Ethylene-Mediated Modulation of Bud Phenology, Cold Hardiness, and Hormone Biosynthesis in Peach (Prunus persica)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Location and Plant Materials
2.2. Experimental Design and Treatments
2.3. Chilling and Heat Requirements
2.4. Leaf Abscission Assessments
2.5. Endogenous Ethylene Levels in Leaves and Buds after Ethephon Application
2.6. Evaluation of Bloom Progression
2.7. Evaluation of Fruit Set, Fruit Size and Tree Injury
2.8. Determination of Bud Cold Hardiness
2.9. Quantification of Phytohormones
2.10. RNA Extraction and Gene Expression Analyses
2.11. Data Analyses
3. Results
3.1. Accumulation of CH and GDH
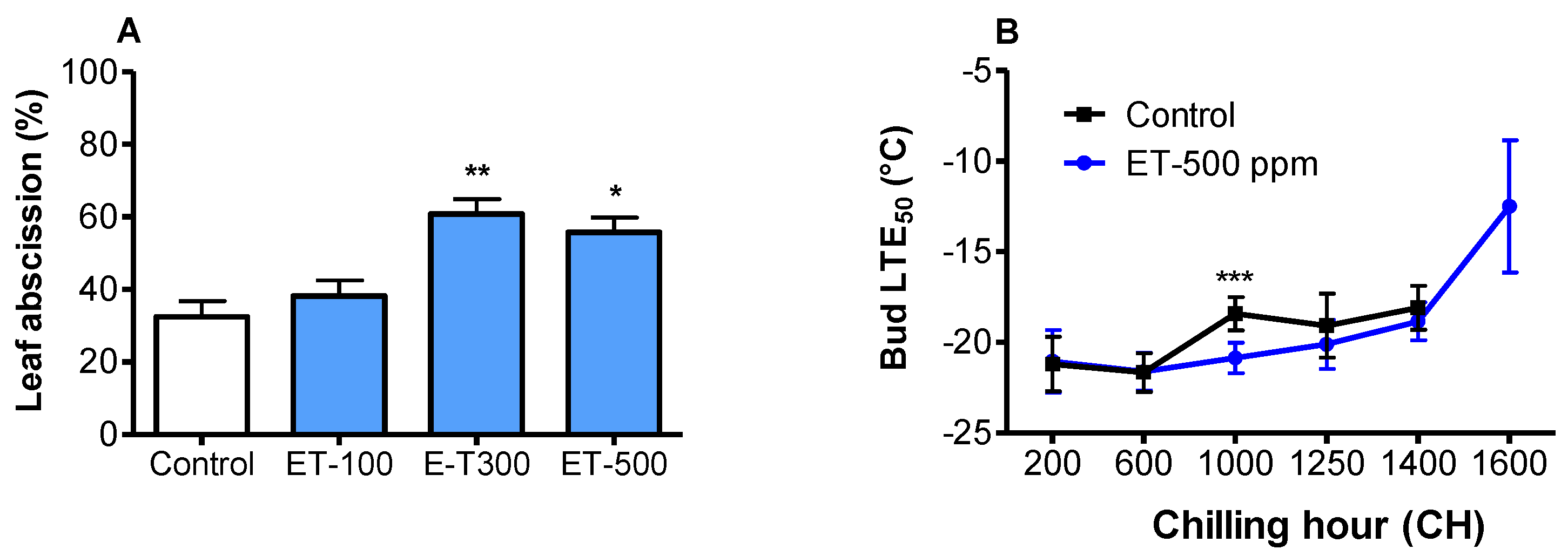
3.2. Ethephon Accelerated Leaf Abscission
3.3. Ethephon Improved Cold Hardiness
3.4. Ethylene Production in Leaves and Buds
3.5. Ethephon Increased Chilling and Heat Requirements
3.6. Ethephon Delayed Bloom in Peach
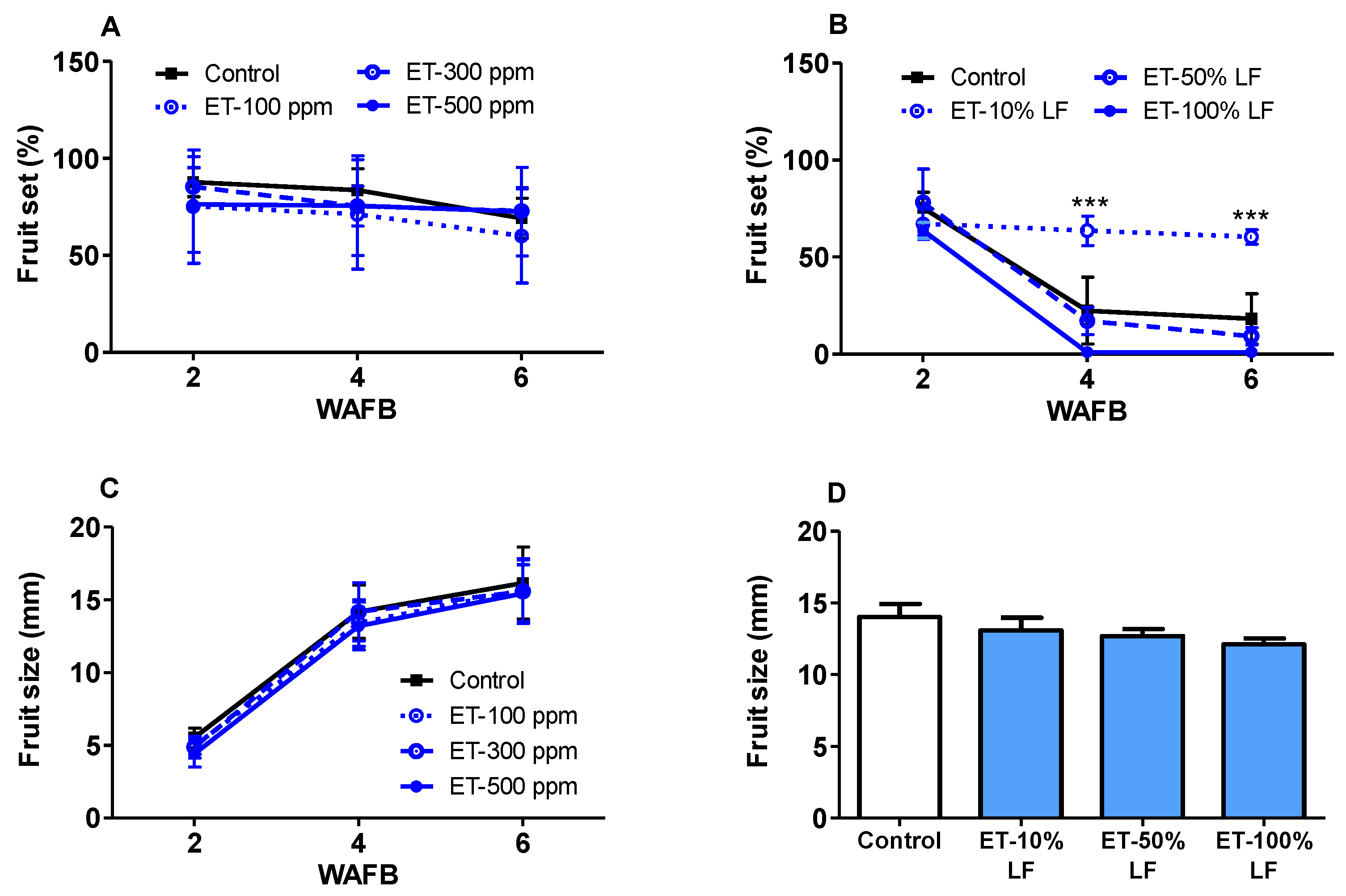
3.7. Ethephon Effects on Fruit Set and Fruit Size
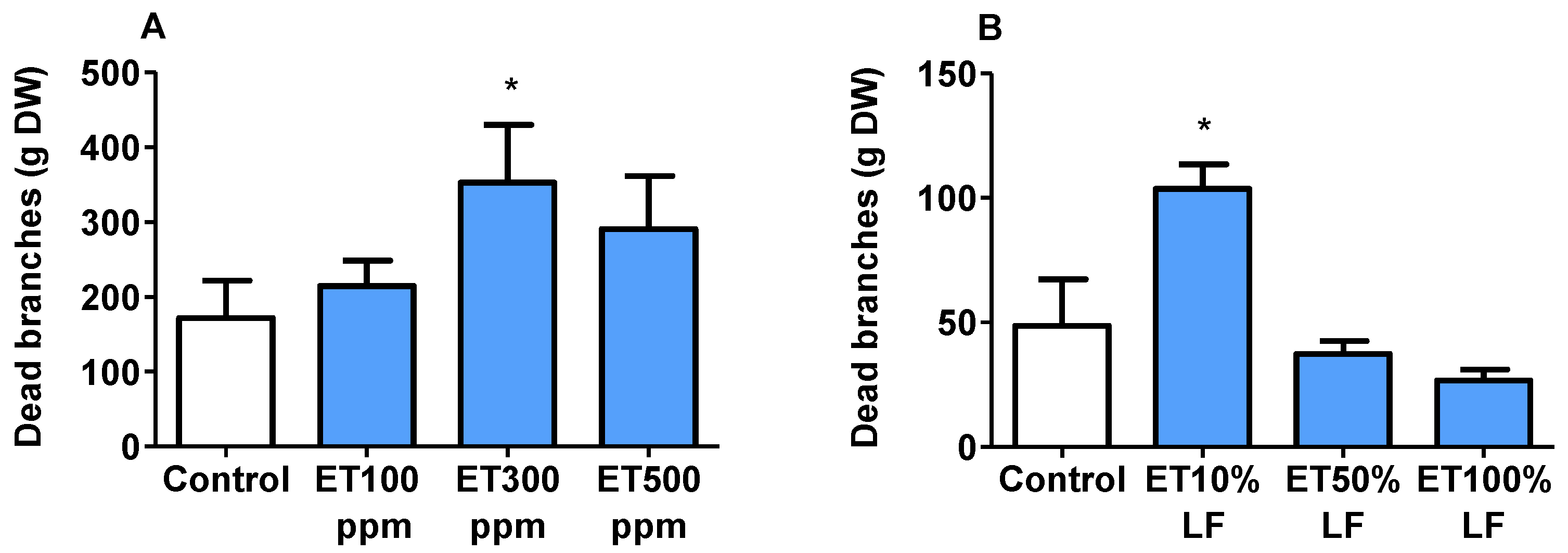
3.8. Tree Injuries Associated with Ethephon
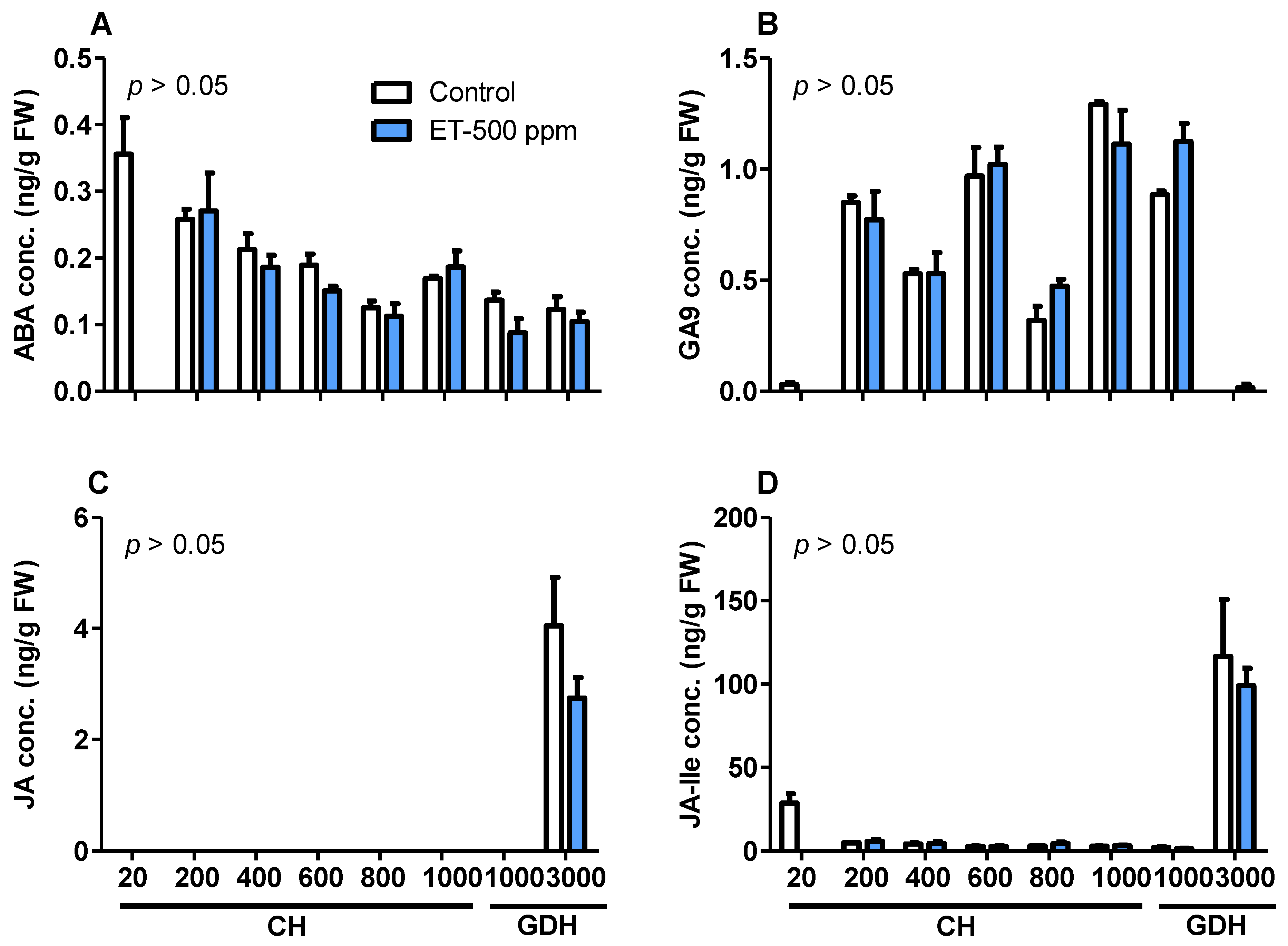
3.9. Phytohormone Accumulation Profiles during Dormancy
3.10. Expression of Genes That Regulate ABA, GA, ET and JA Biosynthesis
4. Discussion
4.1. Floral Bud Phenology and Cold Hardiness as Modulated by Ethephon
4.2. Ethephon Effects on Fruit Set, Fruit Size and Tree Health
4.3. Endogenous Hormonal Changes Associated with Bloom Delay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vitasse, Y.; François, C.; Delpierre, N.; Dufrêne, E.; Kremer, A.; Chuine, I.; Delzon, S. Assessing the effects of climate change on the phenology of European temperate trees. Agric. For. Meteorol. 2011, 151, 969–980. [Google Scholar] [CrossRef]
- Vitasse, Y.; Schneider, L.; Rixen, C.; Christen, D.; Rebetez, M. Increase in the risk of exposure of forest and fruit trees to spring frosts at higher elevations in Switzerland over the last four decades. Agric. For. Meteorol. 2018, 248, 60–69. [Google Scholar] [CrossRef]
- Hoffmann, H.; Rath, T. Future Bloom and Blossom Frost Risk for Malus domestica Considering Climate Model and Impact Model Uncertainties. PLoS ONE 2013, 8, e75033. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Sherif, S.M. Combating Spring Frost with Ethylene. Front. Plant Sci. 2019, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Nzokou, P.; Nikiema, P. The Influence of Three Plant Growth Regulators on Susceptibility to Cold Injury Following Warm Winter Spells in Fraser Fir [Abies fraseri (Pursh) Poir] and Colorado Blue Spruce (Picea pungens). HortScience 2008, 43, 742–746. [Google Scholar] [CrossRef] [Green Version]
