Evaluation of Mouthwash Containing Citrus hystrix DC., Moringa oleifera Lam. and Azadirachta indica A. Juss. Leaf Extracts on Dental Plaque and Gingivitis
Abstract
1. Introduction
2. Results
2.1. Total Phenolic Content
2.2. Trolox Equivalent Antioxidant Capacity (TEAC)
2.3. GC-MS Analysis of Chemical Constituents in Crude Leaf Ethanolic Extracts
2.4. Quality Control Assessment of Formulated Mouthwashes
2.5. Baseline Information of Participants
2.6. GI and PI Scores from Baseline to Day 15
2.7. Change in Number of Oral Microbial Colonies
3. Discussion
4. Materials and Methods
4.1. Plant Preparation and Extraction
4.2. Determination of Total Phenolic Content by Folin-Ciocalcteu Method
4.3. Determination of Trolox Equivalent Antioxidant Capacity (TEAC)
4.4. Gas Chromatography-Mass Spectrometry Analysis (GC-MS)
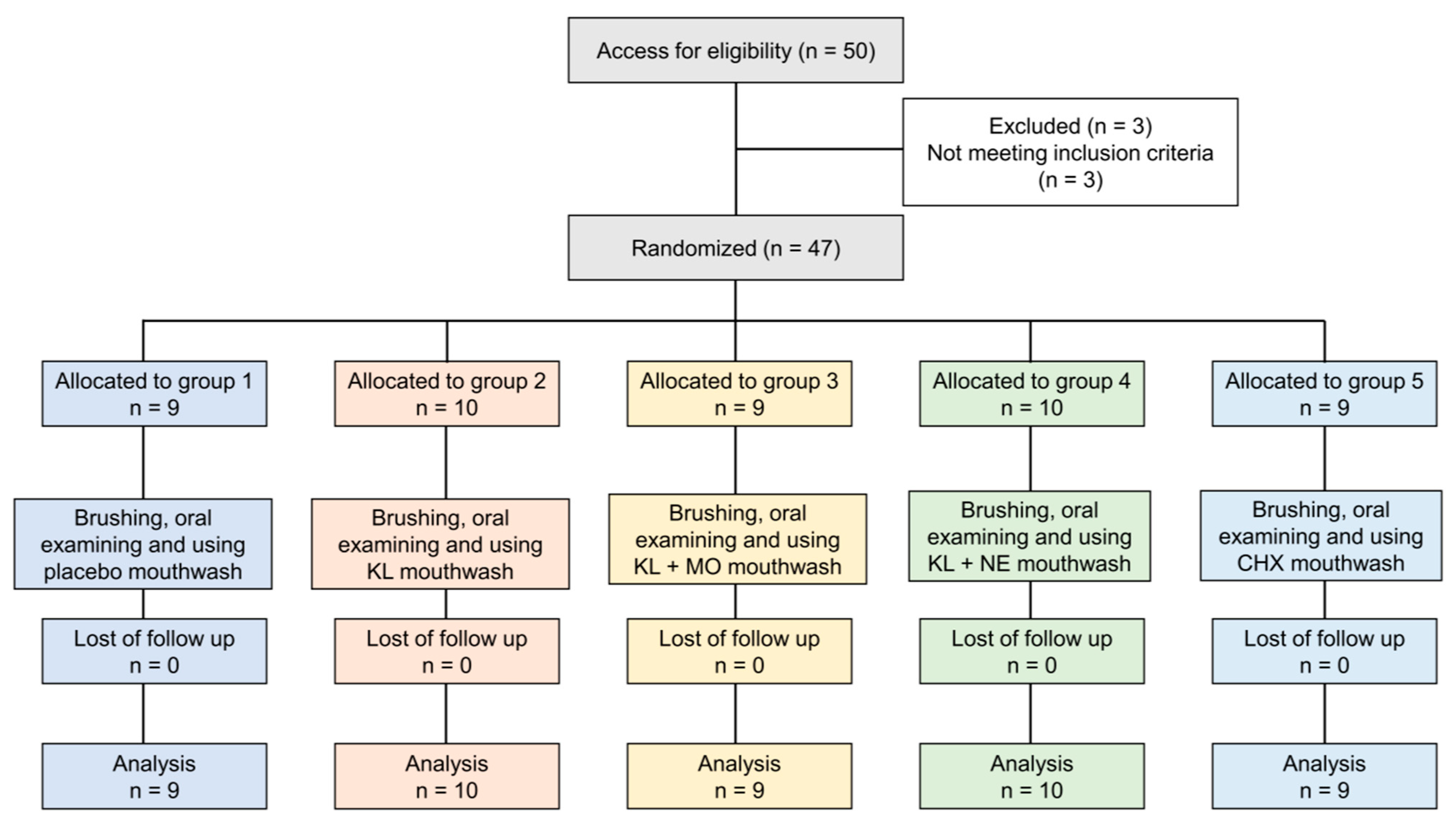
4.5. Study Design
4.6. Participants
4.7. Interventions
4.8. Mouthwash Descriptions
4.9. Assessment and Outcome
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-M.; An, J. Cytokines, inflammation, and pain. Int. Anesthesiol. Clin. 2007, 45, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Schett, G.; Neurath, M.F. Resolution of chronic inflammatory disease: Universal and tissue-specific concepts. Nat. Commun. 2018, 9, 3261. [Google Scholar] [CrossRef] [PubMed]
- Hasturk, H.; Kantarci, A.; Van Dyke, T.E. Oral inflammatory diseases and systemic inflammation: Role of the macrophage. Front. Immunol. 2012, 3, 118. [Google Scholar] [CrossRef]
- Löe, H. The Gingival Index, the Plaque Index and the Retention Index Systems. J. Periodontol. 1967, 38, 610–616. [Google Scholar] [CrossRef]
- Kinane, D.F.; Stathopoulou, P.G.; Papapanou, P.N. Periodontal diseases. Nat. Rev. 2017, 3, 17–38. [Google Scholar] [CrossRef] [PubMed]
- Murakami, S.; Mealey, B.L.; Mariotti, A.; Chapple, I.L.C. Dental plaque–induced gingival conditions. J. Clin. Periodontol. 2018, 45, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Adams, D.; Addy, M. Mouthrinses. Adv. Dent. Res. 1994, 8, 291–301. [Google Scholar] [CrossRef] [PubMed]
- Safiaghdam, H.; Oveissi, V.; Bahramsoltani, R.; Farzaei, M.H.; Rahimi, R. Medicinal plants for gingivitis: A review of clinical trials. Iran J. Basic Med. Sci. 2018, 21, 978–991. [Google Scholar] [PubMed]
