Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens
Abstract
:1. Introduction
2. Results
2.1. In Vitro Antagonistic Activity of Bs MBI600 against Forl, P. ultimum, and R. solani
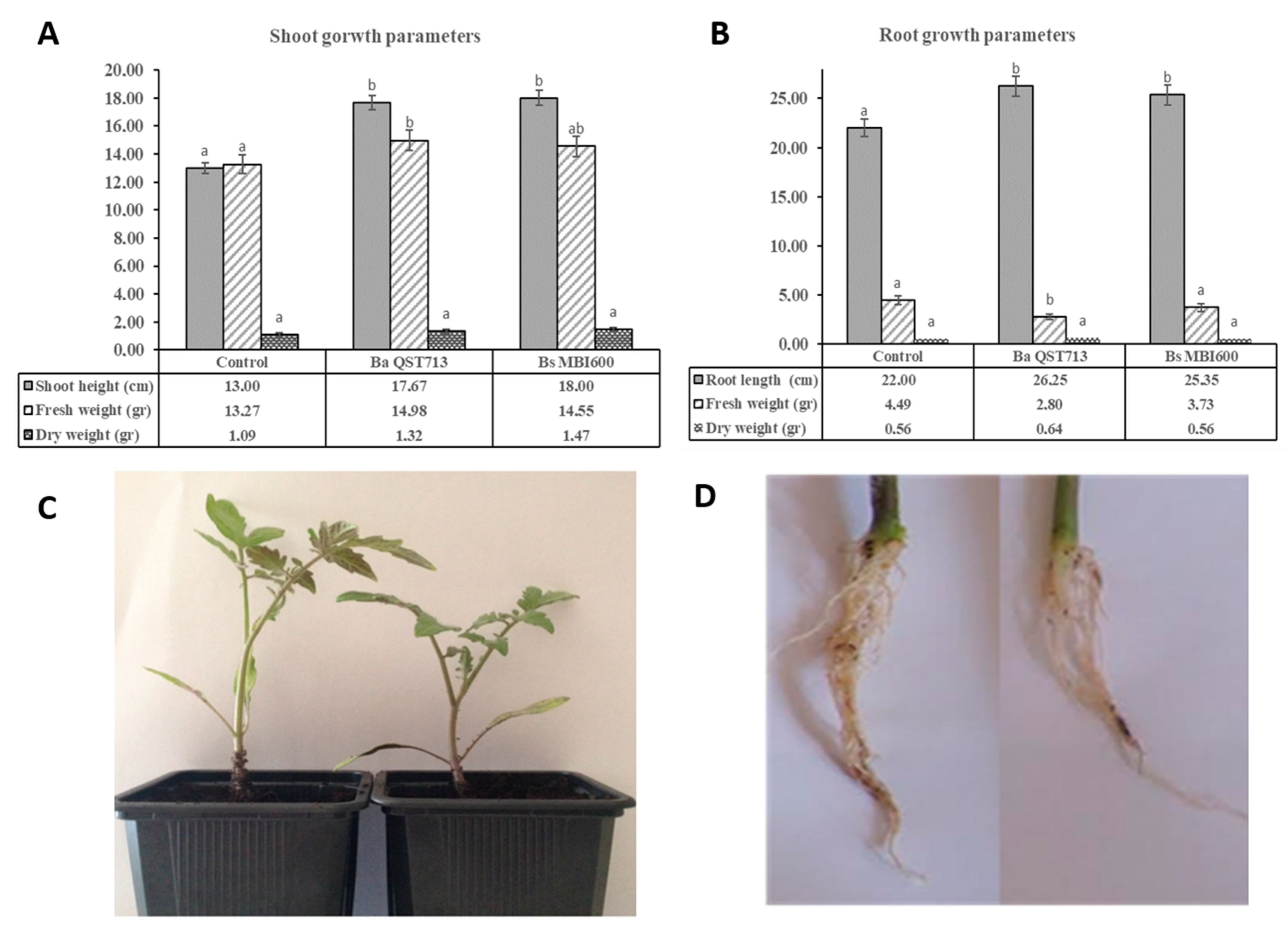
2.2. Growth Characteristics of Tomato Plants Treated with Bs MBI600
2.3. Root Colonization
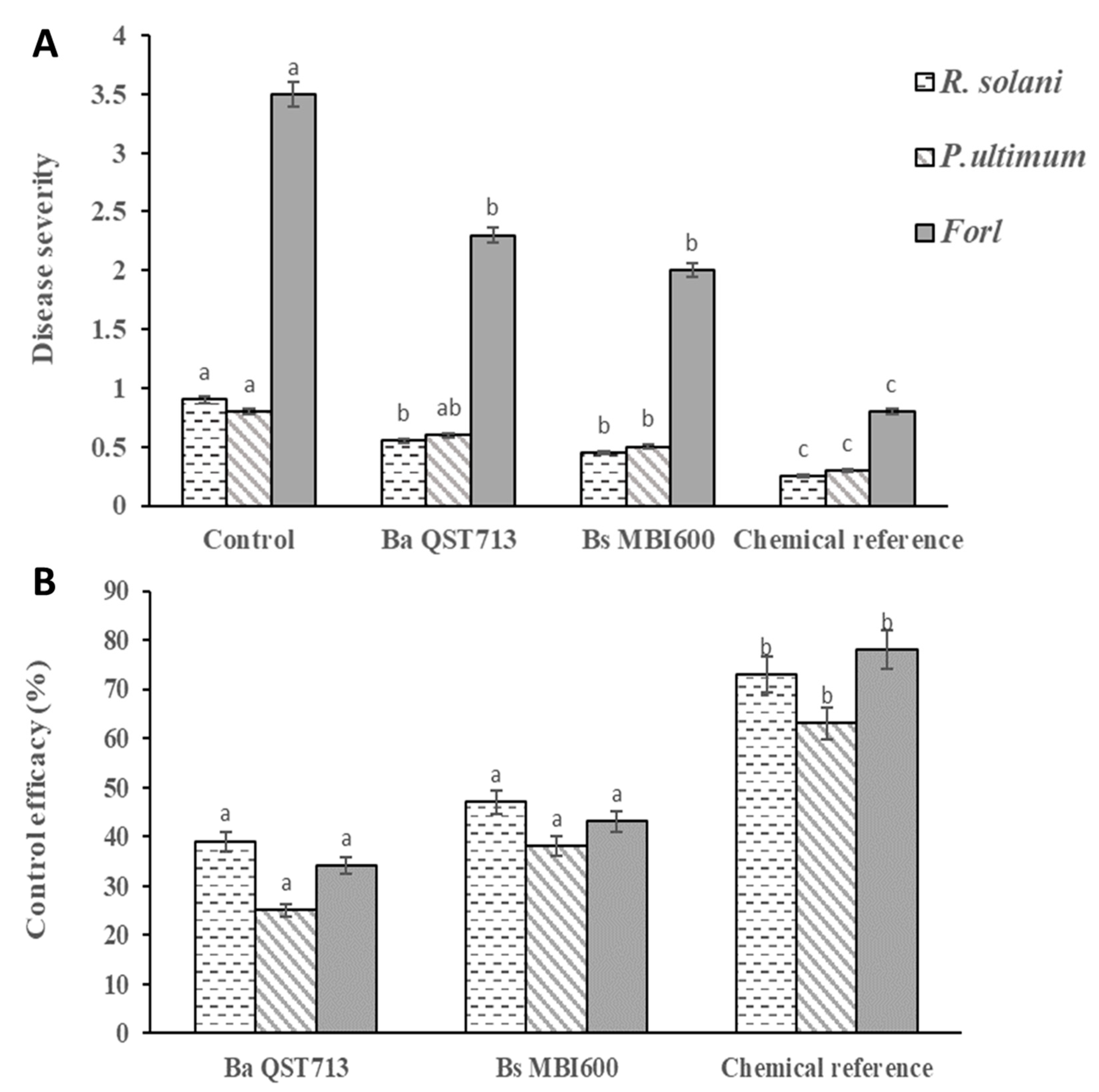
2.4. Biocontrol Activity of Bs MBI600 against Soilborne Tomato Pathogens
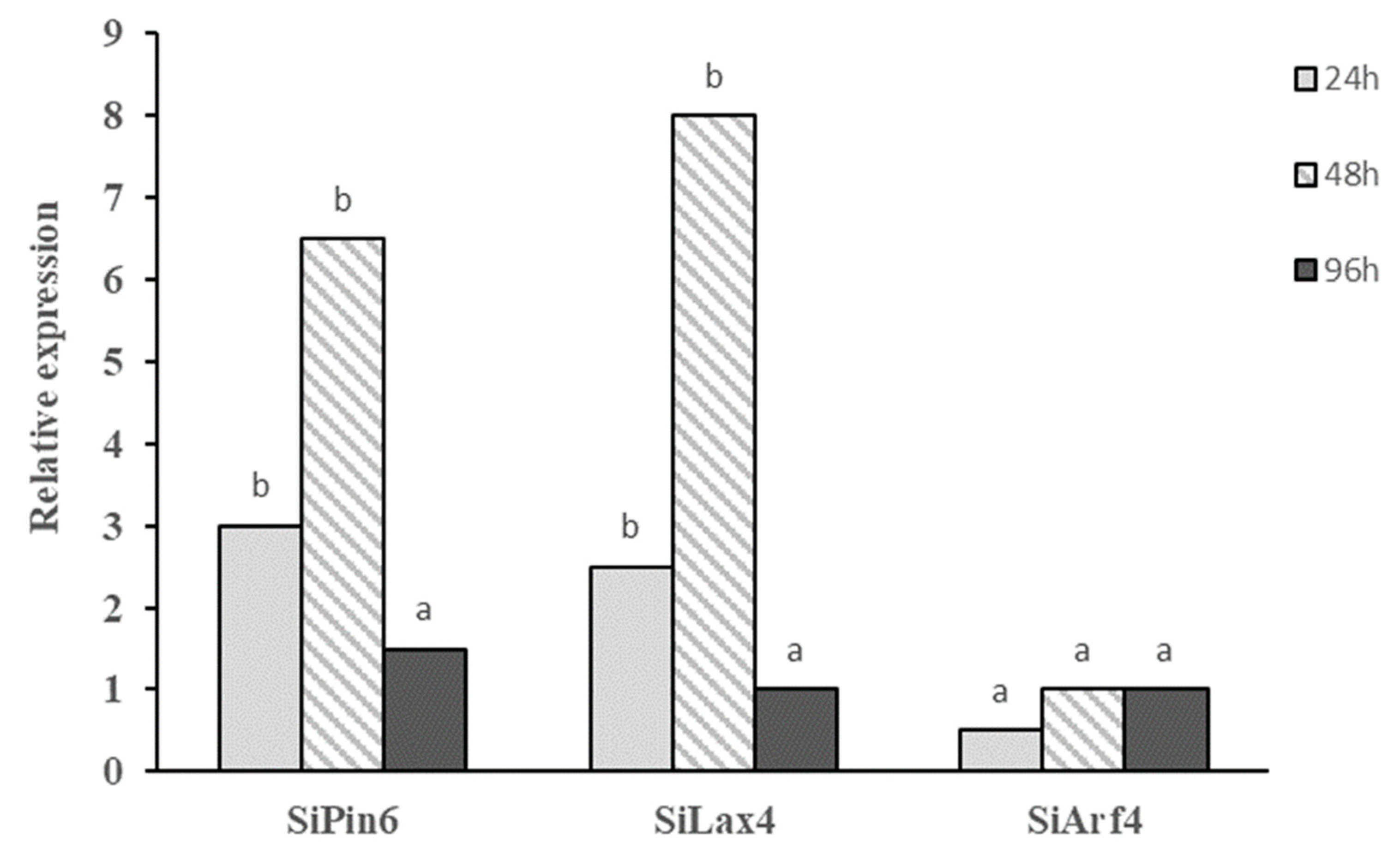
2.5. Induction of Auxin-Related Genes in Tomato Plants Treated with Bs MBI600
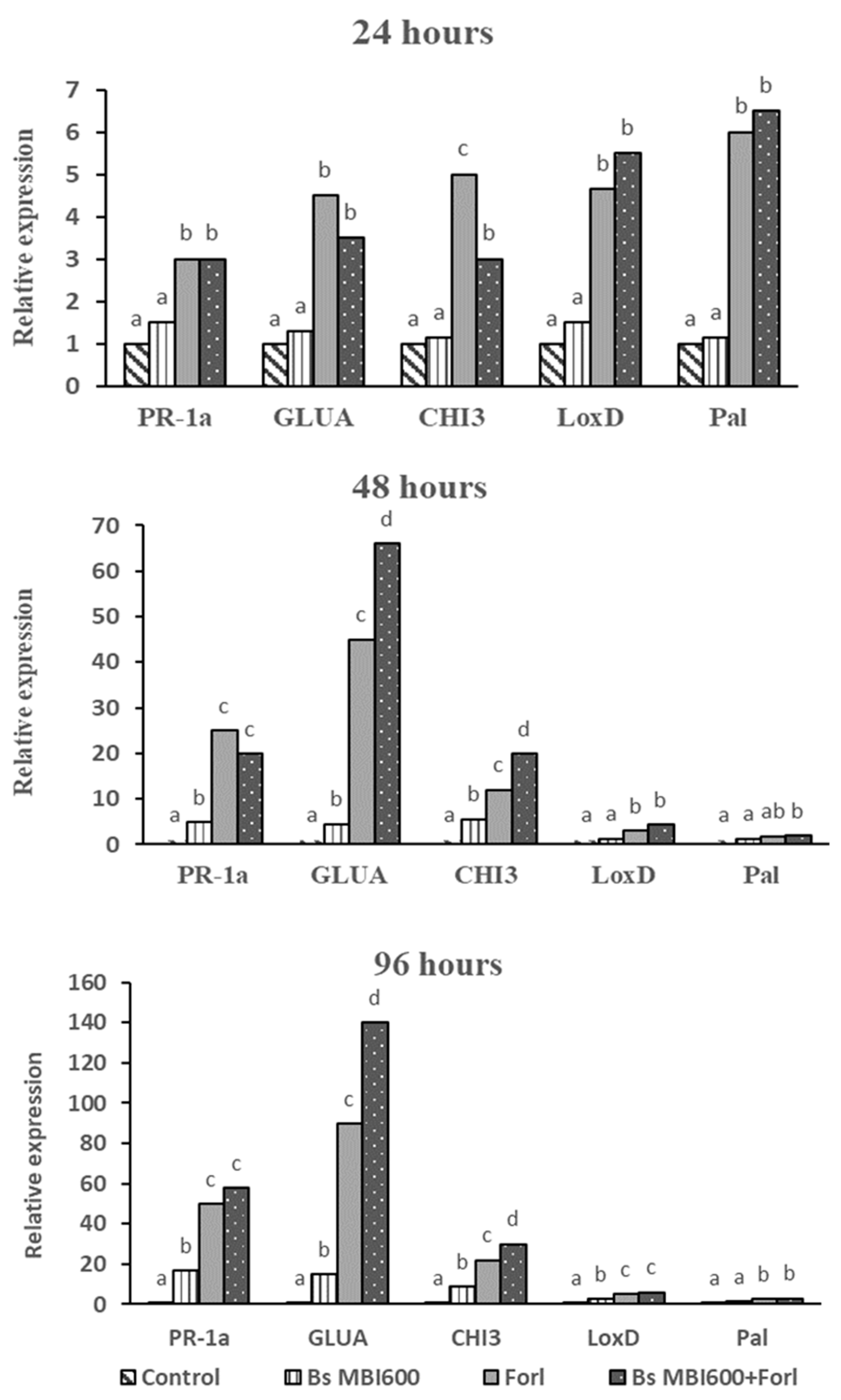
2.6. Induction of Defense-Related Genes in Tomato Plants Treated with Bs MBI600
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Microorganisms
4.3. In Vitro Effects of Bs MBI600 against the Mycelial Growth of Soilborne Pathogens
4.4. Plant Growth Promotion Assays
4.5. Root Colonization Assays in Various Growth Substrates
4.6. In Planta Efficacy of Bs MBI600 for Controlling Soilborne Pathogens of Tomato
4.7. Induction of Defense- and Auxin-Related Genes in Tomato Plants
4.8. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO. Tomato, Food and Agriculture Organization. 2021. Available online: http://www.fao.org/faostat/en/#data/QC/ (accessed on 10 March 2021).
- Manzo, D.; Ferriello, F.; Puopolo, G.; Zoina, A.; D’Esposito, B.; Tardella, L.; Ferrarini, A.; Ercolano, M.R. Fusarium oxysporum f.sp. radicis-lycopersici induces distinct transcriptome reprogramming in resistant and susceptible isogenic tomato lines. BMC Plant Biol. 2016, 16, 53. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, W.R. Fusarium crown and root rot of tomatoes. Phytoprotection 1988, 69, 49–64. [Google Scholar]
- Ogoshi, A. Ecology and pathogenicity of anastomosis and intraspecific groups of Rhizoctonia solani Kuhn. Annu. Rev. Phytopathol. 1987, 25, 125–143. [Google Scholar] [CrossRef]
- Gravel, V.; Martinez, C.; Antoun, H.; Tweddell, R.J. Control of greenhouse tomato root rot [Pythium ultimum] in hydroponic systems, using plant-growth-promoting microorganisms. Can. J. Plant Pathol. 2006, 28, 475–483. [Google Scholar] [CrossRef]
- Gravel, V.; Dorais, M.; Ménard, C. Organic fertilization and its effect on development of sweet pepper transplants. HortScience 2012, 47, 198–204. [Google Scholar] [CrossRef] [Green Version]