- Durner, E.F. Dormant Pruning and Fall Ethephon Application Influence Peach Pistil Hardiness. J. Am. Soc. Hortic. Sci. 1995, 120, 823–829. [Google Scholar] [CrossRef]
- Grijalva-Contreras, L.R.; Martínez-Díaz, G.; Macías-Duarte, R.; Robles-Contreras, F. Effect of ethephon on almond bloom delay, yield, and nut quality under warm climate conditions in northwestern Mexico. Chil. J. Agric. Res. 2011, 71, 34–38. [Google Scholar] [CrossRef]
- Moghadam, E.G.; Mokhtarian, A. Delaying Apricot (cv. Shahroudi) Flower Induction by Growth Regulators Application. J. Appl. Sci. 2006, 6, 266–269. [Google Scholar] [CrossRef]
- Pahwa, K.; Ghai, N. Effect of ethylene on physiological and biochemical parameters in different crop plants—A review. J. Appl. Nat. Sci. 2015, 7, 1064–1069. [Google Scholar] [CrossRef] [Green Version]
- Crisosto, C.H.; Miller, A.N.; Lombard, P.B.; Robbins, S. Effect of Fall Ethephon Applications on Bloom Delay, Flowering, and Fruiting of Peach and Prune. HortScience 1990, 25, 426–428. [Google Scholar] [CrossRef]
- Durner, E.F.; Gianfagna, T.J. Ethephon Prolongs Dormancy and Enhances Supercooling in Peach Flower Buds. J. Am. Soc. Hortic. Sci. 1991, 116, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Crisosto, C.H.; Lombard, P.B.; Fuchigami, L.H. Effect of Fall Ethephon and Hand Defoliation on Dormant Bud Ethylene Levels, Bloom Delay, and Yield Components of ’Redhaven’ Peach. Acta Hortic. 1987, 201, 203–212. [Google Scholar] [CrossRef]
- Funt, R.; Ferree, D. Ethephon Induced Bloom Delay of Peach Trees, Ohio USA. Acta Hortic. 1986, 163–170. [Google Scholar] [CrossRef]
- Krewer, G.; Nesmith, D.S.; Williamson, J.; Maus, B.; Mullinix, B. Ethephon for Bloom Delay of Rabbiteye and Southern Highbush Blueberries. Small Fruits Rev. 2005, 4, 43–57. [Google Scholar] [CrossRef]
- Lang, G.A. Dormancy: A new universal terminology. HortScience 1987, 22, 817–820. [Google Scholar]
- Fan, S.; Bielenberg, D.G.; Zhebentyayeva, T.N.; Reighard, G.L.; Okie, W.R.; Holland, D.; Abbott, A.G. Mapping quantitative trait loci associated with chilling requirement, heat requirement and bloom date in peach (Prunus persica). New Phytol. 2010, 185, 917–930. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, J.H. Chilling requirement of peach varieties. J. Am. Soc. Hortic. Sci. 1950, 56, 122–128. [Google Scholar]
- Richardson, E.A.; Seeley, S.D.; Walker, D.R. A model for estimating the completion of rest for Redhaven and El-berta peach trees. HortScience 1974, 9, 331–332. [Google Scholar]
- Fishman, S.; Erez, A.; Couvillon, G.A. The temperature dependence of dormancy breaking in plants: Mathematical analysis of a two-step model involving a cooperative transition. J. Theor. Biol. 1987, 124, 473–483. [Google Scholar] [CrossRef]
