Heterogeneous Impact of Water Warming on Exotic and Native Submerged and Emergent Plants in Outdoor Mesocosms
Abstract
:1. Introduction
2. Results
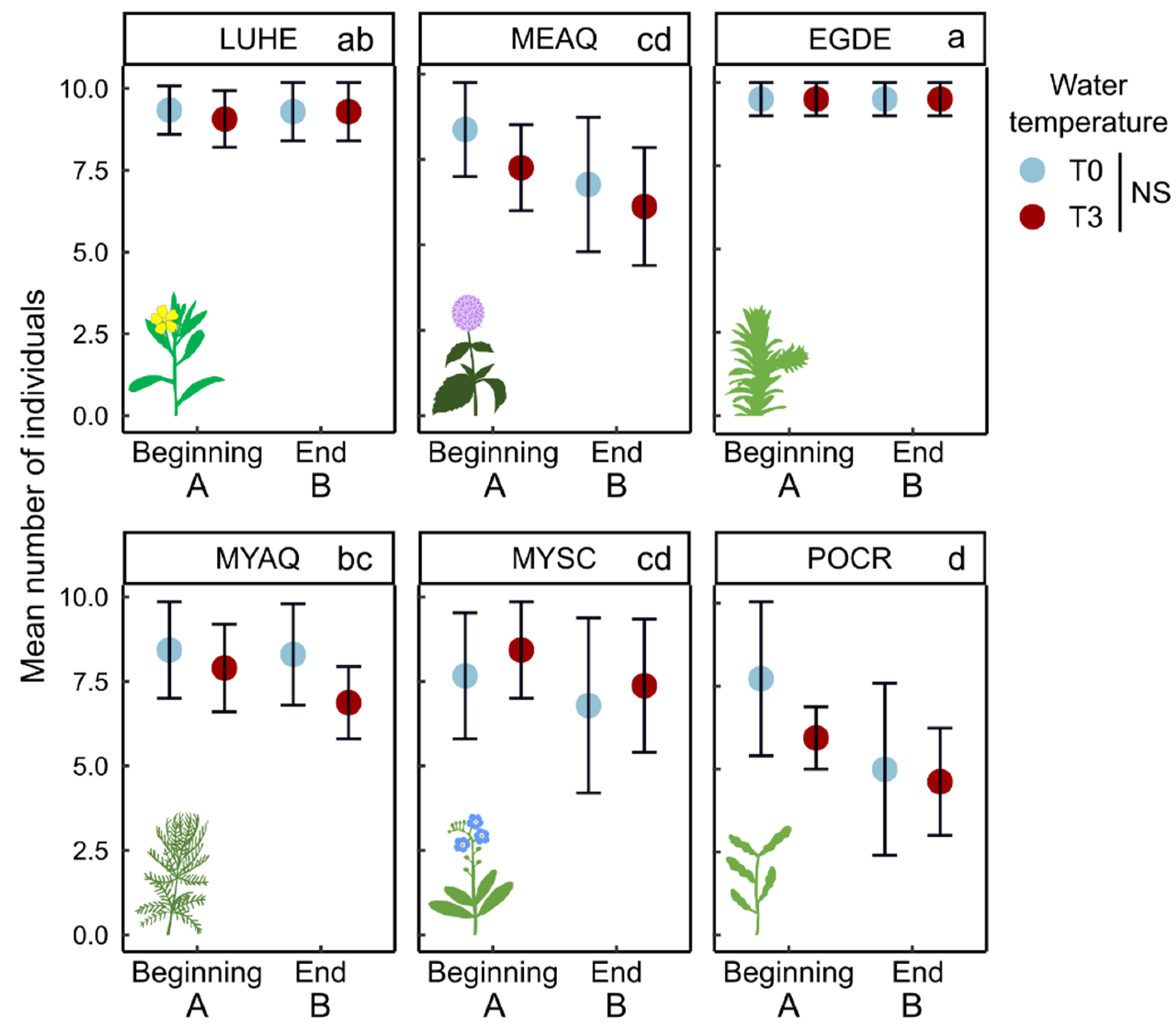
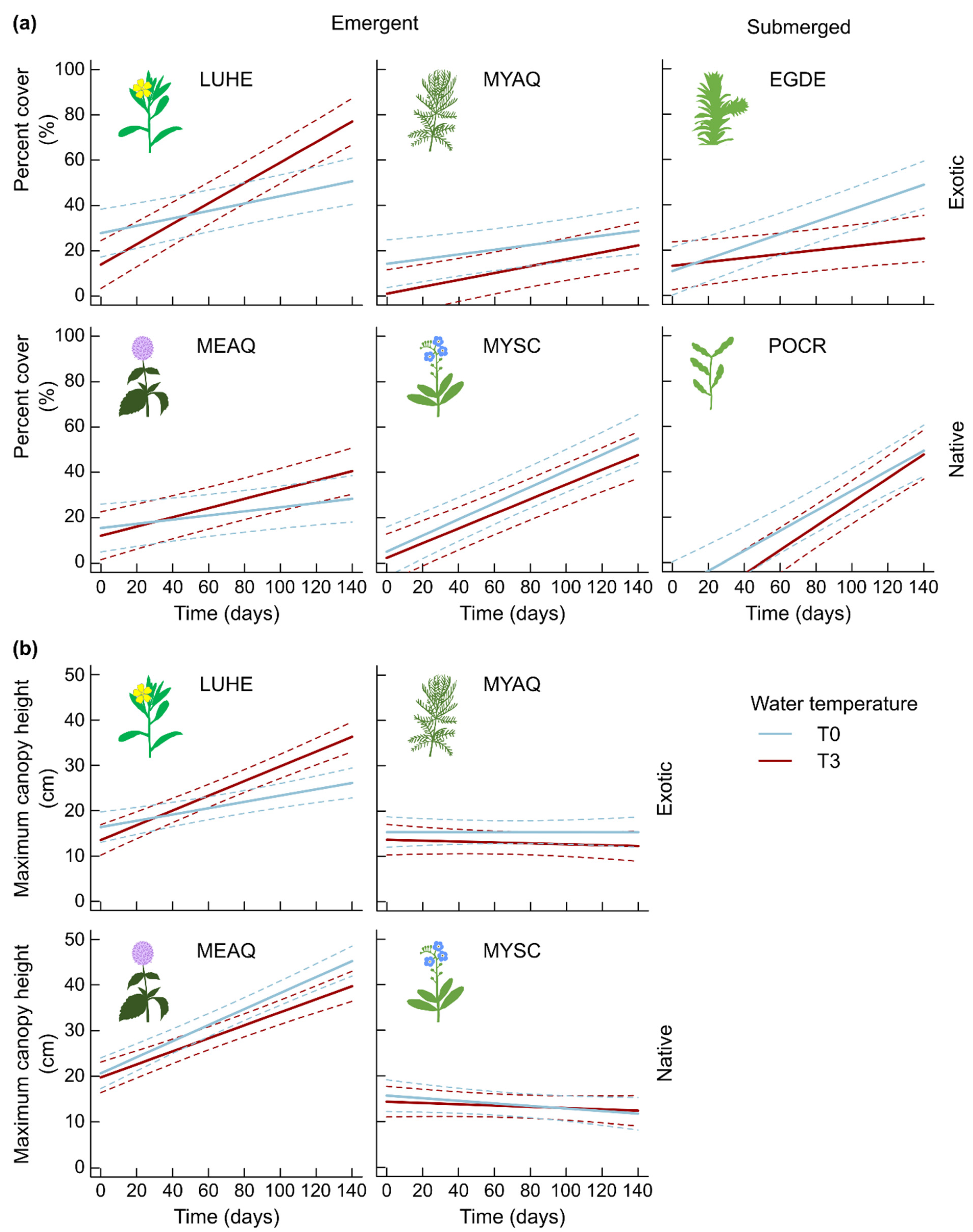
