Determination of Fertility-Related Traits in Muscadine Grape Population
Abstract
:1. Introduction
2. Results and Discussion
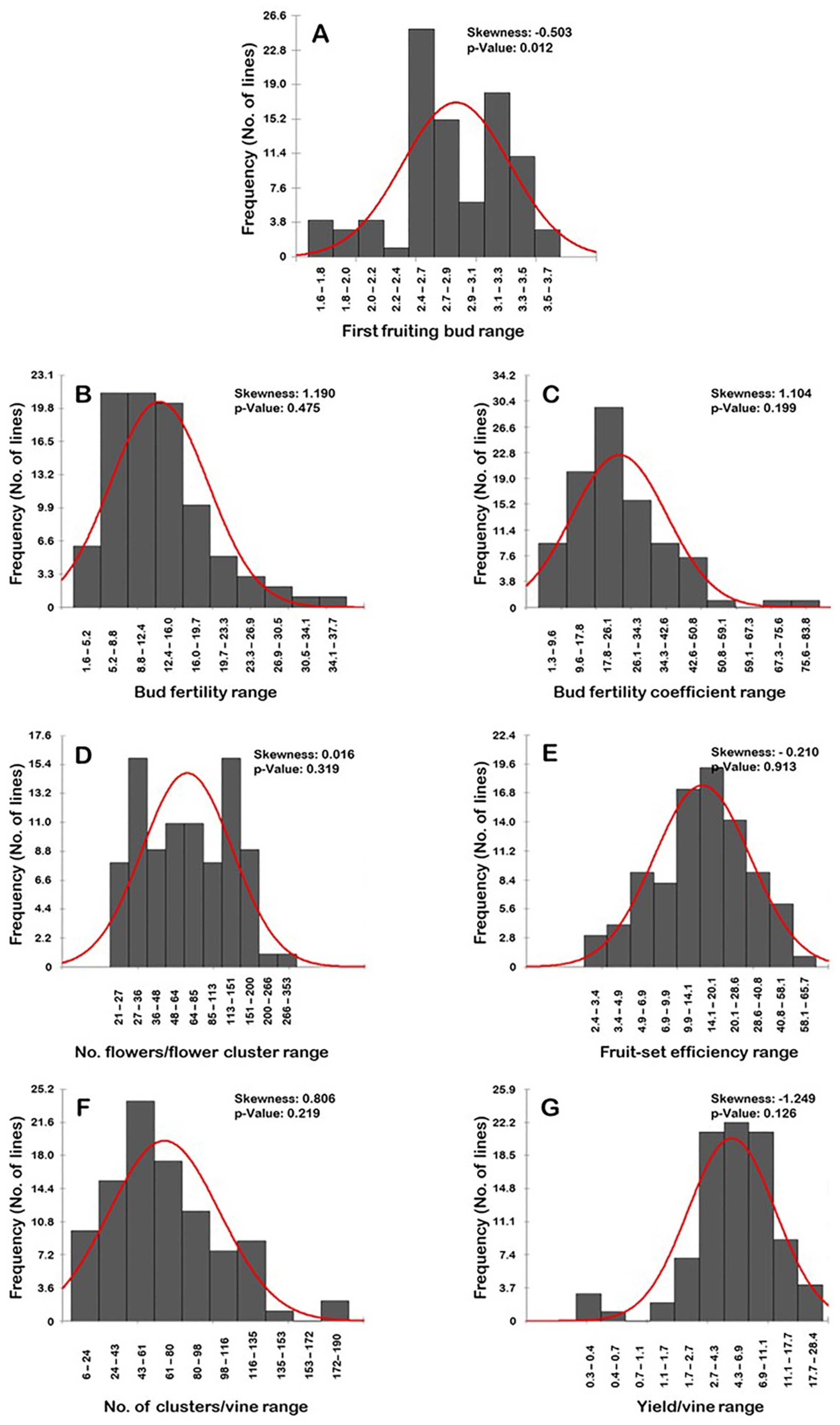
2.1. First Fruiting Bud Trait
2.2. Bud Fertility Trait
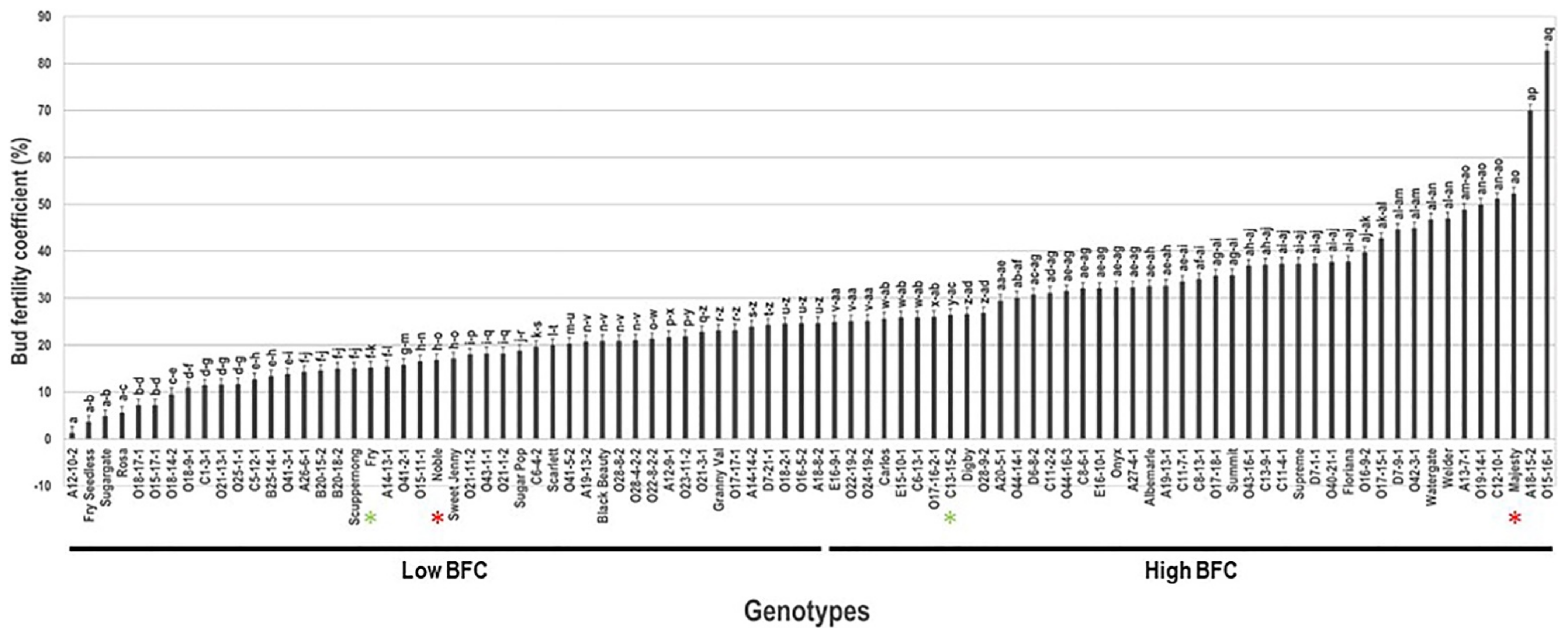
2.3. Bud Fertility Coefficient Trait
2.4. Number of Flowers/Flower Cluster
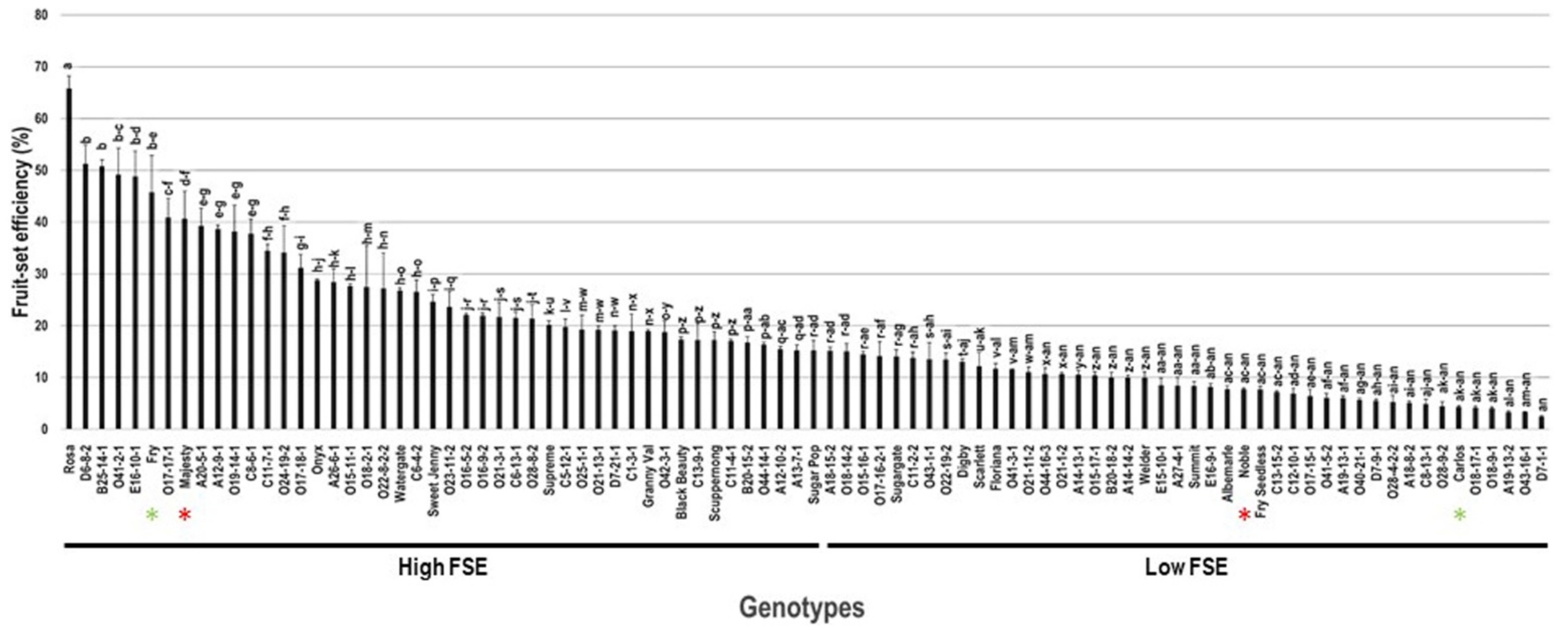
2.5. Fruit-Set Efficiency
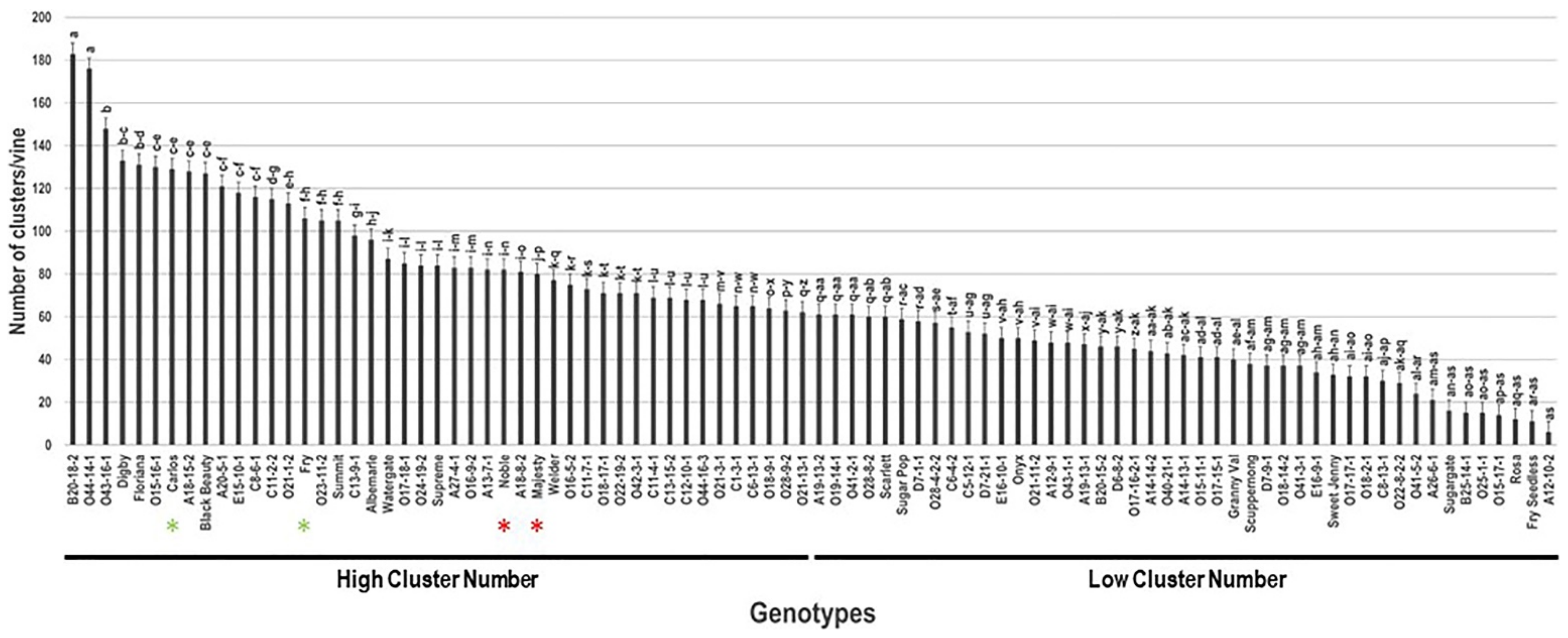
2.6. Number of Clusters/Vine (N.C/V)
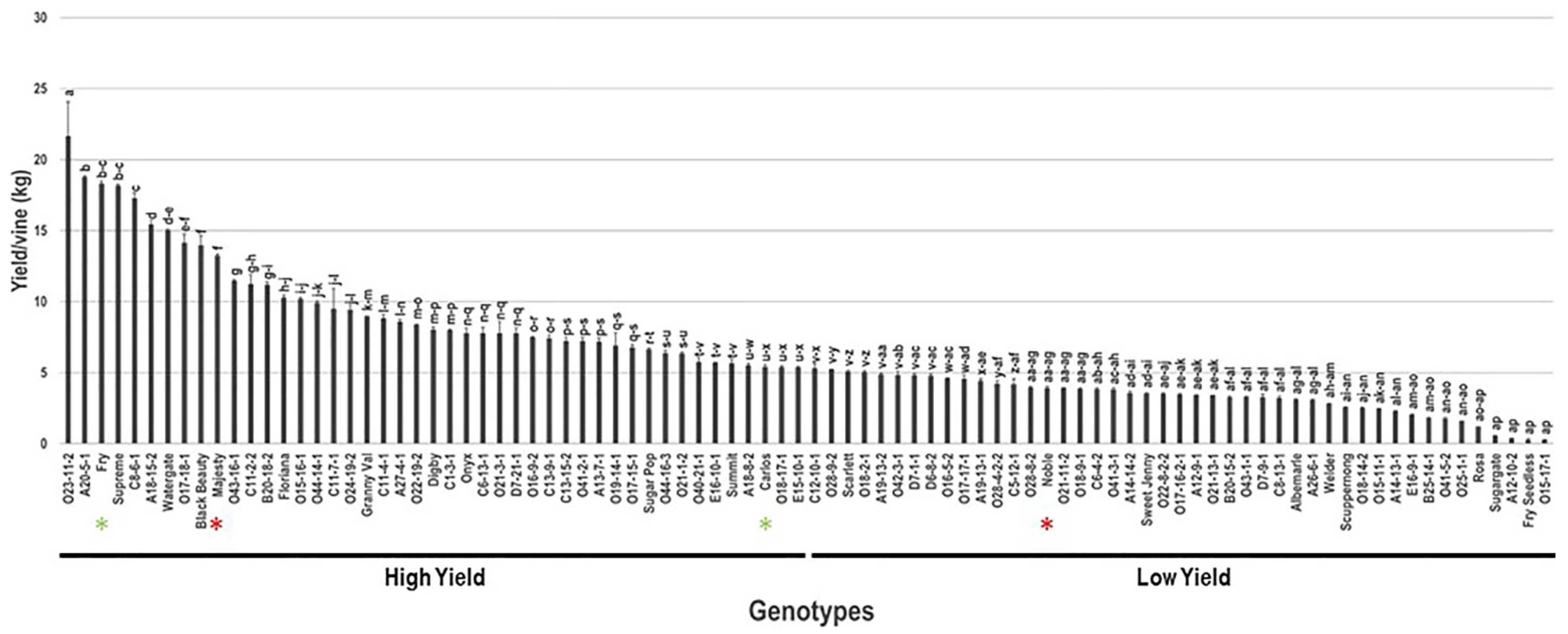
2.7. Yield/Vine (Y/V)
2.8. Distribution Frequency
2.9. Cross-Correlation Analysis between Muscadine Traits
3. Materials and Methods
3.1. Plant Material
3.2. Fertility-Related Traits
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Boss, P.K.; Thomas, M.R. Tendrils, inflorescences and fruitfulness: A molecular perspective. Aust. J. Grape Wine Res. 2000, 6, 168–174. [Google Scholar] [CrossRef]
- Jones, G.V.; White, M.A.; Cooper, O.R.; Storchmann, K. Climate change and global wine quality. Clim. Chang. 2005, 73, 319–343. [Google Scholar] [CrossRef]