- McGovern, R.J. Management of tomato diseases caused by Fusarium oxysporum. Crop Prot. 2015, 73, 78–92. [Google Scholar] [CrossRef]
- Abdallah, R.A.B.; Stedel, C.; Garagounis, C.; Nefzi, A.; Jabnoun-Khiareddine, H.; Papadopoulou, K.K.; Daami-Remadi, M. Involvement of lipopeptide antibiotics and chitinase genes and induction of host defense in suppression of Fusarium wilt by endophytic Bacillus spp. in tomato. Crop Prot. 2017, 99, 45–58. [Google Scholar] [CrossRef]
- Myresiotis, C.K.; Karaoglanidis, G.S.; Vryzas, Z.; Papadopoulou-Mourkidou, E. Evaluation of plant growth-promoting rhizobacteria, acibenzolar-S-methyl, and hymexazol for integrated control of Fusarium crown and root rot on tomato. Pest Manag. Sci. 2012, 68, 404–411. [Google Scholar] [CrossRef]
- Kamou, N.N.; Cazorla, F.; Kandylas, G.; Lagopodi, A.L. Induction of defense-related genes in tomato plants after treatments with the biocontrol agents Pseudomonas chlororaphis ToZa7 and Clonostachys rosea IK726. Arch. Microbiol. 2020, 202, 257–267. [Google Scholar] [CrossRef]
- Choudhary, D.K.; Johri, B.N. Interactions of Bacillus spp. and plants—With special reference to induced systemic resistance (ISR). Microbiol. Res. 2009, 164, 493–513. [Google Scholar] [CrossRef]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pieterse, C.M.; Van der Does, D.; Zamioudis, C.; Leon-Reyes, A.; Van Wees, S.C. Hormonal modulation of plant immunity. Annu. Rev. Cell Dev. Biol. 2012, 28, 489–521. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.Q.; Zhao, D.L.; Shen, L.L.; Jing, C.L.; Zhang, C.S. Application and mechanisms of Bacillus subtilis in biological control of plat disease. In Role of Rhizospheric Microbes in Soil; Meena, V.S., Ed.; Springer Nature Ltd.: Singapore, 2018; pp. 225–250. [Google Scholar]
- Dimopoulou, A.; Theologidis, I.; Liebmann, B.; Kalantidis, K.; Vassilakos, N.; Skandalis, N. Bacillus amyloliquefaciens MBI600 differentially induces tomato defense signaling pathways depending on plant part and dose of application. Sci. Rep. 2019, 9, 19120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Bucio, J.; Campos-Cuevas, J.C.; Hernández-Calderón, E.; Velásquez-Becerra, C.; Farías-Rodríguez, R.; Macías-Rodríguez, L.I.; Valencia-Cantero, E. Bacillus megaterium rhizobacteria promote growth and alter root-system architecture through an auxin- and ethylene-independent signaling mechanism in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2007, 20, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugtenberg, B.; Kamilova, F. Plant-growth-promoting rhizobacteria. Annu. Rev. Microbiol. 2009, 63, 541–556. [Google Scholar] [CrossRef] [Green Version]
