Assessment of Nutritional and Quality Properties of Leaves and Musts in Three Local Spanish Grapevine Varieties Undergoing Controlled Climate Change Scenarios
Abstract
:1. Introduction
2. Results
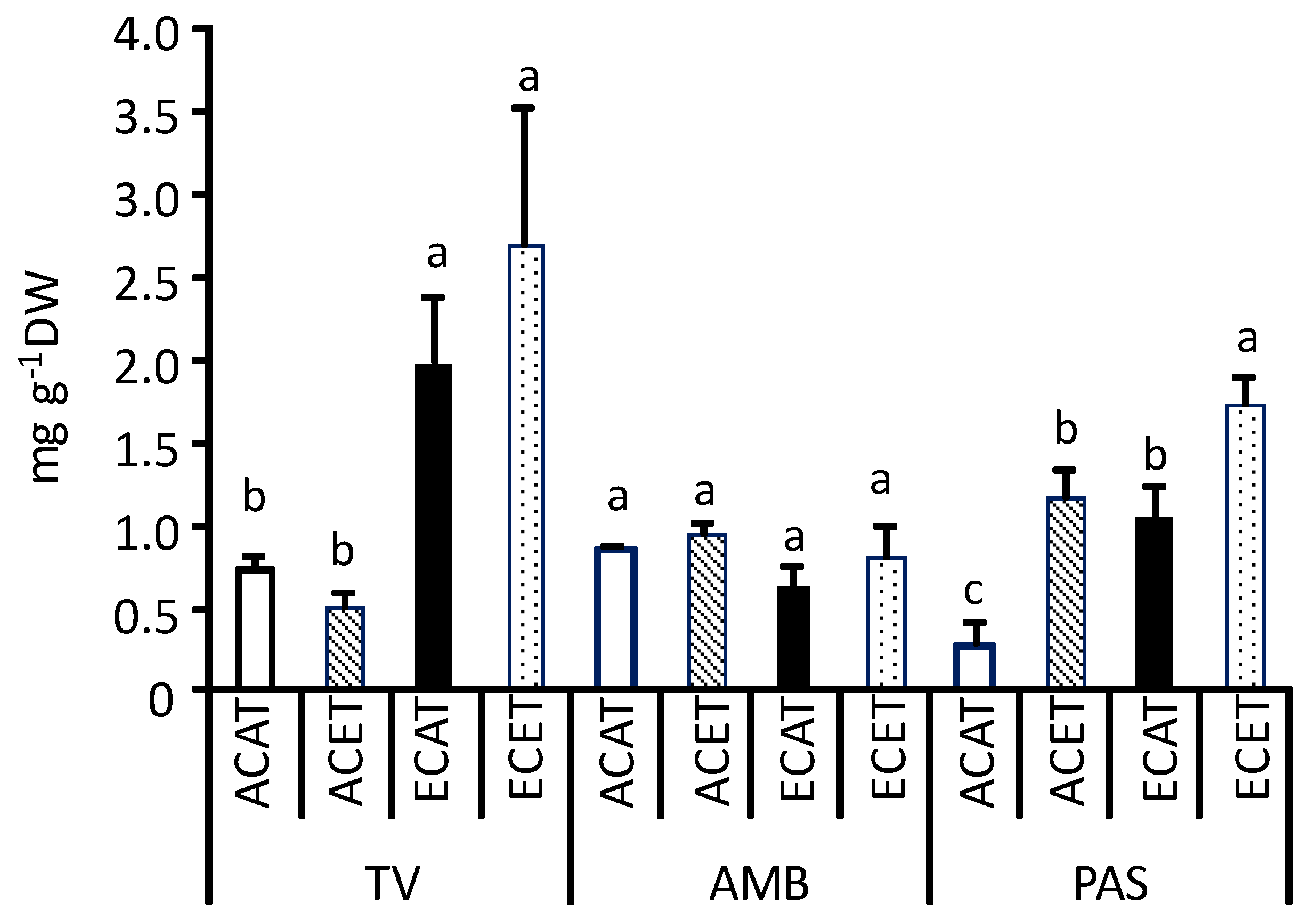
2.1. Nutritional Traits of Leaves
2.2. Quality Traits of Must
3. Discussion
3.1. Nutritional Traits of Leaves
3.2. Quality Traits of Must
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Experimental Design
4.3. Harvest of Leaves and Determinations of Metabolites
4.4. Harvest of Berries and Quality Determinations
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunet, M.; Saladié, P.D.O.; Jones, P.D.; Sigró, J.; Aguilar, E.; Moberg, A.; Lister, D.; Walther, A.; López, D.; Almarza, C. The development of a new dataset of Spanish daily adjusted temperature series (SDATS) (1850–2003). Int. J. Climatol. 2006, 26, 1777–1802. [Google Scholar] [CrossRef]
- IPCC Intergovernmental Panel on Climate Change. Global warming of 1.5 °C. In An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development and Efforts to Eradicate Poverty; Masson-Delmotte, T.W.V., Zhai, P., Pörtner, H.O., Masson-Delmotte, V., Zhai, P., Pörtner, H.O., Roberts, D., Skea, J., Shukla, P.R., Pirani, A., et al., Eds.; IPCC: Geneva, Switzerland, 2018; Available online: www.ipcc.ch (accessed on 7 March 2021).