- Anderson, J.; Richardson, E.; Kesner, C. Validation of Chill Unit and Flower Bud Phenology Models for ’Montmorency’ Sour Cherry. Acta Hortic. 1986, 184, 71–78. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud Dormancy in Perennial Fruit Tree Species: A Pivotal Role for Oxidative Cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Devitt, M.L.; Stafstrom, J.P. Cell cycle regulation during growth-dormancy cycles in pea axillary buds. Plant Mol. Biol. 1995, 29, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Wisniewski, M.; Lindow, S.E.; Ashworth, E.N. Observations of Ice Nucleation and Propagation in Plants Using Infrared Video Thermography. Plant Physiol. 1997, 113, 327–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.; Jikumaru, Y.; Kamiya, Y. Profiling of Hormones and Related Metabolites in Seed Dormancy and Germination Studies. Methods Mol. Biol. 2011, 773, 99–111. [Google Scholar] [CrossRef]
- Sherif, S.M.; Shukla, M.R.; Murch, S.J.; Bernier, L.; Saxena, P.K. Simultaneous induction of jasmonic acid and disease-responsive genes signifies tolerance of American elm to Dutch elm disease. Sci. Rep. 2016, 6, 21934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisniewski, M.; Lightner, G.; Davis, G.; Schiavone, M. System configuration for microcomputer-controlled, low-temperature differential thermal analysis. Comput. Electron. Agric. 1990, 5, 223–232. [Google Scholar] [CrossRef]
- Olien, W.B.; Bukovac, M.J. The effect of temperature on rate of ethylene evolution from ethephon and from ethephon-treated leaves of sour cherry. J. Am. Soc. Hortic. Sci. 1978, 103, 199–202. [Google Scholar]
- Flore, J.A.M.J.B. Factors influencing absorption of 14C(2-chloroethyl-phosphonic acid by leaves of cherry. J. Am. Soc. Hortic. Sci. 1982, 107, 965–968. [Google Scholar]
- Liu, J.; Sherif, S.M. Hormonal Orchestration of Bud Dormancy Cycle in Deciduous Woody Perennials. Front. Plant Sci. 2019, 10, 1136. [Google Scholar] [CrossRef] [PubMed]
- Wasternack, C.; Hause, B. Jasmonates: Biosynthesis, perception, signal transduction and action in plant stress response, growth and development. An update to the 2007 review in Annals of Botany. Ann. Bot. 2013, 111, 1021–1058. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, D.; Campoy, J.; Egea, J. Chilling and heat requirements of apricot cultivars for flowering. Environ. Exp. Bot. 2007, 61, 254–263. [Google Scholar] [CrossRef]
- Alburquerque, N.; García-Montiel, F.; Carrillo, A.; Burgos, L. Chilling and heat requirements of sweet cherry cultivars and the relationship between altitude and the probability of satisfying the chill requirements. Environ. Exp. Bot. 2008, 64, 162–170. [Google Scholar] [CrossRef]