- Zamioudis, C.; Mastranesti, P.; Dhonukshe, P.; Blilou, I.; Pieterse, C.M.J. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. bacteria. Plant Physiol. 2013, 162, 304–318. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Y.; Li, Y.; Wang, X.; Nan, W.; Hu, Y.; Zhang, H.; Zhao, C.; Wang, F.; Li, P.; et al. Endophytic microbes Bacillus sp. LZR216-regulated root development is dependent on polar auxin transport in Arabidopsis seedlings. Plant Cell Rep. 2015, 34, 1075–1087. [Google Scholar] [CrossRef] [PubMed]
- Franco-Sierra, N.D.; Posada, L.F.; Santa-Maria, G.; Romero-Tabarez, M.; Villegas-Escobar, V.; Alvarez, J.C. Bacillus subtilis EA-CB0575 genome reveals clues for plant growth promotion and potential for sustainable agriculture. Funct. Integr. Genom. 2020, 20, 575–589. [Google Scholar] [CrossRef]
- Shafi, J.; Tian, H.; Ji, M. Bacillus species as versatile weapons for plant pathogens: A review. Biotechnol. Bioeng. Equip. 2017, 31, 446–459. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.M.; Radhakrishnan, R.; Lee, I.J. Bacillus amyloliquefaciens subsp. plantarum GR53, a potent biocontrol agent resists Rhizoctonia disease on Chinese cabbage through hormonal and antioxidants regulation. World J. Microbiol. Biotechnol. 2015, 31, 1517–1527. [Google Scholar] [CrossRef] [PubMed]
- Solanki, M.K.; Kumar, S.; Pandey, A.K.; Srivastava, S.; Singh, R.K.; Kashyap, P.L.; Srivastava, A.K.; Arora, D.K. Diversity and antagonistic potential of Bacillus spp. associated to the rhizosphere of tomato for the management of Rhizoctonia solani. Biocontrol. Sci. Technol. 2012, 22, 203–217. [Google Scholar] [CrossRef]
- de Curtis, F.; Lima, G.; Vitullo, D.; De Cicco, V. Biocontrol of Rhizoctonia solani and Sclerotium rolfsii on tomato by delivering antagonistic bacteria through a drip irrigation system. Crop Prot. 2010, 29, 663–670. [Google Scholar] [CrossRef]