- Buakaew, W.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Nuengchamnong, N.; Suphrom, N.; Usuwanthim, K. Phytochemical Constituents of Citrus hystrix DC. Leaves Attenuate Inflammation via NF-κB Signaling and NLRP3 Inflammasome Activity in Macrophages. Biomolecules 2021, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Luetragoon, T.; Pankla Sranujit, R.; Noysang, C.; Thongsri, Y.; Potup, P.; Suphrom, N.; Nuengchamnong, N.; Usuwanthim, K. Bioactive Compounds in Moringa oleifera Lam. Leaves Inhibit the Pro-Inflammatory Mediators in Lipopolysaccharide-Induced Human Monocyte-Derived Macrophages. Molecules 2020, 25, 191. [Google Scholar] [CrossRef] [PubMed]
- Kooltheat, N.; Kamuthachad, L.; Anthapanya, M.; Samakchan, N.; Sranujit, R.P.; Potup, P.; Ferrante, A.; Usuwanthim, K. Kaffir lime leaves extract inhibits biofilm formation by Streptococcus mutans. Nutrition 2016, 32, 486–490. [Google Scholar] [CrossRef]
- Lee, J.-W.; Ryu, H.W.; Park, S.-Y.; Park, H.A.; Kwon, O.-K.; Yuk, H.J.; Shrestha, K.K.; Park, M.; Kim, J.H.; Lee, S.; et al. Protective effects of neem (Azadirachta indica A. Juss.) leaf extract against cigarette smoke- and lipopolysaccharide-induced pulmonary inflammation. Int. J. Mol. Med. 2017, 40, 1932–1940. [Google Scholar] [CrossRef] [PubMed]
- Jiang, K.; Chen, L.-L.; Wang, S.-F.; Wang, Y.; Li, Y.; Gao, K. Anti-inflammatory Terpenoids from the Leaves and Twigs of Dysoxylum gotadhora. J. Nat. Prod. 2015, 78, 1037–1044. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.-Q.; Wang, S.-F.; Li, Y.; Song, Q.-Y.; Gao, K. Terpenoids with anti-inflammatory activity from Abies chensiensis. Fitoterapia 2016, 111, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.P.; Islam, T.; Santos, P.S.; Ferreira, P.B.; Oliveira, G.L.S.; Alencar, M.V.O.B.; Paz, M.F.C.J.; Ferreira, L.F.; Feitosa, C.M.; Citó, A.M.G.L.; et al. Evaluation of Antioxidant Activity of Phytol Using Non-and Pre-Clinical Models. Curr. Pharm. Biotechnol. 2016, 17, 1278–1284. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.O.; Sousa, F.B.M.; Damasceno, S.R.B.; Carvalho, N.S.; Silva, V.G.; Oliveira, F.R.M.A.; Sousa, D.P.; Aragão, K.S.; Barbosa, A.L.; Freitas, R.M.; et al. Phytol, a diterpene alcohol, inhibits the inflammatory response by reducing cytokine production and oxidative stress. Fundam. Clin. Pharmacol. 