- Urrestarazu, J.; Miranda, C.; Santesteban, L.G.; Royo, J.B. Recovery and identification of grapevine varieties cultivated in old vineyards from Navarre (Northeastern Spain). Sci. Hortic. 2015, 191, 65–73. [Google Scholar] [CrossRef]
- Loureiro, M.D.; Moreno-Sanz, P.; Suárez, B. Agronomical characterization of minority grapevine cultivars from Asturias (Spain). Ciéncia Téc. Vitiv. 2017, 32, 102–114. [Google Scholar] [CrossRef]
- Jiménez, C.; Peiró, R.; Yuste, A.; García, J.; Martínez-Gil, F.; Gisbert, C. Looking for old grapevine varieties. Vitis 2019, 58, 59–60. [Google Scholar]
- Ocete, C.A.; Arroyo, R.; Lovicu, G.; Rodríguez-Miranda, A.; Valle, J.M.; Cantos, M.; García, J.L.; Lara, M.; González de Canales, F.; Llompart, J.; et al. An inventory of the relic Eurasian wild grapevine populational nuclei in Huelva province (Andalusia, Spain). Vitis 2019, 58, 53–57. [Google Scholar]
- Jiménez-Cantizano, A.; Muñoz-Martín, A.; Amores-Arrocha, A.; Sancho-Galán, P.; Palacios, V. Identification of red grapevine cultivars (Vitis vinifera L.) preserved in ancient vineyards in Axarquia (Andalusia, Spain). Plants 2020, 9, 1572. [Google Scholar] [CrossRef]
- Venios, X.; Korkas, E.; Nisiotou, A.; Banilas, G. Grapevine responses to heat stress and global warming. Plants 2020, 9, 1754. [Google Scholar] [CrossRef]
- Antolín, M.C.; Toledo, M.; Pascual, I.; Irigoyen, J.J.; Goicoechea, N. The exploitation of local Vitis vinifera L. biodiversity as a valuable tool to cope with climate change maintaining berry quality. Plants 2021, 10, 71. [Google Scholar] [CrossRef]
- Kizildeniz, T.; Mekni, I.; Santesteban, H.; Pascual, I.; Morales, F.; Irigoyen, J.J. Effects of climate change including elevated CO2 concentration, temperature and water deficit on growth, water status, and yield quality of grapevine (Vitis vinifera L.) cultivars. Agric. Water Manag. 2015, 159, 155–164. [Google Scholar] [CrossRef] [Green Version]
- Gutiérrez-Gamboa, G.; Díaz-Gálvez, I.; Verdugo-Vásquez, N.; Moreno-Simunovic, Y. Leaf-to-fruit ratios in Vitis vinifera L.cv. ‘Sauvignon Blanc’, ‘Carmenère’, ‘Cabernet Sauvignon’, and ‘Syrah’ growing in Maule Valley (Chile): Influence on yield and fruit composition. Agriculture 2019, 9, 176. [Google Scholar] [CrossRef] [Green Version]
- Alem, H.; Torregrosa, L.; Rigou, P.; Schneider, R.; Ojeda, H. Effect of the plant sink/source balance on the metabolic content of the Vitis vinifera L. red grape. Eur. J. Agron. 2021, 122, 126168. [Google Scholar] [CrossRef]
- Stoll, M.; Bischo-Schaefer, M.; Lafontaine, M.; Tittmann, S.; Henschke, J. Impact of various leaf area modifications on berry maturation in Vitis vinifera L. cv. Riesling. Acta Hortic. 2013, 978, 293–299. [Google Scholar] [CrossRef]
- Gurbuz, Y. Determination of nutritive value of leaves of several Vitis vinifera varieties as a source of alternative feedstuff for sheep using in vitro and in situ measurements. Small Rum. Res. 2007, 71, 59–66. [Google Scholar] [CrossRef]
- Andelković, M.; Radovanović, B.; Milenkovic, A.A.; Radovanović, V. Phenolic compounds and bioactivity of healthy and infected grapevine leaf extracts from red varieties Merlot and Vranac (Vitis vinifera L.). Plant Food Hum. Nutr. 2015, 70, 317–323. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Marques, A.P.; Torres, V.M.; Bernardes Silva, A.; Matos, A.R.; Cordeiro, C.; Figueiredo, A.; Sousa Silva, M. Vitis vinifera ‘Pinot noir’ leaves as a source of bioactive nutraceutical compounds. Food Funct. 2019, 10, 3822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harb, J.; Alseekh, S.; Tohge, T.; Fernie, A.R. Profiling of primary metabolites and flavonols in leaves of two table grape varieties collected from semiarid and temperate regions. Phytochemistry 2015, 117, 444–455. [Google Scholar] [CrossRef]