- Alonso, J.M.; Ansón, J.M.; Espiau, M.T.; Sociasi, C.R. Determination of endodormancy break in almond flower buds by a correlation model using the average temperature of different day intervals and its application to the estimation of chill and heat requirements and blooming date. J. Am. Soc. Hortic. Sci. 2005, 130, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Egea, J.; Ortega, E.; Martínez-Gómez, P.; Dicenta, F. Chilling and heat requirements of almond cultivars for flowering. Environ. Exp. Bot. 2003, 50, 79–85. [Google Scholar] [CrossRef]
- Fadón, E.; Fernandez, E.; Behn, H.; Luedeling, E. A Conceptual Framework for Winter Dormancy in Deciduous Trees. Agronomy 2020, 10, 241. [Google Scholar] [CrossRef] [Green Version]
- Torres, E.; Giné-Bordonaba, J.; Asín, L. Thinning flat peaches with ethephon and its effect on endogenous ethylene production and fruit quality. Sci. Hortic. 2021, 278, 109872. [Google Scholar] [CrossRef]
- Coston, D.C.; Krewer, G.W.; Elkner, T.E.; Williamson, J.G.; Sims, E.T.J. Chemical treatments to delay bloom in peach prunus persica. J. Am. Soc. Hortic. Sci. 1985, 110, 874–877. [Google Scholar]
- Irving, D.E. ‘Fantasia’ nectarine: Effects of autumn-applied ethephon on blossoming and cropping. Exp. Agric. 1987, 15, 67–72. [Google Scholar] [CrossRef] [Green Version]
- Crisosto, C.H. Fall Ethephon Delays-Bloom in ‘Redhaven’ Peach by Delaying Flower Differentiation and Development during Dormancy. J. Am. Soc. Hortic. Sci. 1989, 114, 881–884. [Google Scholar]
- Grossmann, K.; Hansen, H. Ethylene-triggered abscisic acid: A principle in plant growth regulation? Physiol. Plant. 2001, 113, 9–14. [Google Scholar] [CrossRef]
- Rodrigo, M.-J.; Alquezar, B.; Zacarías, L. Cloning and characterization of two 9-cis-epoxycarotenoid dioxygenase genes, differentially regulated during fruit maturation and under stress conditions, from orange (Citrus sinensis L. Osbeck). J. Exp. Bot. 2006, 57, 633–643. [Google Scholar] [CrossRef] [Green Version]
- Doğramacı, M.; Foley, M.E.; Chao, W.S.; Christoffers, M.J.; Anderson, J.V.; Dogramaci, M. Induction of endodormancy in crown buds of leafy spurge (Euphorbia esula L.) implicates a role for ethylene and cross-talk between photoperiod and temperature. Plant Mol. Biol. 2013, 81, 577–593. [Google Scholar] [CrossRef]
- Shefferson, R.P. The evolutionary ecology of vegetative dormancy in mature herbaceous perennial plants. J. Ecol. 2009, 97, 1000–1009. [Google Scholar] [CrossRef]
- Saniewski, M.; Ueda, J.; Miyamoto, K.; Horbowicz, M.; Puchalski, J. Hormonal control of gummosis in Rosaceae. J. Fruit Ornam. Plant Res. 2006, 14, 137–143. [Google Scholar]