2014, 28, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Woo, E.-R.; Lee, D.G. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic. Res. 2016, 50, 1309–1318. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.M.S.; Heimfarth, L.; Pereira, E.W.M.; Oliveira, F.S.; Menezes, I.R.A.; Coutinho, H.D.M.; Picot, L.; Antoniolli, A.R.; Quintans, L.; Quintans-Júnior, L.J. Phytol, a Chlorophyll Component, Produces Antihyperalgesic, Anti-inflammatory, and Antiarthritic Effects: Possible NFκB Pathway Involvement and Reduced Levels of the Proinflammatory Cytokines TNF-α and IL-6. J. Nat. Prod. 2020, 83, 1107–1117. [Google Scholar] [CrossRef]
- Abbas, M.H.; Al-Yaseen, A.; Alhamadi, W. Prevalence of Staphylococcus Aureus among gingivitis in patient with orthodontic wires in Kufa City/Iraq. Pak. J. Biotechnol. 2017, 14, 91–96. [Google Scholar]
- Rams, T.E.; Feik, D.; Slots, J. Staphylococci in human periodontal diseases. Oral. Microbiol. Immunol. 1990, 5, 29–32. [Google Scholar] [CrossRef] [PubMed]
- McCarney, R.; Warner, J.; Iliffe, S.; van Haselen, R.; Griffin, M.; Fisher, P. The Hawthorne Effect: A randomised, controlled trial. BMC Med. Res. Methodol. 2007, 7, 30. [Google Scholar] [CrossRef] [PubMed]
- Marsh, P.D. Dental plaque as a biofilm and a microbial community—Implications for health and disease. BMC Oral Health 2006, 6 (Suppl. S1), S14. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Rao, R.S.; Majumdar, B.; Anil, S. Clinical Appearance of Oral Candida Infection and Therapeutic Strategies. Front. Microbiol. 2015, 6, 1391. [Google Scholar] [CrossRef]
- Alzohairy, M.A. Therapeutics Role of Azadirachta indica (Neem) and Their Active Constituents in Diseases Prevention and Treatment. Evid. Based Complement. Altern. Med. 2016, 2016, 7382506. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Al-Toubi, W.A.S.; Weli, A.M.; Al-Riyami, Q.A.; Al-Sabahi, J.N. Identification and characterization of chemical compounds in different crude extracts from leaves of Omani neem. J. Taibah Univ. Sci. 2013, 7, 181–188. [Google Scholar] [CrossRef]