- Lima, A.; Bento, A.; Baraldi, I.; Malheiro, R. Selection of grapevine leaf varieties for culinary process based on phytochemical composition and antioxidant properties. Food Chem. 2016, 212, 291–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, A.; Pereira, J.A.; Baraldi, I.; Malheiro, R. Cooking impact in color, pigments and volatile composition of grapevine leaves (Vitis vinifera L. var. Malvasia Fina and Touriga Franca). Food Chem. 2017, 221, 1197–1205. [Google Scholar] [CrossRef] [Green Version]
- Nassiri-Asl, M.; Hosseinzadeh, H. Review of the pharmacological effects of Vitis vinifera (Grape) and its bioactive compounds. Phytother. Res. 2009, 23, 1197–1204. [Google Scholar] [CrossRef]
- Lacerda, D.S.; Costa, P.C.; Funchal, C.; Dani, C.; Gomez, R. Benefits of vine leaf on different biological systems, in grape and wine biotechnology. IntechOpen 2016, 6, 125–143. [Google Scholar]
- Loizzo, M.R.; Sicari, V.; Pellicanò, T.; Xiao, J.; Poiana, M.; Tundis, R. Comparative analysis of chemical composition, antioxidant and anti-proliferative activities of Italian Vitis vinifera by-products for a sustainable agro-industry. Food Chem. Toxicol. 2019, 127, 127–134. [Google Scholar] [CrossRef]
- Torres, N.; Antolín, M.C.; Garmendia, I.; Goicoechea, N. Nutritional properties of Tempranillo grapevine leaves are affected by clonal diversity, mycorrhizal symbiosis and air temperature regime. Plant Physiol. Biochem. 2018, 130, 542–554. [Google Scholar] [CrossRef]
- Torres, N.; Plano, D.; Antolín, M.C.; Sanmartín, C.; Domínguez-Fernández, M.; De Peña, M.-P.; Encío, I.; Goicoechea, N. Potential biomedical reuse of vegetative residuals from mycorrhized grapevines subjected to warming. Arch. Agron. Soil Sci. 2019, 65, 1341–1353. [Google Scholar] [CrossRef]
- Moreno, D.; Valdés, E.; Uriarte, D.; Gamero, E.; Talaverano, I.; Vilanova, M. Early leaf removal applied in warm climatic conditions: Impact on Tempranillo wine volatiles. Food Res. Int. 2017, 98, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Kizildeniz, T.; Pascual, I.; Irigoyen, J.J.; Morales, F. Future CO2, warming and water deficit impact white and red Tempranillo grapevine: Photosynthetic acclimation to elevated CO2 and biomass allocation. Physiol. Plant. 2021. [Google Scholar] [CrossRef]
- Lebon, G.; Wojnarowiez, G.; Holzapfel, B.; Fontaine, F.; Vaillant-Gaveau, N.; Clément, C. Sugars and flowering in the grapevine (Vitis vinifera L.). J. Exp. Bot. 2008, 59, 2565–2578. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stoev, K.; Ivantchev, V. Données nouvelles sur le problème de la translocation descendante et ascendante des produits de la photosynthèse de la vigne. Vitis 1977, 16, 253–262. [Google Scholar]
- Aranjuelo, I.; Sanz-Sáez, A.; Jauregui, I.; Irigoyen, J.J.; Araus, J.L.; Sánchez-Díaz, M.; Erice, G. Harvest index, a parameter conditioning responsiveness of wheat plants to elevated CO2. J. Exp. Bot. 2013, 64, 1879–1892. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Jiang, Y.; Zhou, G. Response and adaption of photosynthesis, respiration, and antioxidant systems to elevated CO2 with environmental stress in plants. Front. Plant Sci. 2015, 6, 701. [Google Scholar] [CrossRef] [Green Version]