- Samaras, A.; Nikolaidis, M.; Antequera-Gómez, M.L.; Cámara-Almirón, J.; Romero, D.; Moschakis, T.; Amoutzias, G.D.; Karaoglanidis, G.S. Whole genome sequencing and root colonization studies reveal novel insights in the biocontrol potential and growth promotion by Bacillus subtilis MBI 600 on cucumber. Front. Microbiol. 2021, 11, 3437. [Google Scholar] [CrossRef]
- Kumar, K.V.K.; Yellareddygari, S.K.; Reddy, M.S.; Kloepper, J.W.; Lawrence, K.S.; Zhou, X.G.; Miller, M.E. Efficacy of Bacillus subtilis MBI 600 against sheath blight caused by Rhizoctonia solani and on growth and yield of rice. Rice Sci. 2012, 19, 55–63. [Google Scholar] [CrossRef] [Green Version]
- Beris, D.; Theologidis, I.; Skandalis, N.; Vassilakos, N. Bacillus amyloliquefaciens strain MBI600 induces salicylic acid dependent resistance in tomato plants against Tomato spotted wilt virus and Potato virus Y. Sci. Rep. 2018, 8, 10320. [Google Scholar] [CrossRef] [Green Version]
- Samaras, A.; Efthimiou, K.; Roumeliotis, E.; Karaoglanidis, G.S. Biocontrol potential and plant-growth-promoting effects of Bacillus amyloliquefaciens MBI 600 against Fusarium oxysporum f. sp. radicis-lycopersici on tomato. Acta Hort. 2018, 1207, 139–145. [Google Scholar] [CrossRef]
- Raza, W.; Ling, N.; Zhang, R.; Huang, Q.; Xu, Y.; Shen, Q. Success evaluation of the biological control of Fusarium wilts of cucumber, banana, and tomato since 2000 and future research strategies. Crit. Rev. Biotechnol. 2017, 37, 202–212. [Google Scholar] [CrossRef] [PubMed]
- Fousia, S.; Paplomatas, E.J.; Tjamos, S.E. Bacillus subtilis QST 713 confers protection to tomato plants against Pseudomonas syringae pv tomato and induces plant defence-related genes. J. Phytopathol. 2016, 164, 264–270. [Google Scholar] [CrossRef]
- Ambreetha, S.; Chinnadurai, C.; Marimuthu, P.; Balachandar, D. Plant-associated Bacillus modulates the expression of auxin-responsive genes of rice and modifies the root architecture. Rhizosphere 2018, 5, 57–66. [Google Scholar] [CrossRef]
- Spaepen, S.; Vanderleyden, J.; Remans, R. Indole-3-acetic acid in microbial and microorganism-plant signaling. FEMS Microbiol. Rev. 2007, 31, 425–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhage, A.; van Wees, S.C.; Pieterse, C.M. Plant immunity: It’s the hormones talking, but what do they say? Plant Physiol. 2010, 154, 536–540. [Google Scholar] [CrossRef] [PubMed]