- Chatterjee, A.; Saluja, M.; Singh, N.; Kandwal, A. To evaluate the antigingivitis and antipalque effect of an Azadirachta indica (neem) mouthrinse on plaque induced gingivitis: A double-blind, randomized, controlled trial. J. Indian Soc. Periodontol. 2011, 15, 398–401. [Google Scholar] [CrossRef] [PubMed]
- Elgamily, H.; Moussa, A.; Elboraey, A.; El-Sayed, H.; Al-Moghazy, M.; Abdalla, A. Microbiological Assessment of Moringa Oleifera Extracts and Its Incorporation in Novel Dental Remedies against Some Oral Pathogens. Open Access Maced J. Med. Sci. 2016, 4, 585–590. [Google Scholar] [CrossRef] [PubMed]
- Kooltheat, N.; Sranujit, R.P.; Chumark, P.; Potup, P.; Laytragoon-Lewin, N.; Usuwanthim, K. An ethyl acetate fraction of Moringa oleifera Lam. Inhibits human macrophage cytokine production induced by cigarette smoke. Nutrients 2014, 6, 697–710. [Google Scholar] [CrossRef] [PubMed]
- Agouillal, F.; Moghrani, H.; Nasrallah, N.; El Enshasy, H. A Review of Genetic Taxonomy, Biomolecules Chemistry and Bioactivities of Citrus hystrix DC. Biosci. Biotechnol. Res. Asia 2017, 14, 285–305. [Google Scholar] [CrossRef]
- Singh, A.; Negi, M.S.; Rajagopal, J.; Bhatia, S.; Tomar, U.K.; Srivastava, P.S.; Lakshmikumaran, M. Assessment of genetic diversity in Azadirachta indica using AFLP markers. Theor. Appl. Genet. 1999, 99, 272–279. [Google Scholar] [CrossRef]
- Rufai, S.; Hanafi, M.M.; Rafii, M.Y.; Ahmad, S.; Arolu, I.W.; Ferdous, J. Genetic Dissection of New Genotypes of Drumstick Tree (Moringa oleifera Lam.) Using Random Amplified Polymorphic DNA Marker. BioMed Res. Int. 2013, 2013, 604598. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, E.A.; Gillespie, K.M. Estimation of total phenolic content and other oxidation substrates in plant tissues using Folin–Ciocalteu reagent. Nat. Protoc. 2007, 2, 875–877. [Google Scholar] [CrossRef]
- Rahman, M.M.; Islam, M.B.; Biswas, M.; Khurshid Alam, A.H.M. In vitro antioxidant and free radical scavenging activity of different parts of Tabebuia pallida growing in Bangladesh. BMC Res. Notes 2015, 8, 621. [Google Scholar] [CrossRef] [PubMed]
- Kadam, P.; Bhalerao, S. Sample size calculation. Int. J. Ayurveda Res. 2010, 1, 55–57. [Google Scholar] [PubMed]

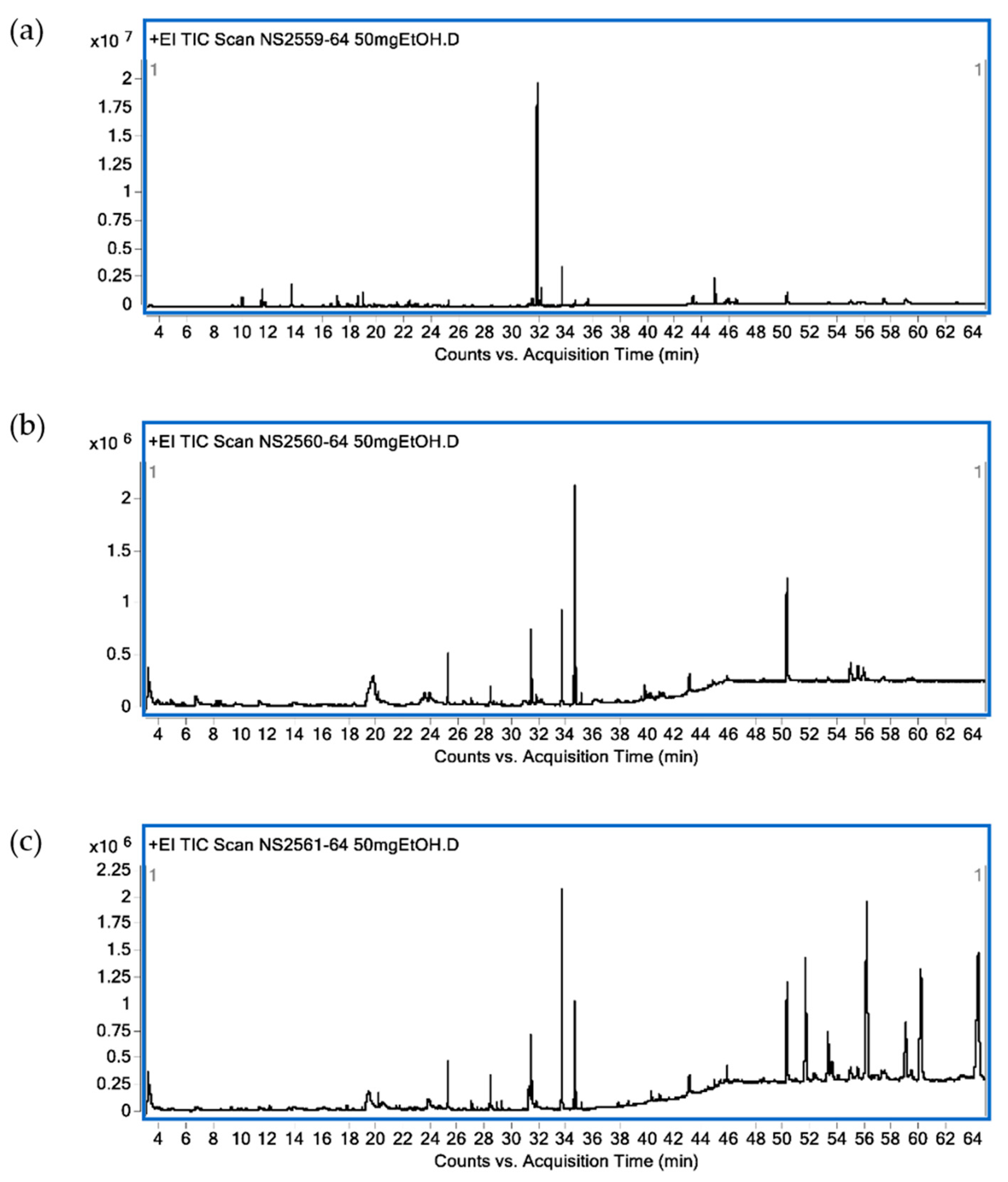

| RT (min) | Identified Compounds | Classification | Relative Area (%) | ||
|---|---|---|---|---|---|