- Leisner, C.P. Review: Climate change impacts on food security- focus on perennial cropping systems and nutritional value. Plant Sci. 2020, 293, 110412. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Morales, F.; Sánchez-Díaz, M.; Delrot, S.; Aguirreolea, J.; Gomès, E.; Pascual, I. Climate change conditions (elevated CO2 and temperature) and UV-B radiation affect grapevine (Vitis vinifera cv. Tempranillo) leaf carbon assimilation, altering fruit ripening rates. Plant Sci. 2015, 236, 168–176. [Google Scholar] [CrossRef]
- Ozden, M.; Demirel, U.; Kahraman, A. Effects of proline on antioxidant system in leaves of grapevine (Vitis vinifera L.) exposed to oxidative stress by H2O2. Sci. Hortic. 2009, 119, 163–168. [Google Scholar] [CrossRef]
- Aarti, P.D.; Tanaka, R.; Tanaka, A. Effects of oxidative stress on chlorophyll biosynthesis in cucumber (Cucumis sativus) cotyledons. Physiol. Plant. 2006, 128, 186–197. [Google Scholar] [CrossRef]
- Fernandes, T.M.; Gomes, B.B.; Lanfer-Marquez, U.M. Apparent absorption of chlorophyll from spinach in an assay with dogs. Innov. Food Sci. Emerg. 2007, 8, 426–432. [Google Scholar] [CrossRef]
- Rao, A.V.; Rao, L.G. Carotenoids and human health. Pharmacol. Res. 2007, 55, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Vagiri, M.; Johansson, E.; Rumpunen, K. Phenolic compounds in black currant leaves—An interaction between the plant and foliar diseases? J. Plant Interact. 2017, 12, 193–199. [Google Scholar] [CrossRef] [Green Version]
- Nogales, A.; Santos, E.S.; Abreu, M.M.; Arán, D.; Victorino, G.; Pereira, H.S.; Lopes, C.M.; Viegas, W. Mycorrhizal inoculation differentially affects grapevine’s performance in copper contaminated and non-contaminated soils. Front. Plant Sci. 2019, 9, 1906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panzella, L. Natural phenolic compounds for health, food andcosmetic applications. Antioxidants 2020, 9, 427. [Google Scholar] [CrossRef]
- Chang, J.-D.; Mantri, N.; Sun, B.; Jiang, L.; Chen, P.; Jiang, B.; Jiang, Z.; Zhang, J.; Shen, J.; Lu, H.; et al. Effects of elevated CO2 and temperature on Gynostemma pentaphyllum physiology and bioactive compounds. J. Plant Physiol. 2016, 196–197, 41–52. [Google Scholar] [CrossRef]
- Wohlfahrt, Y.; Tittmann, S.; Schmidt, D.; Rauhut, D.; Honermeier, B.; Stoll, M. The effect of elevated CO2 on berry development and bunch structure of Vitis vinifera L. cvs. Riesling and Cabernet Sauvignon. Appl. Sci. 2020, 10, 2486. [Google Scholar] [CrossRef] [Green Version]
- Sweetman, C.; Sadras, V.O.; Hancock, R.D.; Soole, K.L.; Ford, C.M. Metabolic effects of elevated temperature on organic acid degradation in ripening Vitis vinifera fruit. J. Exp. Bot. 2014, 65, 5975–5988. [Google Scholar] [CrossRef]
- Martin, C.; Zhang, Y.; Tonelli, C.; Petroni, K. Plants, Diet, and Health. Annu. Rev. Plant Biol. 2013, 64, 19–46. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, H.M.; Rentzsch, M.; Breuer, M. Anthocyanins reduce fungal growth in fruits. Nat. Prod. Commun. 2008, 3, 1267–1272. [Google Scholar] [CrossRef] [Green Version]
- Arrizabalaga-Arriazu, M.; Gomès, E.; Morales, F.; Irigoyen, J.; Pascual, I.; Hilbert, G. High temperature and elevated carbón dioxide modify berry composition of different clones of grapevine (Vitis vinifera L.) cv. Tempranillo. Front. Plant Sci. 2020, 11, 603687. [Google Scholar] [CrossRef]
- Caminero, L.; Villar, C.A.; Ostiz, I.O.; Navarra, E. Cepas Singulares de Navarra. 2013. Available online: https://www.navarraagraria.com/categories/item/403-cepas-singulares-de-navarra-prospeccion-yrecopilacion-del-vinedo-de-esta-region (accessed on 15 January 2020).
