional Module in Antifungal Plant Immunity. Proc. Natl. Acad. Sci. USA 2010, 107, 21896–21901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giamoustaris, A.; Mithen, R.F. Glucosinolates and Disease Resistance in Oilseed Rape (Brassica napus ssp. oleifera). Plant Pathol. 2010, 46, 271–275. [Google Scholar] [CrossRef]
- Robin, A.H.K.; Laila, R.; Abuyusuf, M.; Park, J.I.; Nou, I.S. Leptosphaeria Maculans Alters Glucosinolate Accumulation and Expression of Aliphatic and Indolic Glucosinolate Biosynthesis Genes in Blackleg Disease-Resistant and -Susceptible Cabbage Lines at the Seedling Stage. Front. Plant Sci. 2020, 11, 1134. [Google Scholar] [CrossRef]
- Pedras, M.S.; Zheng, Q.A.; Gadagi, R.S.; Rimmer, S.R. Phytoalexins and Polar Metabolites from the Oilseeds Canola and Rapeseed: Differential Metabolic Responses to the Biotroph Albugo Candida and to Abiotic Stress. Phytochemistry 2008, 69, 894–910. [Google Scholar] [CrossRef]
- Hiruma, K.; Onozawa-Komori, M.; Takahashi, F.; Asakura, M.; Bednarek, P.; Okuno, T.; Schulze-Lefert, P.; Takano, Y. Entry Mode-dependent Function of an Indole Glucosinolate Pathway in Arabidopsis for Nonhost Resistance against Anthracnose Pathogens. Plant Cell 2010, 22, 2429–2443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kidd, B.N.; Kadoo, N.Y.; Dombrecht, B.; Tekeoglu, M.; Kazan, K. Auxin Signaling and Transport Promote Susceptibility to the Root-infecting Fungal Pathogen Fusarium Oxysporum in Arabidopsis. Mol. Plant Microbe Interact. MPMI 2011, 24, 733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Q.H.; Stephen, S.; Kazan, K.; Jin, G.; Fan, L.; Taylor, J.; Dennis, E.S.; Helliwell, C.A.; Wang, M.B. Characterization of the Defense Transcriptome Responsive to Fusarium Oxysporum-infection in Arabidopsis Using RNA-seq. Gene 2013, 512, 259–266. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Peltier, A.J.; Meng, J.; Osborn, T.C.; Grau, C.R. Evaluation of Sclerotinia Stem Rot Resistance in Oilseed Brassica napus Using a Petiole Inoculation Technique Under Greenhouse Conditions. Plant Dis. 2004, 88, 1033–1039. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Zhao, Q.; Yang, Q.; Liu, H.; Li, Q.; Yi, X.; Cheng, Y.; Guo, L.; Fan, C.; Zhou, Y. Comparative Transcriptomic Analysis Uncovers the Complex Genetic Network for Resistance to Sclerotinia Sclerotiorum in Brassica Napus. Sci. Rep. 2016, 6, 19007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stotz, H.U.; Sawada, Y.; Shimada, Y.; Hirai, M.Y.; Sasaki, E.; Krischke, M.; Brown, P.D.; Saito, K.; Kamiya, Y. Role of Camalexin, Indole Glucosinolates, and Side Chain Modification of Glucosinolate-derived Isothiocyanates in Defense of Arabidopsis against Sclerotinia Sclerotiorum. Plant J. 2011, 67, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Anthony, M.A.; Celenza, J.L.; Armstrong, A.; Frey, S.D. Indolic Glucosinolate Pathway Provides Resistance to Mycorrhizal Fungal Colonization in a Non-host Brassicaceae. Ecosphere 2020, 11, 4. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Yang, H.; Ren, L.; Chen, W.; Liu, L.; Liu, F.; Zeng, L.; Yan, R.; Chen, K.; Fang, X. Jasmonic Acid-Mediated Aliphatic Glucosinolate Metabolism Is Involved in Clubroot Disease Development in Brassica napus L. Front. Plant Sci. 2018, 9, 750. [Google Scholar] [CrossRef]
- Castillo, N.; Pastor, V.; Chavez, A.; Arro, M.; Boronat, A.; Flors, V.; Ferrer, A.; Altabella, T. Inactivation of UDP-Glucose Sterol Glucosyltransferases Enhances Arabidopsis Resistance to Botrytis cinerea. Front. Plant Sci. 2019, 10, 1162. [Google Scholar] [CrossRef] [Green Version]
- Tinte, M.M.; Steenkamp, P.A.; Piater, L.A.; Dubery, I.A. Lipopolysaccharide Perception in Arabidopsis Thaliana: Diverse LPS Chemotypes from Burkholderia Cepacia, Pseudomonas Syringae and Xanthomonas Campestris Trigger Differential Defence-related Perturbations in the Metabolome. Plant Physiol. Biochem. 2020, 156, 267–277. [Google Scholar] [CrossRef]
- Brader, G.; Mikkelsen, M.D.; Halkier, B.A.; Tapio Palva, E. Altering Glucosinolate Profiles Modulates Disease Resistance in Plants. Plant J. 2006, 46, 758–767. [Google Scholar] [CrossRef] [PubMed]
- Mishina, T.E.; Zeier, J. Bacterial Non-host Resistance: Interactions of Arabidopsis with Non-adapted Pseudomonas Syringae Strains. Physiol. Plant 2007, 131, 448–461. [Google Scholar] [CrossRef]
- Truman, W.; Bennettt, M.; Kubigsteltig, I.; Turnbull, C.; Grant, M. Arabidopsis Systemic Immunity Uses Conserved Defense Signaling Pathways and is Mediated by Jasmonates. Proc. Natl. Acad. Sci. USA 2007, 104, 1075–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aires, A.; Dias, C.S.P.; Carvalho, R.; Oliveira, M.H.; Monteiro, A.A.; Simões, M.V.; Rosa, E.A.S.; Bennett, R.N.; Saavedra, M.J. Correlations between Disease Severity, Glucosinolate Profiles and Total Phenolics and Xanthomonas Campestris pv. Campestris Inoculation of Different Brassicaceae. Sci. Hortic. 2011, 129, 503–510. [Google Scholar] [CrossRef]
- Clay, N.K.; Adio, A.M.; Denoux, C.; Jander, G.; Ausubel, F.M. Glucosinolate Metabolites Required for an Arabidopsis Innate Immune Response. Science 2009, 323, 95–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geng, X.; Cheng, J.; Gangadharan, A.; Mackey, D. The Coronatine Toxin of Pseudomonas Syringae is a Multifunctional Suppressor of Arabidopsis Defense. Plant Cell 2012, 24, 4763–4774. [Google Scholar] [CrossRef] [Green Version]