- Li, Z.; Zhu, W.; Fan, Y.-C.; Ye, J.-L.; Li, G.-H. Effects of pre- and post-treatment with ethephon on gum formation of peach gummosis caused by Lasiodiplodia theobromae. Plant Pathol. 2014, 63, 1306–1315. [Google Scholar] [CrossRef]
- Skirycz, A.; Claeys, H.; De Bodt, S.; Oikawa, A.; Shinoda, S.; Andriankaja, M.; Maleux, K.; Eloy, N.; Coppens, F.; Yoo, S.-D.; et al. Pause-and-Stop: The Effects of Osmotic Stress on Cell Proliferation during Early Leaf Development in Arabidopsis and a Role for Ethylene Signaling in Cell Cycle Arrest. Plant Cell 2011, 23, 1876–1888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalcsits, L.A.; Silim, S.; Tanino, K. Warm temperature accelerates short photoperiod-induced growth cessation and dormancy induction in hybrid poplar (Populus × spp.). Trees 2009, 23, 971–979. [Google Scholar] [CrossRef]
- Kalberer, S.R.; Wisniewski, M.; Arora, R. Deacclimation and reacclimation of cold-hardy plants: Current understanding and emerging concepts. Plant Sci. 2006, 171, 3–16. [Google Scholar] [CrossRef]
- Shin, H.; Oh, S.-I.; Kim, M.-A.; Yun, S.K.; Oh, Y.; Son, I.-C.; Kim, H.-S.; Kim, D. Relationship between cold hardiness and dehydrin gene expression in peach shoot tissues under field conditions. Hortic. Environ. Biotechnol. 2015, 56, 280–287. [Google Scholar] [CrossRef]
- Rubio, S.; Dantas, D.; Bressan-Smith, R.; Pérez, F.J. Relationship Between Endodormancy and Cold Hardiness in Grapevine Buds. J. Plant Growth Regul. 2016, 35, 266–275. [Google Scholar] [CrossRef]
- Duan, X.; Cai, C.; Yang, Y.; Chen, F.; Sang, Z.; Ma, L. Fall Ethephon Application Enhances the Freezing Tolerance of Magnolia wufengensis During Overwintering. Forests 2019, 10, 868. [Google Scholar] [CrossRef] [Green Version]
- Durner, E.F. Cryoprotection of deacclimating peach flower buds by ethephon alteration of pistil carbohydrate content. Cryobiology 1989, 26, 290–296. [Google Scholar] [CrossRef]
- Ciardi, J.A.; Deikman, J.; Orzolek, M.D. Increased ethylene synthesis enhances chilling tolerance in tomato. Physiol. Plant. 1997, 101, 333–340. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, H.; Pan, X.; Chen, X.; Zhang, Z.; Lu, X.; Huang, R. Overexpression of ethylene response factor TERF2 confers cold tolerance in rice seedlings. Transgenic Res. 2011, 20, 857–866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Huang, R. Enhanced tolerance to freezing in tobacco and tomato overexpressing transcription factor TERF2/LeERF2 is modulated by ethylene biosynthesis. Plant Mol. Biol. 2010, 73, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments. Plant Signal. Behav. 2012, 7, 1456–1466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavelienė, V.; Pakalniškytė, L.; Novickienė, L. Regulation of proline and ethylene levels in rape seedlings for freezing tolerance. Open Life Sci. 2014, 9, 1099–1107. [Google Scholar] [CrossRef] [Green Version]