- Mullins, M.G. Test-plants for investigations of the physiology of fruiting in Vitis vinifera L. Nature 1966, 209, 419–420. [Google Scholar] [CrossRef]
- Ollat, N.; Gény, L.; Soyer, J.P. Les boutures fructifères de vigne: Validation d’un modèle d’étude de la physiologie de la vigne. I. Principales caractéristiques de l’appareil végétatif. J. Int. Sci. Vigne Vin 1998, 32, 1–9. [Google Scholar]
- Coombe, B.G. Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Morales, F.; Pascual, I.; Sánchez-Díaz, M.; Aguirreolea, J.; Irigoyen, J.J.; Goicoechea, N.; Antolín, M.C.; Oyarzun, M.; Urdiain, A. Methodological advances: Using greenhouses to simulate climate change scenarios. Plant Sci. 2014, 226, 30–40. [Google Scholar] [CrossRef]
- Rawson, H.M.; Gifford, R.M.; Condon, B.N. Temperature gradient chambers for research on global environment change. I. Portable chambers for research on short-stature vegetation. Plant Cell Environ. 1995, 18, 1048–1054. [Google Scholar] [CrossRef]
- Kliewer, W.M.; Dokoozlian, N.K. Leaf area/crop weight ratios of grapevines: Influence on fruit composition and wine quality. Am. J. Enol. Vitic. 2005, 56, 170–181. [Google Scholar]
- Séstak, Z.; Càtsky, J.; Jarvis, P. Plant Photosynthetic Production: Manual of Methods; Dr. W. Junk N.V.: The Hague, The Netherlands, 1971. [Google Scholar]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. In Methods in Enzymology; Colowick, S.P., Kaplan, N.O., Eds.; Academic: San Diego, CA, USA, 1987; pp. 350–382. [Google Scholar]
- Jarvis, C.E.; Walker, J.R.L. Simultaneous, rapid, spectrophotometric determination of total starch, amylose and amylopectin. J. Sci. Food Agric. 1993, 63, 53–57. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Yemm, E.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irigoyen, J.; Emerich, D.W.; Sánchez-Díaz, M. Water stress induced changes in concentrations of proline and total soluble sugars in nodulated alfalfa (Medicago sativa) plants. Physiol. Plant. 1992, 84, 55–60. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.; Contour-Ansel, D.; Bernhard-Reversat, F. High performance liquid chromatography of water-soluble phenolics in leaf litter of three Eucalyptus hybrids (Congo). Plant Sci. 2002, 163, 217–222. [Google Scholar] [CrossRef]
- Waterman, P.T.; Mole, S. Analysis of Phenolic Plant Metabolites; Blackwell Scientific Publication: London, UK, 1994. [Google Scholar]
- OIV. Compendium of International Methods of Analysis of Wines and Musts; International Organisation of Vine and Wine: Paris, France, 2018; Volume 1, ISBN 979-10-91799-80-5. [Google Scholar]
- Saint-Cricq, N.; Vivas, N.; Glories, Y. Maturité phénolique: Définition et contrôle. Rev. Fr. Oenol. 1998, 173, 22–25. [Google Scholar]
- Ribéreau-Gayon, J.; Stonestreet, E. Le dosage des anthocyanes dans le vin rouge. Bull. Soc. Chim. Fr. 1965, 9, 2649–2652. [Google Scholar]