| C. hystrix | M. oleifera | A. indica | |||
| 9.29 | trans-Linalool oxide | Monoterpene | 0.26 | - | - |
| 10.00 | β-Linalool | Monoterpene | 1.61 | - | - |
| 11.36 | Isopulegol | Monoterpene | 1.06 | - | - |
| 11.51 | Citronellal | Monoterpene | 3.16 | - | - |
| 11.68 | Isopregol | Monoterpene | 0.71 | - | - |
| 13.63 | Citronellol | Monoterpene | 4.28 | - | - |
| 14.37 | cis-Geraniol | Monoterpene | 0.17 | - | - |
| 16.01 | Citronellic acid | Monoterpene | 0.31 | - | - |
| 16.56 | 2-(2-Hydroxy-2-propanyl)-5-methylcyclohexanol | Monoterpene | 0.59 | - | - |
| 17.02 | Citronellol acetate | Monoterpene | 1.94 | - | - |
| 17.15 | Menthoglycol | Monoterpene | 0.25 | - | - |
| 17.75 | Copaene | Sesquiterpene | 0.50 | - | 0.15 |
| 17.97 | Ethoxycitronellal | Monoterpene | 0.18 | - | - |
| 18.51 | (2E)-1-Ethoxy-3,7-dimethyl-2,6-octadiene | Monoterpene | 1.88 | - | - |
| 18.91 | β-Caryophyllene | Sesquiterpene | 2.64 | - | - |
| 19.33 | Sucrose | Disaccharide | 0.75 | 24.20 | 4.66 |
| 19.77 | α-Caryophyllene | Sesquiterpene | 0.40 | - | - |
| 20.1 | 1-Dodecanol | Fatty alcohol | - | 0.91 | 0.58 |
| 21.43 | δ-Cadinene | Sesquiterpene | 0.78 | - | - |
| 22.05 | Elemol | Sesquiterpene | 0.20 | - | - |
| 22.31 | trans-Nerolidol | Sesquiterpene | 1.08 | - | - |
| 22.77 | (-)-Spathulenol | Sesquiterpene | 0.38 | - | - |
| 22.92 | Caryophyllene oxide | Sesquiterpene | 0.41 | - | - |
| 23.70 | Ethyl α-d-glucopyranoside | Glycoside | - | 1.59 | 1.31 |
| 25.20 | Dodecyl acrylate | Fatty ester | 0.95 | 4.24 | 1.70 |
| 28.30 | Phytol acetate | Diterpene | - | 1.42 | 1.26 |
| 29.17 | 3,7,11,15-Tetramethyl-2-hexadecen-1-ol | Diterpene | - | - | 0.31 |
| 30.47 | Isophytol | Diterpene | 0.13 | - | - |
| 31.35 | Ethyl palmitate | Fatty acid ethyl ester | 1.21 | 6.09 | 2.45 |
| 31.72 | Lauryl 3-mercaptopropionate | Fatty acid ester | - | 0.59 | 0.21 |
| 31.75 | Tetraprenol | Diterpene | 47.42 | - | - |
| 32.00 | trans-Geranylgeraniol | Diterpene | 3.22 | - | - |
| 33.57 | Phytol | Diterpene | 6.57 | 8.39 | 7.56 |
| 34.44 | Ethyl-9,12-octadecadienoate | Fatty acid ethyl ester | 0.32 | 2.33 | 0.30 |
| 34.56 | Ethyl linolenate | Fatty acid ethyl ester | 1.05 | 18.89 | 3.85 |
| 34.99 | Ethyl stearate | Fatty acid ethyl ester | 0.22 | 0.88 | 0.23 |