- Turnbull, C.G.N.; Sinclair, E.R.; Anderson, K.L.; Nissen, R.J.; Shorter, A.J.; Lanham, T.E. Routes of Ethephon Uptake in Pineapple (Ananas comosus) and Reasons for Failure of Flower Induction. J. Plant Growth Regul. 1999, 18, 145–152. [Google Scholar] [CrossRef]
- Souza, F.; Alves, E.; Pio, R.; Castro, E.; Reighard, G.; Freire, A.I.; Mayer, N.A.; Pimentel, R. Influence of Temperature on the Development of Peach Fruit in a Subtropical Climate Region. Agronomy 2019, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Durner, E.F.; Gianfagna, T.J.; Rooney, F.X.; Teiger, G.S.; Seiler, M.J.; Cantarella, M.J. Harvest Date and Size Distribution of Peach Fruit Are Altered with Fall-applied Ethephon. HortScience 1990, 25, 911–913. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.; Halaly, T.; Acheampong, A.K.; Takebayashi, Y.; Jikumaru, Y.; Kamiya, Y.; Or, E. Abscisic acid (ABA) regulates grape bud dormancy, and dormancy release stimuli may act through modification of ABA metabolism. J. Exp. Bot. 2015, 66, 1527–1542. [Google Scholar] [CrossRef]
- Ewang, D.; Egao, Z.; Edu, P.; Exiao, W.; Etan, Q.; Echen, X.; Eli, L.; Egao, D. Expression of ABA Metabolism-Related Genes Suggests Similarities and Differences Between Seed Dormancy and Bud Dormancy of Peach (Prunus persica). Front. Plant Sci. 2016, 6, 1248. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Niu, Q.; He, L.; Teng, Y.; Bai, S. Abscisic Acid (ABA) Promotes the Induction and Maintenance of Pear (Pyrus pyrifolia White Pear Group) Flower Bud Endodormancy. Int. J. Mol. Sci. 2018, 19, 310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, S.; Böhlenius, H.; Moritz, T.; Nilsson, O. GA4 Is the Active Gibberellin in the Regulation of LEAFY Transcription and Arabidopsis Floral Initiation. Plant Cell 2006, 18, 2172–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rinne, P.L.; Welling, A.; Vahala, J.; Ripel, L.; Ruonala, R.; Kangasjärvi, J.; van der Schoot, C. Chilling of Dormant Buds Hyperinduces FLOWERING LOCUS T and Recruits GA-Inducible 1,3-β-Glucanases to Reopen Signal Conduits and Release Dormancy in Populus. Plant Cell 2011, 23, 130–146. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.; Gao, Z.; Wang, L.; Zhong, W.; Ni, Z.; Zhang, Z. Comparative proteomic and transcriptomic approaches to address the active role of GA4 in Japanese apricot flower bud dormancy release. J. Exp. Bot. 2013, 64, 4953–4966. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Acheampong, A.K.; Shi, Z.; Halaly, T.; Kamiya, Y.; Ophir, R.; Galbraith, D.W.; Or, E. Distinct gibberellin functions during and after grapevine bud dormancy release. J. Exp. Bot. 2018, 69, 1635–1648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widemann, E.; Smirnova, E.; Aubert, Y.; Miesch, L.; Heitz, T. Dynamics of Jasmonate Metabolism upon Flowering and across Leaf Stress Responses in Arabidopsis thaliana. Plants 2016, 5, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciejewska, B.; Kopcewicz, J. Inhibitory Effect of Methyl Jasmonate on Flowering and Elongation Growth in Pharbitis nil. J. Plant Growth Regul. 2002, 21, 216–223. [Google Scholar] [CrossRef]
- Niwa, T.; Suzuki, T.; Takebayashi, Y.; Ishiguro, R.; Higashiyama, T.; Sakakibara, H.; Ishiguro, S. Jasmonic acid facilitates flower opening and floral organ development through the upregulated expression of SlMYB21 transcription factor in tomato. Biosci. Biotechnol. Biochem. 2018, 82, 292–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, K.; Yazici, C.; Muradoğlu, F. Effect of jasmonic acid on germination dormant and nondormant apple seeds. Asian J. Chem. 2007, 19, 1098–1102. [Google Scholar]
- Singh, P.; Dave, A.; Vaistij, F.E.; Worrall, D.; Holroyd, G.H.; Wells, J.G.; Kaminski, F.; Graham, I.A.; Roberts, M.R. Jasmonic acid-dependent regulation of seed dormancy following maternal herbivory in Arabidopsis. New Phytol. 2017, 214, 1702–1711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howe, G.T.; Horvath, D.P.; Dharmawardhana, P.; Priest, H.D.; Mockler, T.C.; Strauss, S.H. Extensive Transcriptome Changes During Natural Onset and Release of Vegetative Bud Dormancy in Populus. Front. Plant Sci. 2015, 6, 989. [Google Scholar] [CrossRef] [Green Version]
- Heitz, T.; Smirnova, E.; Widemann, E.; Aubert, Y.; Pinot, F.; Ménard, R. The Rise and Fall of Jasmonate Biological Activities. Prokaryotic Cytoskelet. 2016, 86, 405–426. [Google Scholar] [CrossRef]
- Ionescu, I.A.; López-Ortega, G.; Burow, M.; Bayo-Canha, A.; Junge, A.; Gericke, O.; Møller, B.L.; Sánchez-Pérez, R. Transcriptome and Metabolite Changes during Hydrogen Cyanamide-Induced Floral Bud Break in Sweet Cherry. Front. Plant Sci. 2017, 8, 1233. [Google Scholar] [CrossRef] [PubMed]
- E Farmer, E.; Goossens, A. Jasmonates: What ALLENE OXIDE SYNTHASE does for plants. J. Exp. Bot. 2019, 70, 3373–3378. [Google Scholar] [CrossRef]
- Suttle, J.C. Involvement of Ethylene in Potato Microtuber Dormancy. Plant Physiol. 1998, 118, 843–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sumitomo, K.; Narumi, T.; Satoh, S.; Hisamatsu, T. Involvement of the ethylene response pathway in dormancy induction in chrysanthemum. J. Exp. Bot. 2008, 59, 4075–4082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruttink, T.; Arend, M.; Morreel, K.; Storme, V.; Rombauts, S.; Fromm, J.; Bhalerao, R.; Boerjan, W.; Rohde, A. A Molecular Timetable for Apical Bud Formation and Dormancy Induction in Poplar. Plant Cell 2007, 19, 2370–2390. [Google Scholar] [CrossRef] [Green Version]