- Lobell, D.B.; Field, C.B.; Cahill, K.N.; Bonfils, C. Impacts of future climate change on California perennial crop yields: Model projections with climate and crop uncertainties. Agric. For. Meteorol. 2006, 141, 208–218. [Google Scholar] [CrossRef] [Green Version]
- White, M.A.; Diffenbaugh, N.; Jones, G.V.; Pal, J.; Giorgi, F. Extreme heat reduces and shifts United States premium wine production in the 21st century. Proc. Natl. Acad. Sci. USA 2006, 103, 11217–11222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olien, W.C.; Hegwood, C.P. Muscadine—A classic southeastern fruit. HortScience 1990, 25, 726–831. [Google Scholar] [CrossRef] [Green Version]
- Sandhu, A.K.; Gu, L. Antioxidant capacity, phenolic content, and profiling of phenolic compounds in the seeds, skin, and pulp of Vitis rotundifolia (muscadine grapes) as determined by HPLC-DAD-ESI-MS n. J. Agric. Food Chem. 2010, 58, 4681–4692. [Google Scholar] [CrossRef] [PubMed]
- Fanizza, G.; Lamaj, F.; Costantini, L.; Chaabane, R.; Grando, M. QTL analysis for fruit yield components in table grapes (Vitis vinifera). Theor. Appl. Genet. 2005, 111, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Jackson, R.S. Wine Science: Principles and Applications; Academic Press: Cambridge, MA, USA, 2020; pp. 947–978. [Google Scholar] [CrossRef]