- Barah, P.; Winge, P.; Kusnierczyk, A.; Tran, D.H.; Bones, A.M. Molecular Signatures in Arabidopsis Thaliana in Response to Insect Attack and Bacterial Infection. PLoS ONE 2013, 8, e58987. [Google Scholar] [CrossRef] [Green Version]
- Sun, Q.; Zhang, E.; Liu, Y.; Xu, Z.; Hui, M.; Zhang, X.; Cai, M. Transcriptome Analysis of Two Lines of Brassica Oleracea in Response to Early Infection with Xanthomonas Campestris pv. Campestris. Can. J. Plant Pathol. 2020, 43, 127–139. [Google Scholar] [CrossRef]
- Liu, M.; Wu, F.; Wang, S.; Lu, Y.; Chen, X.; Wang, Y.; Gu, A.; Zhao, J.; Shen, S. Comparative Transcriptome Analysis Reveals Defense Responses against Soft Rot in Chinese Cabbage. Hortic. Res. 2019, 6, 68. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deutsch, C.A.; Tewksbury, J.J.; Tigchelaar, M.; Battisti, D.S.; Merrill, S.C.; Huey, R.B.; Naylor, R.L. Increase in Crop Losses to Insect Pests in a Warming Climate. Science 2018, 361, 916–919. [Google Scholar] [CrossRef] [Green Version]
- Brown, P.D.; Morra, M.J. Control of Soil-Borne Plant Pests Using Glucosinolate-Containing Plants. Adv. Agron. 1997, 61, 168–231. [Google Scholar]
- Strauss, S.Y.; Lambrix, I. Optimal Defence Theory and Flower Petal Colour Predict Variation in the Secondary Chemistry of Wild Radish. J. Ecol. 2004, 92, 132–141. [Google Scholar] [CrossRef]
- Vadassery, J.; Reichelt, M.; Hause, B.; Gershenzon, J.; Boland, W.; Mithofer, A. CML42-mediated Calcium Signaling Coordinates Responses to Spodoptera Herbivory and Abiotic Stresses in Arabidopsis. Plant Physiol. 2012, 159, 1159–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mewis, I.; Appel, H.M.; Hom, A.; Raina, R.; Schultz, J.C. Major Signaling Pathways Modulate Arabidopsis Glucosinolate Accumulation and Response to Both Phloem-feeding and Chewing Insects. Plant Physiol. 2005, 138, 1149–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahuja, I.; van Dam, N.M.; Winge, P.; Traelnes, M.; Heydarova, A.; Rohloff, J.; Langaas, M.; Bones, A.M. Plant Defence Responses in Oilseed Rape MINELESS Plants after Attack by the Cabbage Moth Mamestra Brassicae. J. Exp. Bot. 2015, 66, 579–592. [Google Scholar] [CrossRef] [Green Version]
- Santolamazza-Carbone, S.; Sotelo, T.; Velasco, P.; Cartea, M.E. Antibiotic Properties of the Glucosinolates of Brassica oleracea var. acephala similarly Affect Generalist and Specialist Larvae of Two Lepidopteran Pests. J. Pest Sci. 2016, 89, 195–206. [Google Scholar] [CrossRef]