2.1. Morphological Traits
2.2. Content Evaluation of Some Metabolic Compounds
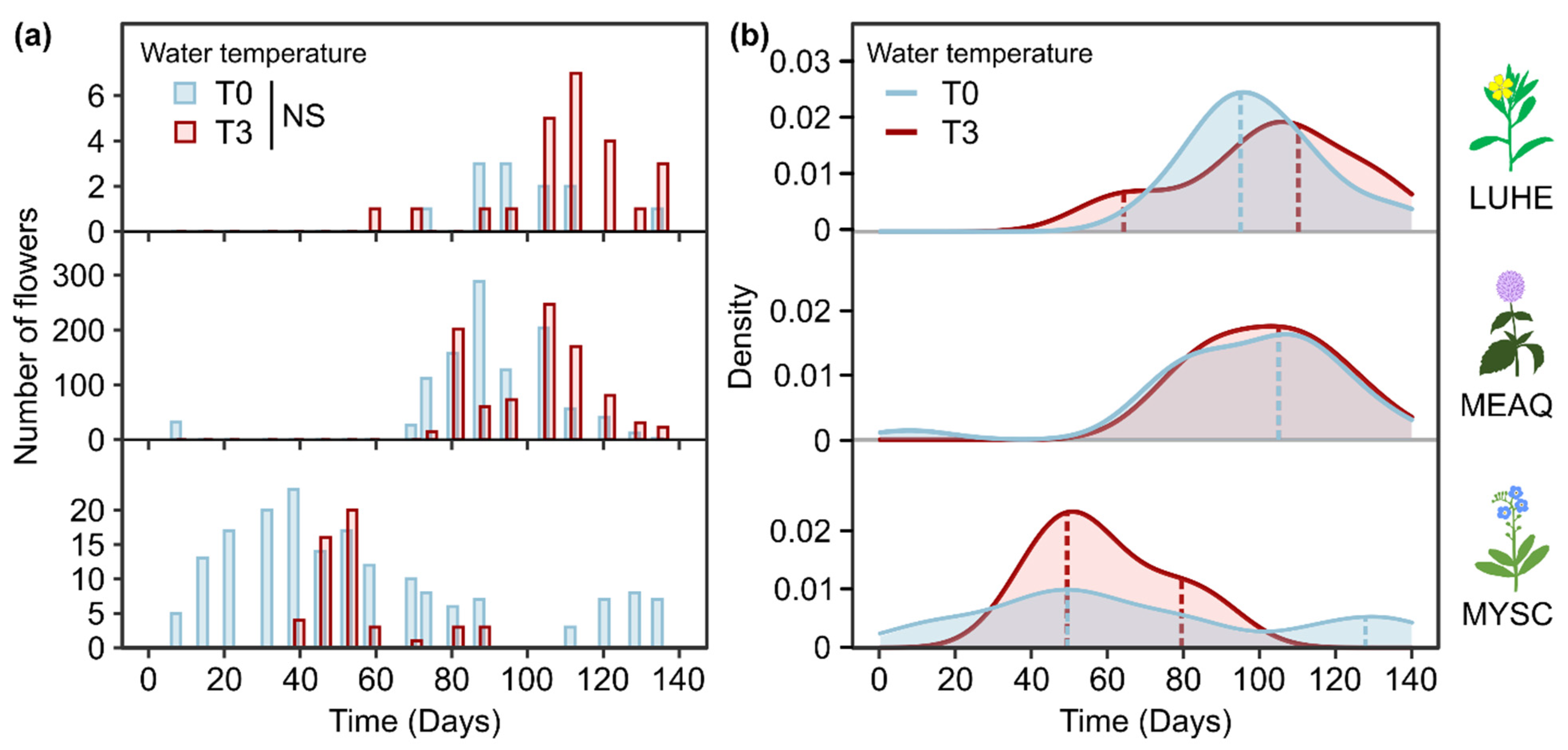
2.3. Flowering Phenology
3. Discussion
3.1. Warmer Water Has Impacts on Growth, Phenology and Metabolism