- Boss, P.K.; Thomas, M.R. Association of dwarfism and floral induction with a grape ‘green revolution’ mutation. Nature 2002, 416, 847–850. [Google Scholar] [CrossRef] [PubMed]
- Boss, P.K.; Buckeridge, E.J.; Poole, A.; Thomas, M.R. New insights into grapevine flowering. Funct. Plant Biol. 2003, 30, 593–606. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, C.; Mullins, M.G. Physiology of flowering in the grapevine—A review. Am. J. Enol. Vitic. 1981, 32, 47–63. [Google Scholar]
- Xylem, P. Bud development and fruitfulness of grapevines. Raisin Prod. Man. 2000, 3393, 24. [Google Scholar]
- Shikhamany, S. Physiology and cultural practices to produce seedless grapes in tropical environments. In Proceedings of the Congresso Brasileiro de Viticultura e Enologia, Bento Goncalves, RS, Brasil, 7–10 December 1999; Volume 9, pp. 43–48. [Google Scholar]
- Leão, P.C.d.S.; Silva, E.E.G.d. Brotação e fertilidade de gemas em uvas sem sementes no Vale do São Francisco. Rev. Bras. Frutic. 2003, 25, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Doligez, A.; Bertrand, Y.; Dias, S.; Grolier, M.; Ballester, J.-F.; Bouquet, A.; This, P. QTLs for fertility in table grape (Vitis vinifera L.). Tree Genet. Genomes 2010, 6, 413–422. [Google Scholar] [CrossRef]
- Campbell, J.; Sarkhosh, A.; Habibi, F.; Ismail, A.; Gajjar, P.; Zhongbo, R.; Tsolova, V.; El-Sharkawy, I. Biometrics assessment of cluster- and berry-related traits of muscadine grape population. Plants 2021, 10, 1067. [Google Scholar] [CrossRef] [PubMed]
- Campbell, J.; Sarkhosh, A.; Habibi, F.; Gajjar, P.; Ismail, A.; Tsolova, V.; El-Sharkawy, I. Evaluation of biochemical juice attributes and color-related traits in muscadine grape population. Foods 2021, 10, 1101. [Google Scholar] [CrossRef] [PubMed]
- Mendonca, P.; Darwish, A.G.; Tsolova, V.; El-Sharkawy, I.; Soliman, K.F. The anticancer and antioxidant effects of muscadine grape extracts on racially different triple-negative breast cancer cells. Anticancer Res. 2019, 39, 4043–4053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, J.E.; Kerslake, F.; Dambergs, R.; Close, D.C. Spur