| 40.15 | Glycerol β-palmitate | Fatty acid ester | - | - | 0.46 |
| 42.97 | α-Glyceryl linolenate | Fatty acid ester | - | 1.82 | 1.01 |
| 43.5 | 4-(2,3-Dihydroxy-3-methylbutoxy)furo(3,2-g)chromen-7-one | Furanocoumarin | 0.52 | - | - |
| 44.84 | Squalene | Triterpene | 4.12 | - | 0.21 |
| 50.14 | α-Tocopherol | Triterpene | 3.26 | 14.80 | 5.87 |
| 51.52 | Astaxanthin | Tetraterpene | - | - | 8.56 |
| 53.2 | Stigmasterol | Sterol | 0.54 | - | 3.97 |
| 54.83 | β-Sitosterol | Sterol | 1.57 | 3.79 | 1.29 |
| 55.36 | 24-n-propylidenecholesterol | Sterol | - | 3.11 | 0.66 |
| 55.44 | Dihydrolanosterol | Triterpene | 0.93 | - | - |
| 55.82 | β-Amyrin | Triterpene | - | 2.82 | - |
| 56.05 | Phorbol | Diterpene | - | - | 16.81 |
| 57.31 | Lupeol | Triterpene | 2.10 | - | - |
| 58.91 | Olean-12-ene-3,15,16,21,22,28-hexol | Triterpene | - | - | 5.37 |
| 58.92 | Cycloeucalenol acetate | Triterpene | 2.32 | - | - |
| 60.03 | 14,15β-epoxy-3β,5-dihydroxy-5β-Bufa-20,22-dienolide | Diterpene | - | - | 12.28 |
| 64.25 | Olean-12-ene-3,16,21,22,23,28-hexol | Triterpene | - | - | 18.94 |
| Test Parameters | Test Procedure | Specification | Mouthwash | ||
|---|---|---|---|---|---|
| KL | KL + MO | KL + NE | |||
| Turbidity | Organoleptic | Transparent | Transparent | Transparent | Transparent |
| Color | Organoleptic | Green | Green | Green | Green |
| pH | pH Meter | 5.5–8.5 | 6.6 | 6.7 | 6.8 |
| Lead (Pb) | Based on AOAC (2016) method | <10.0 ppm | Not detected | Not detected | Not detected |
| Arsenic (As) | Based on AOAC (2016) method | <4.0 ppm | <0.5 ppm | Not detected | <0.5 ppm |
| Mercury (Hg) | Based on AOAC (2016) method | <0.5 ppm | Not detected | Not detected | Not detected |
| Clostridium spp. | USP 41 <62> | Absent/1 g. | Absent/1 g. | Absent/1 g. | Absent/1 g. |
| Staphylococcus aureus | USP 41 <62> | Absent/1 g. | Absent/1 g. | Absent/1 g. | Absent/1 g. |
| Pseudomonas aeruginosa | USP 41 <62> | Absent/1 g. | Absent/1 g. | Absent/1 g. | Absent/1 g. |
| Candida albicans | USP 41 <62> | Absent/1 g. | Absent/1 g. | Absent/1 g. | Absent/1 g. |
| Parameters | Placebo (n = 9) | KL (n = 10) | KL + MO (n = 9) | KL + NE (n = 10) | CHX (n = 9) | p-Value |
|---|---|---|---|---|---|---|
| Age (years) | 23.11 ± 4.43 | 21.40 ± 1.71 | 22.89 ± 5.33 | 23.90 ± 8.14 | 26.44 ± 10.42 | 0.6749 a |