- Paponov, I.A.; Teale, W.D.; Trebar, M.; Blilou, I.; Palme, K. The PIN auxin efflux facilitators: Evolutionary and functional perspectives. Trends Plant Sci. 2005, 10, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Pattison, R.J.; Catalá, C. Evaluating auxin distribution in tomato (Solanum lycopersicum) through an analysis of the PIN and AUX/LAX gene families. Plant J. 2012, 70, 585–598. [Google Scholar] [CrossRef]
- Zouine, M.; Fu, Y.; Chateigner-Boutin, A.-L.; Mila, I.; Frasse, P.; Wang, H.; Audran, C.; Roustan, J.-P.; Bouzayen, M. Characterization of the tomato ARF gene family uncovers a multi-levels post-transcriptional regulation including alternative splicing. PLoS ONE 2014, 9, e84203. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Eisenreich, A.; Schneider, K.; Heinemeyer, I.; Junge, H. Comparative analysis of the complete genome sequence of the plant growth–promoting bacterium Bacillus amyloliquefaciens FZB42. Nat. Biotechnol. 2007, 25, 1007–1014. [Google Scholar] [CrossRef] [Green Version]
- Posada, L.F.; Alvarez, J.C.; Romero-Tabarez, M.; de-Bashan, L.; Villegas-Escobar, V. Enhanced molecular visualization of root colonization and growth promotion by Bacillus subtilis EA-CB0575 in different growth systems. Int. J. Microbiol. Res. 2018, 217, 69–80. [Google Scholar] [CrossRef]
- De Souza, R.; Ambrosini, A.; Passaglia, L. Plant growth promoting-bacteria as inoculants in agricultural soils. Genet. Mol. Biol. 2015, 38, 401–419. [Google Scholar] [CrossRef]
- Vacheron, J.; Desbrosses, G.; Bouffaud, M.L.; Touraine, B.; Moënne-Loccoz, Y.; Muller, D.; Prigent-Combaret, C. Plant growth-promoting rhizobacteria and root system functioning. Front. Plant Sci. 2013, 4, 356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Zhang, Z.; Ling, N.; Yuan, Y.; Zheng, X.; Shen, B.; Shen, Q. Bacillus subtilis SQR 9 can control Fusarium wilt in cucumber by colonizing plant roots. Biol. Fertil. Soils 2011, 47, 495–506. [Google Scholar] [CrossRef]
- Hassen, A.I.; Labuschagne, N. Root colonization and growth enhancement in wheat and tomato by rhizobacteria isolated from the rhizoplane of grasses. World J. Microbiol. Biotechnol. 2010, 26, 1837–1846. [Google Scholar] [CrossRef]
- Fan, B.; Chen, X.H.; Budiharjo, A.; Bleiss, W.; Vater, J.; Borriss, R. Efficient colonization of plant roots by the plant growth promoting bacterium Bacillus amyloliquefaciens FZB42, engineered to express green fluorescent protein. J. Biotechnol. 2011, 151, 303–311. [Google Scholar] [CrossRef]