pruning leads to distinctly different phenolic profiles of base sparkling wines than cane pruning. Vitis 2018, 57, 103–109. [Google Scholar] [CrossRef]
- Coombs, W.T. Choosing the right words: The development of guidelines for the selection of the “appropriate” crisis-response strategies. Manag. Commun. Q. 1995, 8, 447–476. [Google Scholar] [CrossRef]
- Friendly, M. Corrgrams: Exploratory displays for correlation matrices. Am. Stat. 2002, 56, 316–324. [Google Scholar] [CrossRef]




| Genotype | Bud Fertility (%) | Bud Fertility Coefficient (%) | No. Flowers/Flower Cluster | Fruit-Set Efficiency | No. Clusters/Vine | Yield/Vine (kg) | Berry Weight (g) | Cluster Weight (g) |
|---|---|---|---|---|---|---|---|---|
| Carlos | 16.7 ± 0.6 | 25.6 ± 1.3 | 186.0 ± 19.1 | 4.3 ± 0.2 | 129 ± 3.9 | 5.4 ± 0.3 | 6.7 ± 0.5 | 41.9 ± 2.9 |
| Noble | 15.6 ± 0.5 | 16.8 ± 0.8 | 158.7 ± 6.5 | 7.8 ± 0.2 | 82 ± 2.5 | 3.9 ± 0.2 | 4.2 ± 0.3 | 47.8 ± 3.3 |
| Fry | 6.0 ± 0.2 | 15.3 ± 0.8 | 32.7 ± 5.9 | 45.7 ± 7.1 | 106 ± 3.2 | 18.3 ± 0.9 | 12.7 ± 0.9 | 172.8 ± 12.1 |