| GI score | 1.21 ± 0.50 | 0.88 ± 0.58 | 1.18 ± 0.54 | 1.03 ± 0.63 | 1.35 ± 0.48 | 0.228 a |
| PI score | 1.63 ± 0.77 | 1.08 ± 0.51 | 1.63 ± 0.65 | 1.33 ± 0.52 | 1.45 ± 0.74 | 0.371 a |
| Sex, Female/Male | 8/1 | 6/4 | 6/3 | 6/4 | 5/4 |
| Microorganisms | Intervals | Placebo | KL | KL + MO | KL + NE | CHX |
|---|---|---|---|---|---|---|
| Staphylococcus spp. | Baseline | 21 ± 38 | 91 ± 188 | 62 ± 69 | 132 ± 148 | 28 ± 39 |
| Day 15 | 78 ± 114 | 109 ± 176 | 46 ± 87 | 83 ± 161 | 27 ± 48 | |
| Accumulative reduction percentage | 12.66 | 30.14 | 45.53 | 33.23 | 54.11 | |
| Candida spp. | Baseline | 1 ± 0 | 0 ± 1 | 1 ± 2 | 1 ± 1 | 8 ± 23 |
| Day 15 | 1 ± 0 | 1 ± 3 | 0 ± 0 | 1 ± 1 | 2 ± 5 | |
| Accumulative reduction percentage | 0 | 0 | 20 | 35.83 | 17.63 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Buakaew, W.; Sranujit, R.P.; Noysang, C.; Sangouam, S.; Suphrom, N.; Thongsri, Y.; Potup, P.; Usuwanthim, K. Evaluation of Mouthwash Containing Citrus hystrix DC., Moringa oleifera Lam. and Azadirachta indica A. Juss. Leaf Extracts on Dental Plaque and Gingivitis. Plants 2021, 10, 1153. https://doi.org/10.3390/plants10061153
Buakaew W, Sranujit RP, Noysang C, Sangouam S, Suphrom N, Thongsri Y, Potup P, Usuwanthim K. Evaluation of Mouthwash Containing Citrus hystrix DC., Moringa oleifera Lam. and Azadirachta indica A. Juss. Leaf Extracts on Dental Plaque and Gingivitis. Plants. 2021; 10(6):1153. https://doi.org/10.3390/plants10061153
Chicago/Turabian StyleBuakaew, Watunyoo, Rungnapa Pankla Sranujit, Chanai Noysang, Supaporn Sangouam, Nungruthai Suphrom, Yordhathai Thongsri, Pachuen Potup, and Kanchana Usuwanthim. 2021. "Evaluation of Mouthwash Containing Citrus hystrix DC., Moringa oleifera Lam. and Azadirachta indica A. Juss. Leaf Extracts on Dental Plaque and Gingivitis" Plants 10, no. 6: 1153. https://doi.org/10.3390/plants10061153
APA StyleBuakaew, W., Sranujit, R. P., Noysang, C., Sangouam, S., Suphrom, N., Thongsri, Y., Potup, P., & Usuwanthim, K. (2021). Evaluation of Mouthwash Containing Citrus hystrix DC., Moringa oleifera Lam. and Azadirachta indica A. Juss. Leaf Extracts on Dental Plaque and Gingivitis. Plants, 10(6), 1153. https://doi.org/10.3390/plants10061153