- Dam, N.; Raaijmakers, C.E.; Putten, W. Root Herbivory Reduces Growth and Survival of the Shoot Feeding Specialist Pieris Rapae on Brassica Nigra. Entomol. Exp. Appl. 2005, 115, 161–170. [Google Scholar]
- Mathur, V.; Ganta, S.; Raaijmakers, C.E.; Reddy, A.S.; Vet, L.E.M.; van Dam, N.M. Temporal Dynamics of Herbivore-induced Responses in Brassica Juncea and Their Effect on Generalist and Specialist Herbivores. Entomol. Exp. Appl. 2011, 139, 215–225. [Google Scholar] [CrossRef]
- van Dam, N.M.; Samudrala, D.; Harren, F.J.; Cristescu, S.M. Real-time Analysis of Sulfur-containing Volatiles in Brassica Plants Infested with Root-feeding Delia Radicum Larvae Using Proton-transfer Reaction Mass Spectrometry. AoB Plants 2012, 2012, pls021. [Google Scholar] [CrossRef]
- Badenes-Pérez, F.R.; Gershenzon, J.; Heckel, D.G. Plant Glucosinolate Content Increases Susceptibility to Diamondback Moth (Lepidoptera: Plutellidae) Regardless of Its Diet. J. Pest Sci. 2019, 93, 491–506. [Google Scholar] [CrossRef]
- Chen, W.; Dong, Y.; Saqib, H.S.A.; Vasseur, L.; Zhou, W.; Zheng, L.; Lai, Y.; Ma, X.; Lin, L.; Xu, X.; et al. Functions of Duplicated Glucosinolate Sulfatases in the Development and Host Adaptation of Plutella Xylostella. Insect Biochem. Mol. Biol. 2020, 119, 103316. [Google Scholar] [CrossRef]
- Ogran, A.; Faigenboim, A.; Barazani, O. Transcriptome Responses to Different Herbivores Reveal Differences in Defense Strategies between Populations of Eruca Sativa. BMC Genom. 2019, 20, 843. [Google Scholar] [CrossRef] [PubMed]
- Gols, R.; van Dam, N.M.; Reichelt, M.; Gershenzon, J.; Raaijmakers, C.E.; Bullock, J.M.; Harvey, J.A. Seasonal and Herbivore-induced Dynamics of Foliar Glucosinolates in Wild Cabbage (Brassica oleracea). Chemoecology 2018, 28, 77–89. [Google Scholar] [CrossRef] [Green Version]
- Robert, C.A.M.; Pellissier, L.; Moreira, X.; Defossez, E.; Pfander, M.; Guyer, A.; van Dam, N.M.; Rasmann, S. Correlated Induction of Phytohormones and Glucosinolates Shapes Insect Herbivore Resistance of Cardamine Species Along Elevational Gradients. J. Chem. Ecol. 2019, 45, 638–648. [Google Scholar] [CrossRef]
- Buckley, J.; Pashalidou, F.G.; Fischer, M.C.; Widmer, A.; Mescher, M.C.; De Moraes, C.M. Divergence in Glucosinolate Profiles between High- and Low-Elevation Populations of Arabidopsis halleri Correspond to Variation in Field Herbivory and Herbivore Behavioral Preferences. Int. J. Mol. Sci. 2019, 20, 174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papazian, S.; Girdwood, T.; Wessels, B.A.; Poelman, E.H.; Dicke, M.; Moritz, T.; Albrectsen, B.R. Leaf Metabolic Signatures Induced by Real and Simulated Herbivory in Black Mustard (Brassica nigra). Metabolomics 2019, 15, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kempema, L.A.; Cui, X.; Holzer, F.M.; Walling, L.L. Arabidopsis Transcriptome Changes in Response to Phloem-Feeding Silverleaf Whitefly Nymphs. Similarities and Distinctions in Responses to Aphids. Plant Physiol. 2007, 143, 849–865. [Google Scholar] [CrossRef] [Green Version]