- Houben, M.; Van De Poel, B. 1-Aminocyclopropane-1-Carboxylic Acid Oxidase (ACO): The Enzyme That Makes the Plant Hormone Ethylene. Front. Plant Sci. 2019, 10, 695. [Google Scholar] [CrossRef] [Green Version]
- Ophir, R.; Pang, X.; Halaly, T.; Venkateswari, J.; Lavee, S.; Galbraith, D.; Or, E. Gene-expression profiling of grape bud response to two alternative dormancy-release stimuli expose possible links between impaired mitochondrial activity, hypoxia, ethylene-ABA interplay and cell enlargement. Plant Mol. Biol. 2009, 71, 403–423. [Google Scholar] [CrossRef] [PubMed]





| Cultivar | Treatment | CR x | Date y | Days z | |
|---|---|---|---|---|---|
| Redhaven (P. persica) | Control | 1046 (62.2) | 22 January | 88 | a |
| ET-100 ppm | 1112 (65.9) | 26 January | 92 | ab | |
| ET-300 ppm | 1147 (67.3) | 28 January | 94 | ab | |
| ET-500 ppm | 1177 (67.8) | 29 January | 95 | b | |
| Sunhigh (P. persica) | Control | 805 (46.9) | 28 December | 94 | a |
| ET-10% | 1145 (68.4) | 27 January | 124 | b | |
| ET-50% | 1115 (65.6) | 26 January | 123 | b | |
| ET-100% | 1092 (63.1) | 25 January | 122 | b | |
| Cultivar | Treatment | HR X | Flowering (F50) y | Days z | |
|---|---|---|---|---|---|
| Redhaven (P. persica) | Control | 4682 | 23 March | 60 | a |
| ET-100 ppm | 4927 | 26 March | 63 | ab | |
| ET-300 ppm | 5260 | 28 March | 65 | b | |
| ET-500 ppm | 5583 | 29 March | 66 | b | |
| Sunhigh (P. persica) | Control | 4960 | 24 March | 61 | a |
| ET 10% | 5842 | 30 March | 67 | b | |
| ET 50% | 5656 | 29 March | 66 | b | |
| ET 100% | 5543 | 28 March | 65 | b | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Islam, M.T.; Sapkota, S.; Ravindran, P.; Kumar, P.P.; Artlip, T.S.; Sherif, S.M. Ethylene-Mediated Modulation of Bud Phenology, Cold Hardiness, and Hormone Biosynthesis in Peach (Prunus persica). Plants 2021, 10, 1266. https://doi.org/10.3390/plants10071266
Liu J, Islam MT, Sapkota S, Ravindran P, Kumar PP, Artlip TS, Sherif SM. Ethylene-Mediated Modulation of Bud Phenology, Cold Hardiness, and Hormone Biosynthesis in Peach (Prunus persica). Plants. 2021; 10(7):1266. https://doi.org/10.3390/plants10071266
Chicago/Turabian StyleLiu, Jianyang, Md Tabibul Islam, Sangeeta Sapkota, Pratibha Ravindran, Prakash P. Kumar, Timothy S. Artlip, and Sherif M. Sherif. 2021. "Ethylene-Mediated Modulation of Bud Phenology, Cold Hardiness, and Hormone Biosynthesis in Peach (Prunus persica)" Plants 10, no. 7: 1266. https://doi.org/10.3390/plants10071266