- Liu, X.; Zhao, H.; Chen, S. Colonization of maize and rice plants by strain Bacillus megaterium C4. Curr. Microbiol. 2006, 52, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Chen, X.H.; Koumoutsi, A.; Scholz, R.; Schneider, K.; Vater, J.; Süssmuth, R.; Borriss, R. Genome analysis of Bacillus amyloliquefaciens FZB42 reveals its potential for biocontrol of plant pathogens. J. Biotechnol. 2009, 140, 27–37. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Liao, M.-J.; Wang, H.-K.; Zheng, M.-Z.; Xu, J.-J.; Guo, J.-H. Bacillus velezensis, a potential and efficient biocontrol agent in control of pepper gray mold caused by Botrytis cinerea. Biol. Control 2018, 126, 147–157. [Google Scholar] [CrossRef]
- Edgar, C.I.; McGrath, K.C.; Dombrecht, B.; Manners, J.M.; Maclean, D.C.; Schenk, P.M.; Kazan, K. Salicylic acid mediates resistance to the vascular wilt pathogen Fusarium oxysporum in the model host Arabidopsis thaliana. Australas. Plant Pathol. 2006, 35, 581–591. [Google Scholar] [CrossRef]
- Liu, H.; Carvalhais, L.C.; Kazan, K.; Schenk, P.M. Development of marker genes for jasmonic acid signaling in shoots and roots of wheat. Plant Signal Behav. 2016, 11, e1176654. [Google Scholar] [CrossRef]
- Tjamos, S.E.; Flemetakis, E.; Paplomatas, E.J.; Katinakis, P. Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. Mol. Plant Microbe Interact. 2005, 18, 555–561. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Han, X.; Feng, D.; Yuan, D.; Huang, L.J. Signaling crosstalk between salicylic acid and ethylene/jasmonate in plant defense: Do we understand what they are whispering? Int. J. Mol. Sci. 2019, 20, 671. [Google Scholar] [CrossRef] [Green Version]
- García-Gutiérrez, L.; Zeriouh, H.; Romero, D.; Cubero, J.; de Vicente, A.; Pérez-García, A. UMAF6639 elicits ISR via JA and SA signalling. Microb. Biotechnol. 2013, 6, 264–274. [Google Scholar] [CrossRef] [Green Version]
- Lakshmanan, V.; Castaneda, R.; Rudrappa, T.; Bais, H.P. Root transcriptome analysis of Arabidopsis thaliana exposed to beneficial Bacillus subtilis FB17 rhizobacteria revealed genes for bacterial recruitment and plant defense independent of malate efflux. Planta 2013, 238, 657–668. [Google Scholar] [CrossRef]