- Agerbirk, N.; Olsen, C.E.; Nielsen, J.K. Seasonal Variation in Leaf Glucosinolates and Insect Resistance in Two Types of Barbarea Vulgaris ssp. arcuata. Phytochemistry 2001, 58, 91–100. [Google Scholar] [CrossRef]
- Kroymann, J.; Donnerhacke, S.; Schnabelrauch, D.; Mitchell-Olds, T. Evolutionary Dynamics of an Arabidopsis Insect Resistance Quantitative Trait Locus. Proc. Natl. Acad. Sci. USA 2003, 100, 14587–14592. [Google Scholar] [CrossRef] [Green Version]
- Ulmer, B.J.; Dosdall, L.M. Glucosinolate Profile and Oviposition Behavior in Relation to the Susceptibilities of Brassicaceae to the Cabbage Seedpod Weevil. Entomol. Exp. Appl. 2010, 121, 203–213. [Google Scholar] [CrossRef]
- Bo, P.; Hopkins, R.; Rask, L.; Meijer, J. Infestation by Cabbage Aphid (Brevicoryne brassicae) on Oilseed Rape (Brassica napus) Causes a Long Lasting Induction of the Myrosinase System. Entomol. Exp. Appl. 2003, 109, 55–62. [Google Scholar]
- Kusnierczyk, A.; Winge, P.; Jorstad, T.S.; Troczynska, J.; Rossiter, J.T.; Bones, A.M. Towards Global Understanding of Plant Defence against Aphids—Timing and Dynamics of Early Arabidopsis Defence Responses to Cabbage Aphid (Brevicoryne brassicae) Attack. Plant Cell Environ. 2008, 31, 1097–1115. [Google Scholar] [CrossRef]
- Beukeboom, L.W. Editor's Choice: March 2020. Entomol. Exp. Appl. 2020, 168, 199. [Google Scholar] [CrossRef]
- de Vos, M.; Jander, G. Myzus Persicae (green peach aphid) Salivary Components Induce Defence Responses in Arabidopsis Thaliana. Plant Cell Environ. 2009, 32, 1548–1560. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, F.; Muller, C. Independent Responses to Ultraviolet Radiation and Herbivore Attack in Broccoli. J. Exp. Bot. 2009, 60, 3467–3475. [Google Scholar] [CrossRef] [Green Version]
- Pfalz, M.; Vogel, H.; Kroymann, J. The Gene Controlling the Indole Glucosinolate Modifier1 Quantitative Trait Locus Alters Indole Glucosinolate Structures and Aphid Resistance in Arabidopsis. Plant Cell 2009, 21, 985–999. [Google Scholar] [CrossRef] [Green Version]
- Hu, J.; Yang, J.J.; Liu, B.M.; Cui, H.Y.; Zhang, Y.J.; Jiao, X.G. Feeding Behavior Explains the Different Effects of Cabbage on MEAM1 and MED Cryptic Species of Bemisia Tabaci. Insect Sci. 2020, 27, 1276–1284. [Google Scholar] [CrossRef]