| Majesty | 34.0 ± 1.2 | 52.3 ± 2.6 | 21.7 ± 4.0 | 40.6 ± 5.3 | 80 ± 2.4 | 13.3 ± 0.7 | 27.6 ± 1.9 | 165.7 ± 11.6 |
| A18-15-2 | 19.7 ± 0.7 | 69.9 ± 3.5 | 75.3 ± 7.8 | 15.1 ± 0.8 | 128 ± 3.8 | 15.4 ± 0.8 | 13.3 ± 0.9 | 120.5 ± 8.4 |
| A20-5-1 | 6.3 ± 0.2 | 29.4 ± 1.5 | 43.7 ± 5.9 | 39.2 ± 3.4 | 121 ± 3.6 | 18.8 ± 0.9 | 9.4 ± 0.7 | 155.2 ± 10.9 |
| B20-18-2 | 9.2 ± 0.3 | 15.0 ± 0.8 | 134.3 ± 23.2 | 10.0 ± 1.0 | 183 ± 5.5 | 11.2 ± 0.6 | 5.2 ± 0.4 | 61.1 ± 4.3 |
| C8-6-1 | 9.4 ± 0.3 | 32.0 ± 1.6 | 57.7 ± 8.5 | 37.7 ± 2.9 | 116 ± 3.5 | 17.3 ± 0.9 | 8.6 ± 0.6 | 149.1 ± 10.4 |
| O15-16-1 | 15.9 ± 0.6 | 82.8 ± 4.1 | 252.0 ± 20.0 | 14.4 ± 0.6 | 130 ± 3.9 | 10.2 ± 0.5 | 3.3 ± 0.2 | 78.3 ± 5.5 |
| O23-11-2 | 7.7 ± 0.3 | 21.9 ± 1.1 | 67.7 ± 4.7 | 23.6 ± 2.9 | 105 ± 3.2 | 22.5 ± 1.1 | 14.1 ± 1.0 | 214.8 ± 15.0 |
| O43-16-1 | 16.7 ± 0.6 | 36.9 ± 1.8 | 194 ± 8.2 | 3.2 ± 0.2 | 148 ± 4.4 | 11.5 ± 0.6 | 15.4 ± 1.2 | 77.5 ± 5.4 |
| O44-14-1 | 15.2 ± 0.5 | 30.1 ± 1.5 | 83.7 ± 1.5 | 16.4 ± 0.4 | 176 ± 5.3 | 9.9 ± 0.5 | 4.6 ± 0.3 | 56.4 ± 3.9 |