- van Wees, S.C.; Van der Ent, S.; Pieterse, C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008, 11, 443–448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jourdan, E.; Henry, G.; Duby, F.; Dommes, J.; Barthélemy, J.P.; Thonart, P.; Ongena, M. Insights into the defense-related events occurring in plant cells following perception of surfactin-type lipopeptide from Bacillus subtilis. Mol. Plant Microbe Interact. 2009, 22, 456–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magno-Pérez-Bryan, M.C.; Martínez-García, P.M.; Hierrezuelo, J.; Rodríguez-Palenzuela, P.; Arrebola, E.; Ramos, C.; de Vicente, A.; Pérez-García, A.; Romero, D. Comparative genomics within the Bacillus genus reveal the singularities of two robust Bacillus amyloliquefaciens biocontrol strains. Mol. Plant Microbe Interact. 2015, 10, 1102–1116. [Google Scholar] [CrossRef] [Green Version]
- Cawoy, H.; Debois, D.; Franzil, L.; de Pauw, E.; Thonart, P.; Ongena, M. Lipopeptides as main ingredients for inhibition of fungal phytopathogens by Bacillus subtilis/amyloliquefaciens. Microb. Biotechnol. 2015, 8, 281–295. [Google Scholar] [CrossRef]
- Jendoubi, W.; Harbaoui, K.; Hamada, D. Salicylic acid-induced resistance against Fusarium oxysporum f.sp radices-lycopercisi in hydroponic grown tomato plants. J. New Sci. 2015, 21, 985–995. [Google Scholar]
- Spoel, S.H.; Koornneef, A.; Claessens, S.M.; Korzelius, J.P.; Van Pelt, J.A.; Mueller, M.J.; Buchala, A.J.; Métraux, J.P.; Brown, R.; Kazan, K.; et al. NPR1 modulates cross-talk between salicylate- and jasmonate-dependent defense pathways through a novel function in the cytosol. Plant Cell 2003, 15, 760–770. [Google Scholar] [CrossRef] [Green Version]
- Romero, D.; Pérez-García, A.; Rivera, M.E.; Cazorla, F.M.; De Vicente, A. Isolation and evaluation of antagonistic bacteria towards the cucurbit powdery mildew fungus Podosphaera fusca. Appl. Microbiol. Biotechnol. 2004, 64, 263–269. [Google Scholar] [CrossRef]
- Hwang, S.F.; Gossen, B.D.; Chang, K.F.; Turnbull, G.D.; Howard, R.J. Effect of seed damage and metalaxyl seed treatment on pythium seedling blight and seed yield of field pea. Can. J. Plant Pathol. 2001, 81, 509–517. [Google Scholar] [CrossRef]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef]