- Anyanga, M.O.; Farman, D.I.; Ssemakula, G.N.; Mwanga, R.O.M.; Stevenson, P.C. Effects of Hydroxycinnamic Acid Esters on Sweetpotato Weevil Feeding and Oviposition and Interactions with Bacillus Thuringiensis Proteins. J. Pest Sci. 2020. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Z.; Tong, H.; Yang, F.; Xing, G.; Wang, L.; Zheng, J.; Zhang, Y.; Su, Q. Tomato Plant Flavonoids Increase Whitefly Resistance and Reduce Spread of Tomato yellow leaf curl virus. J. Econ. Entomol. 2019, 112, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Jost, R.; Altschmied, L.; Bloem, E.; Bogs, J.; Gershenzon, J.; Hahnel, U.; Hansch, R.; Hartmann, T.; Kopriva, S.; Kruse, C.; et al. Expression Profiling of Metabolic Genes in Response to Methyl Jasmonate Reveals Regulation of Genes of Primary and Secondary Sulfur-related Pathways in Arabidopsis Thaliana. Photosynth. Res. 2005, 86, 491–508. [Google Scholar] [CrossRef] [PubMed]
- Pangesti, N.; Reichelt, M.; van de Mortel, J.E.; Kapsomenou, E.; Gershenzon, J.; van Loon, J.J.; Dicke, M.; Pineda, A. Jasmonic Acid and Ethylene Signaling Pathways Regulate Glucosinolate Levels in Plants During Rhizobacteria-Induced Systemic Resistance Against a Leaf-Chewing Herbivore. J. Chem. Ecol. 2016, 42, 1212–1225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, W.; Li, J.; Yu, Q.; Cang, W.; Xu, R.; Wang, Y.; Ji, W. Two Novel Flavin-Containing Monooxygenases Involved in Biosynthesis of Aliphatic Glucosinolates. Front. Plant Sci. 2016, 7, 1292. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Han, M.H.; Guevara-Garcia, A.; Fedoroff, N.V. Mitogen-activated protein kinase signaling in postgermination arrest of development by abscisic acid. Proc. Natl. Acad. Sci. USA 2002, 99, 15812–15817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
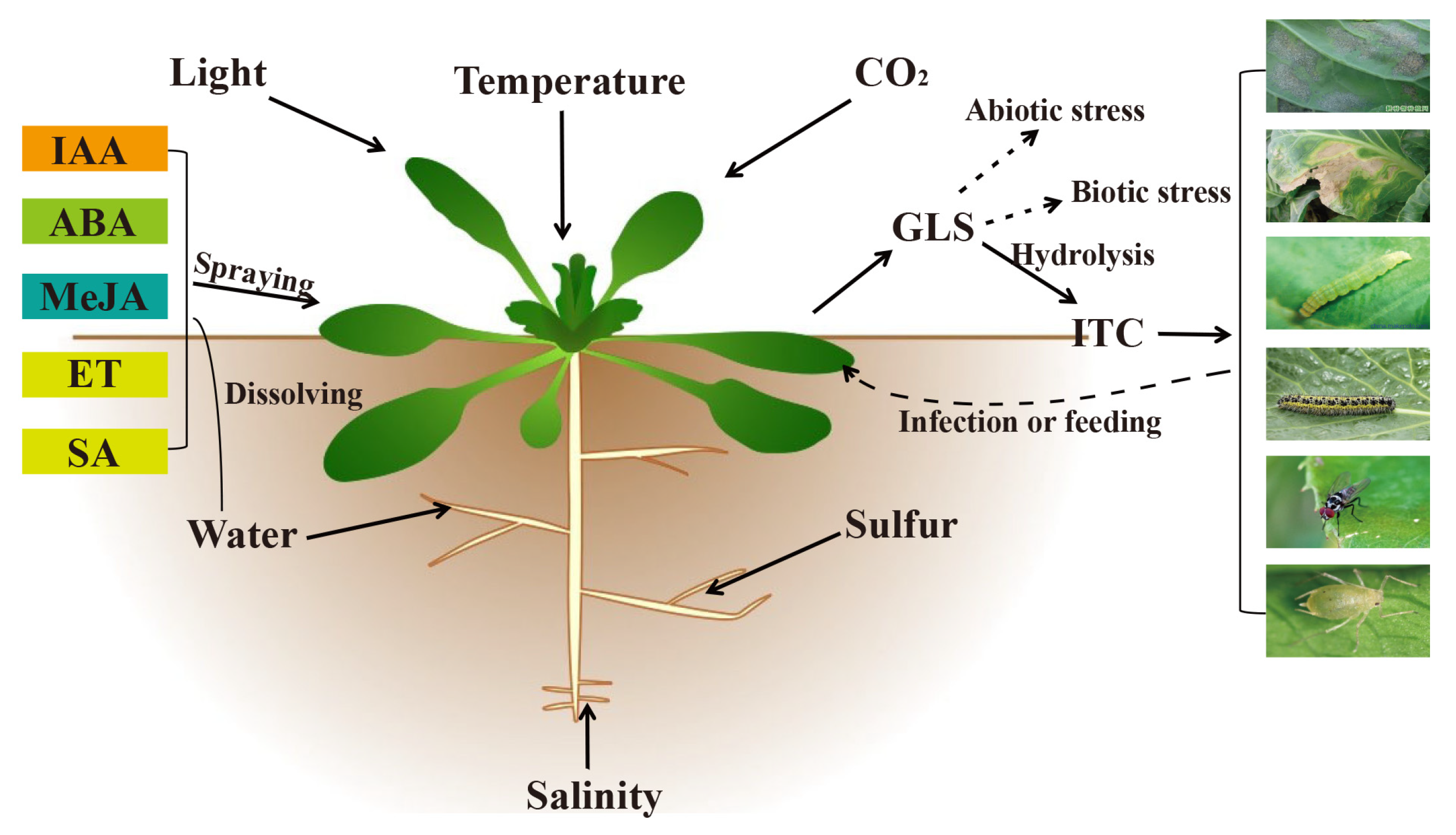
| Component | Species | Names | Correlation | References |
|---|---|---|---|---|
| ITC; Allyl-ITC | Fungal | Alternaria brassicicola | positive | [84,85,86] |
| Plectosphaerella cucumerina | positive | [86] | ||
| Botrytis cinerea | positive | [86] | ||
| Fusarium oxysporum | positive | [86] | ||
| Peronospora parasitica | positive | [86] | ||
| Total GLS | Fungal | Alternaria brassicicola | positive | [92] |
| Leptosphaeria maculans | No; positive | [92,93] | ||
| Sclerotinia sclerotiorum | positive | [7,98,99] | ||
| Arbuscular mycorrhizal fungi | positive | [101] | ||
| Bacteria | Burkholderia cepacia | positive | [104] | |
| Pseudomonas syringae | positive | [104] | ||
| Xanthomonas campestris | positive | [104,111,112] | ||
| Pectobacterium carotovorum | positive | [113] | ||
| Pest | Pieris rapae | positive | [114] | |
| Spodoptera littoralis | positive | [115] | ||