- Nadal, M. Phenolic maturity in red grapes. In Methodologies and Results in Grapevine Research; Delrot, S., Medrano-Gil, H., Or, E., Bavaresco, L., Grando, S., Eds.; Springer Science & Business Media, B.V.: Dordrecht, The Netherlands, 2010; pp. 389–409. [Google Scholar]
- EEC (European Union Commission Regulation) N°2676/90. Community Methods for the Analysis. Off. J. Eur. Comm. 1990, 272, 1–92. [Google Scholar]
- Glories, Y. La couleur des vins rouges. 2e partie: Mesure, origine et interprétation. Connaiss. Vigne Vin 1984, 18, 253–271. [Google Scholar] [CrossRef]
- Kedare, S.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Treatments | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| ACAT | ACET | ECAT | ECET | CO2 | T | CO2 × T | |
| Tinto Velasco (TV) | |||||||
| Total soluble solids (°Brix) | 20.3 a | 18.1 ab | 17.0 b | 16.9 b | * | ns | ns |
| Must pH | 3.95 b | 4.03 ab | 3.84 b | 4.26 a | ns | ** | ns |
| Titratable acidity (g L−1) | 4.85 b | 3.55 ab | 3.05 a | 2.69 c | ns | * | *** |
| Soluble solids/Titratable acidity | 4.30 b | 5.20 ab | 5.66 a | 6.28 a | ns | * | *** |
| Color density | 2.63 a | 2.57 a | 2.53 ab | 1.95 b | ns | ns | ns |
| Tonality index | 0.77 c | 0.92 b | 0.75 c | 1.28 a | *** | *** | *** |
| Ambrosina (AMB) | |||||||
| Total soluble solids (°Brix) | 20.9 | 20.4 | 20.3 | 21.4 | ns | ns | ns |
| Must pH | 3.93 | 3.76 | 3.82 | 4.03 | ns | ns | ns |
| Titratable acidity (g L−1) | 4.64 | 4.88 | 5.38 | 4.15 | ns | ns | ns |
| Soluble solids/Titratable acidity | 4.73 | 4.37 | 4.06 | 5.28 | ns | ns | ns |
| Color density | 2.22 ab | 1.59 b | 2.44 a | 2.40 a | ns | ns | ns |
| Tonality index | 0.78 b | 1.14 a | 0.74 b | 0.84 b | * | ** | * |
| Pasera (PAS) | |||||||
| Total soluble solids (°Brix) | 21.0 a | 21.7 a | 22.9 a | 22.2 a | ns | ns | ns |
| Must pH | 3.91 b | 4.24 a | 4.00 ab | 4.21 a | ns | ** | ns |
| Titratable acidity (g L−1) | 3.94 a | 4.18 a | 4.88 a | 4.68 a | ns | ns | ns |
| Soluble solids/Titratable acidity | 5.46 a | 5.36 a | 4.76 a | 5.09 a | ns | ns | ns |
| Color density | 2.46 a | 2.24 a | 2.18 a | 2.34 a | ns | ns | ns |
| Tonality index | 0.77 b | 0.90 a | 0.80 ab | 0.90 a | ns | ** | ns |
| Treatments | ANOVA | ||||||
|---|---|---|---|---|---|---|---|
| ACAT | ACET | ECAT | ECET | CO2 | T | CO2 × T | |
| Tinto Velasco (TV) | |||||||
| TPI (AU) | 12.3 b | 12.4 b | 22.5 a | 8.6 b | ns | ** | *** |
| Total anthocyanins (mg L−1) | 210 b | 350.8 a | 352.8 a | 148.3 b | ns | ns | *** |
| Extractable anthocyanins (mg L−1) | 157.2 ab | 126.3 b | 182 a | 58.6 c | ns | *** | * |
| EA (%) | 29.4 b | 63.6 a | 49.0 a | 56.8 a | ns | *** | ns |
| SM (%) | 54.0 b | 57.9 ab | 66.4 ab | 71.4 a | * | ns | ns |
| Total antioxidant capacity (mg L−1) | 18.0 a | 119.8 a | 19.7 a | 20.0 a | ns | ns | ns |
| Ambrosina (AMB) | |||||||
| TPI (AU) | 23.1 ab | 20.7 b | 32.4 a | 16.2 b | ns | * | ns |
| Total anthocyanins (mg L−1) | 200.0 a | 98.6 c | 153.8 ab | 133.0 bc | ns | *** | ns |
| Extractable anthocyanins (mg L−1) | 157.4 a | 61.3 c | 152.8 ab | 104.7 bc | ns | *** | ns |