- Vakalounakis, D.J.; Fragkiadakis, G.A. Genetic diversity of Fusarium oxysporum isolates from cucumber: Differentiation by pathogenicity, vegetative compatibility, and RAPD fingerprinting. Phytopathology 1999, 89, 161–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aimé, S.; Alabouvette, C.; Steinberg, C.; Olivain, C. The endophytic strain Fusarium oxysporum Fo47: A good candidate for priming the defense responses in tomato roots. Mol. Plant Microbe Interact. 2013, 26, 918–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
| Treatment | Pathogen | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Fusarium oxysporum f.sp. radicis lycopersici | Pythium ultimum | Rhizoctonia solani | |||||||
| Colony Diameter (mm) | Relative Inhibition | Inhibition Zone a | Colony Diameter (mm) | Relative Inhibition | Inhibition Zone | Colony Diameter (mm) | Relative Inhibition | Inhibition Zone | |
| Control (Pathogen) | 70b b | 0b | - | 90b | 0b | - | 70b | 0b | - |
| Bs MBI600 + Pathogen | 25a | 64.1a | ++ | 65a | 27.8a | - | 65a | 7.2a | + |
| Growing System | Days after Application a | ||
|---|---|---|---|
| 5 | 15 | 20 | |
| Gnotobiotic system | 2 × 105 b b B c | 1.3 × 103 bc B | 3 × 102 c A |
| Commercial Peat mixture | 3.2 × 105 b B | 4 × 102 c A | 2.5 × 102 c A |
| Vegetable soil | 4 × 104 b A | 2 × 103 bc B | 1.7 × 102 c A |
| Hydroponic cubes | 3 × 105 b B | 2.4 × 103 bc B | 4 × 102 c A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samaras, A.; Roumeliotis, E.; Ntasiou, P.; Karaoglanidis, G. Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants 2021, 10, 1113. https://doi.org/10.3390/plants10061113
Samaras A, Roumeliotis E, Ntasiou P, Karaoglanidis G. Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants. 2021; 10(6):1113. https://doi.org/10.3390/plants10061113
Chicago/Turabian StyleSamaras, Anastasios, Efstathios Roumeliotis, Panagiota Ntasiou, and George Karaoglanidis. 2021. "Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens" Plants 10, no. 6: 1113. https://doi.org/10.3390/plants10061113
APA StyleSamaras, A., Roumeliotis, E., Ntasiou, P., & Karaoglanidis, G. (2021). Bacillus subtilis MBI600 Promotes Growth of Tomato Plants and Induces Systemic Resistance Contributing to the Control of Soilborne Pathogens. Plants, 10(6), 1113. https://doi.org/10.3390/plants10061113