| Slug | negative | [93] | ||
| Spodoptera exigua | positive | [116] | ||
| Trichoplusia ni | positive | [117] | ||
| Manduca sexta | positive | [117] | ||
| Mamestra brassicae | positive | [118] | ||
| Pratylenchus penetrans | positive | [118] | ||
| Delia radicum L. | positive | [118] | ||
| Spodoptera litura Fabricius | positive | [121] | ||
| Plutella xylostella L. | positive | [121,123,124] | ||
| Pieris brassicae | positive | [128,129] | ||
| Insect | Phyllotreta nemorum | No | [131] | |
| Psylliodes chrysocephala | positive | [132] | ||
| Ceutorhynchus obstrictus | No | [133] | ||
| Indole GLS | Fungal | Albugo candida | positive | [94] |
| Colletotrichum gloeosporioides | Positive | [95] | ||
| Colletotrichum orbiculare | Positive | [95] | ||
| Fusarium oxysporum | positive | [96,97] | ||
| Plasmodiophora brassicae | positive | [102] | ||
| Bacteria | Pseudomonas syringae | positive | [106,107,108] | |
| Aliphatic GLS | Fungal | Plasmodiophora brassicae | positive | [102] |
| Pest | Spodoptera littoralis | positive | [125] | |
| Pieris brassicae | positive | [125] | ||
| Pieris rapae | positive | [126] | ||
| Insect | Psylliodes chrysocephala | positive | [137] | |
| Aromatic GLS | Fungal | Plasmodiophora brassicae | positive | [102] |
| Pest | Plutella xylostella L. | positive | [60] | |
| Benzenic GLS | Insect | Psylliodes chrysocephala | positive | [136] |
| Indolyl-3-acetonitrile, 4-methoxyglucobrassicin, | Fungal | Albugo candida | positive | [94] |
| Aliphatic isopropyl; methylpropyl GLS | Bacteria | Erwinia carotovora | positive | [105] |
| Indol-3-yl-methyl; 1-methoxy-indol-3-yl-methyl | Pest | Mamestra brassicae | positive | [118] |
| P-hydroxybenzyl; 3-butenyl | Insect | Ceutorhynchus obstrictus | positive | [133] |
| Sinigrin | Pest | Pieris rapae | negative | [119] |
| Glucobrassicin | Pest | Pieris rapae | positive | [119] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants 2021, 10, 1097. https://doi.org/10.3390/plants10061097
Liu Z, Wang H, Xie J, Lv J, Zhang G, Hu L, Luo S, Li L, Yu J. The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants. 2021; 10(6):1097. https://doi.org/10.3390/plants10061097
Chicago/Turabian StyleLiu, Zeci, Huiping Wang, Jianming Xie, Jian Lv, Guobin Zhang, Linli Hu, Shilei Luo, Lushan Li, and Jihua Yu. 2021. "The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance" Plants 10, no. 6: 1097. https://doi.org/10.3390/plants10061097
APA StyleLiu, Z., Wang, H., Xie, J., Lv, J., Zhang, G., Hu, L., Luo, S., Li, L., & Yu, J. (2021). The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants, 10(6), 1097. https://doi.org/10.3390/plants10061097