| EA (%) | 22.9 a | 24.8 a | 8.8 bc | 17.3 ab | ** | ns | ns |
| SM (%) | 65.1 | 76.8 | 75.7 | 73.9 | ns | ns | ns |
| Total antioxidant capacity (mg L−1) | 17.4 b | 21.9 ab | 20.0 ab | 22.8 a | ns | * | ns |
| Pasera (PAS) | |||||||
| TPI (AU) | 32.4 a | 29.3 ab | 21.6 b | 20.3 b | * | ns | ns |
| Total anthocyanins (mg L−1) | 155.2 | 155.3 | 156.1 | 152.7 | ns | ns | ns |
| Extractable anthocyanins (mg L−1) | 152.4 | 139.1 | 131.6 | 134.4 | ns | ns | ns |
| EA (%) | 11.4 b | 18.8 b | 30.4 a | 16.0 b | * | ns | ns |
| SM (%) | 77.5 | 80.3 | 66.2 | 67.2 | ns | ns | ns |
| Total antioxidant capacity (mg L−1) | 21.5 | 20.3 | 22.6 | 19.9 | ns | ns | ns |
| Code | Passport | Variety/Local Name | Origin | Genotype | VMC4F3-1 | VVIN16 | VVIV37 | VVIV67 | VVMD27 | VVIP31 | VVS2 | ZAG79 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T73 | CS0073 | Tinto Velasco/unknown | Los Arcos (Navarra) | GEN 0062 | 187 | 206 | 151 | 151 | 158 | 158 | 358 | 375 | 179 | 185 | 184 | 190 | 131 | 131 | 237 | 251 |
| T46 | CS0046 | Ambrosina/Tinta | Los Arcos (Navarra) | GEN 0906 | 167 | 187 | 151 | 153 | 163 | 165 | 362 | 372 | 179 | 194 | 184 | 190 | 131 | 131 | 251 | 251 |
| T85 | CS0085 | Parrel/Pasera | Ablitas (Navarra) | GEN 0101 | 179 | 187 | 153 | 159 | 161 | 171 | 364 | 372 | 179 | 194 | 176 | 180 | 141 | 151 | 251 | 257 |
| Variety | Code | Reproductive Cycle | Bunch Mass (g FW Bunch−1) | Berry Mass (g FW Berry−1) |
|---|---|---|---|---|
| Tinto Velasco (TV) | T73 | Medium | 153 | 2.01 |
| Ambrosina (AMB) | T46 | Long | 205 | 1.38 |
| Pasera (PAS) | T85 | Long | 331 | 2.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goicoechea, N.; Jiménez, L.; Prieto, E.; Gogorcena, Y.; Pascual, I.; Irigoyen, J.J.; Antolín, M.C. Assessment of Nutritional and Quality Properties of Leaves and Musts in Three Local Spanish Grapevine Varieties Undergoing Controlled Climate Change Scenarios. Plants 2021, 10, 1198. https://doi.org/10.3390/plants10061198
Goicoechea N, Jiménez L, Prieto E, Gogorcena Y, Pascual I, Irigoyen JJ, Antolín MC. Assessment of Nutritional and Quality Properties of Leaves and Musts in Three Local Spanish Grapevine Varieties Undergoing Controlled Climate Change Scenarios. Plants. 2021; 10(6):1198. https://doi.org/10.3390/plants10061198
Chicago/Turabian StyleGoicoechea, Nieves, Leyre Jiménez, Eduardo Prieto, Yolanda Gogorcena, Inmaculada Pascual, Juan José Irigoyen, and María Carmen Antolín. 2021. "Assessment of Nutritional and Quality Properties of Leaves and Musts in Three Local Spanish Grapevine Varieties Undergoing Controlled Climate Change Scenarios" Plants 10, no. 6: 1198. https://doi.org/10.3390/plants10061198
APA StyleGoicoechea, N., Jiménez, L., Prieto, E., Gogorcena, Y., Pascual, I., Irigoyen, J. J., & Antolín, M. C. (2021). Assessment of Nutritional and Quality Properties of Leaves and Musts in Three Local Spanish Grapevine Varieties Undergoing Controlled Climate Change Scenarios. Plants, 10(6), 1198. https://doi.org/10.3390/plants10061198