| Average * | 12.9 ± 6.5 | 26.4 ± 13.8 | 84.1 ± 56.5 | 18.7 ± 13.3 | 67.7 ± 36.9 | 6.6 ± 4.5 | 12.2 ± 5.6 | 96.9 ± 42.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, J.; Gajjar, P.; Ismail, A.; Habibi, F.; Darwish, A.G.; Tsolova, V.; Sarkhosh, A.; El-Sharkawy, I. Determination of Fertility-Related Traits in Muscadine Grape Population. Plants 2021, 10, 1175. https://doi.org/10.3390/plants10061175
Campbell J, Gajjar P, Ismail A, Habibi F, Darwish AG, Tsolova V, Sarkhosh A, El-Sharkawy I. Determination of Fertility-Related Traits in Muscadine Grape Population. Plants. 2021; 10(6):1175. https://doi.org/10.3390/plants10061175
Chicago/Turabian StyleCampbell, Jiovan, Pranavkumar Gajjar, Ahmed Ismail, Fariborz Habibi, Ahmed G. Darwish, Violeta Tsolova, Ali Sarkhosh, and Islam El-Sharkawy. 2021. "Determination of Fertility-Related Traits in Muscadine Grape Population" Plants 10, no. 6: 1175. https://doi.org/10.3390/plants10061175
APA StyleCampbell, J., Gajjar, P., Ismail, A., Habibi, F., Darwish, A. G., Tsolova, V., Sarkhosh, A., & El-Sharkawy, I. (2021). Determination of Fertility-Related Traits in Muscadine Grape Population. Plants, 10(6), 1175. https://doi.org/10.3390/plants10061175