3.1.1. Few Changes in Morphological Traits Related to Growth
3.1.2. Warming Effects on Primary and Secondary Metabolisms
3.1.3. Impacts of Extended and Shortened Flowering Duration
3.2. Comparison between Species Depending on Species Biogeographic Origin and Growth Form
4. Materials and Methods
4.1. Plant Collection
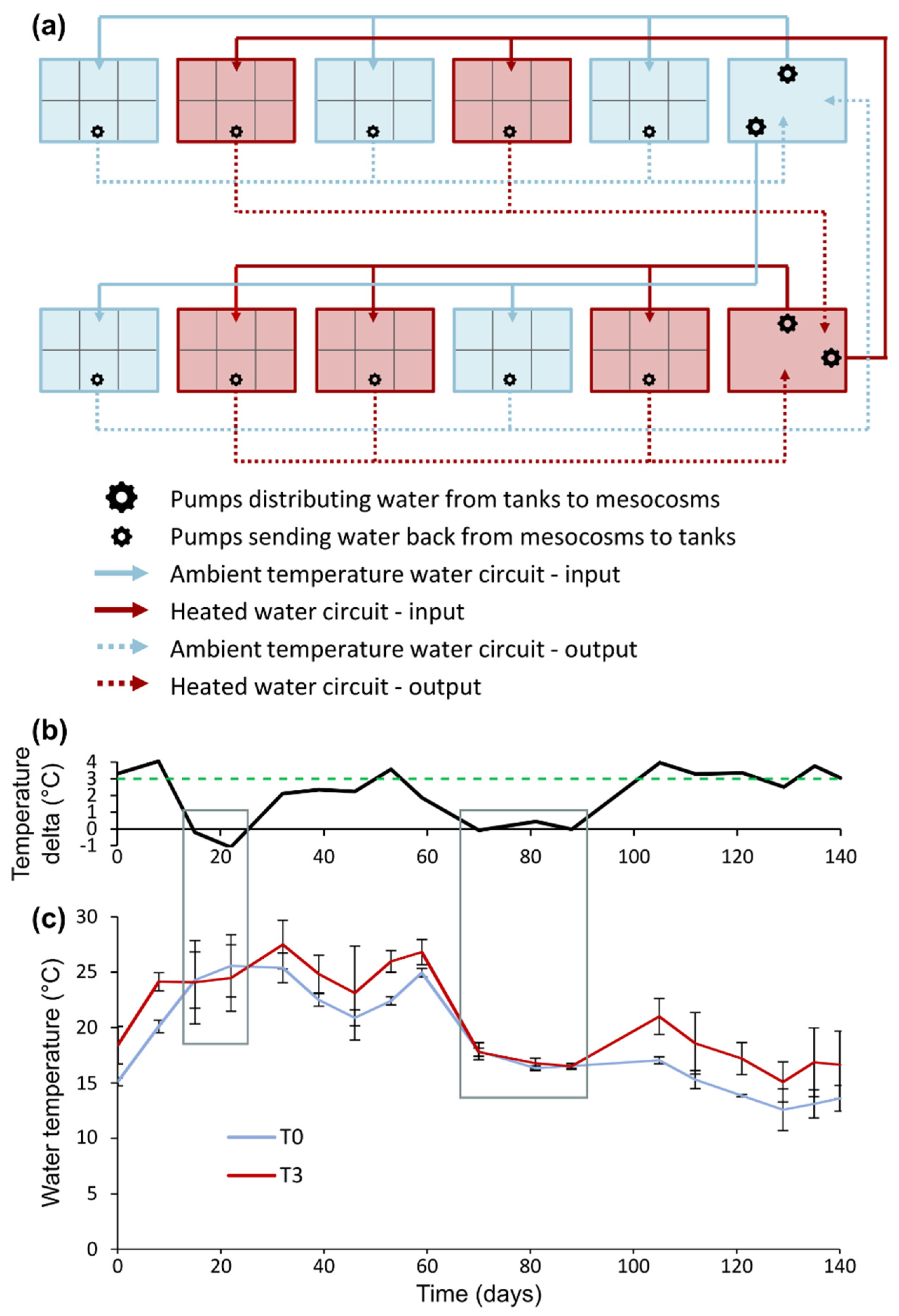
4.2. Experimental Design
4.3. Repeated Measurements of Growth, Phenology and Water Quality
4.4. Estimation of Pigment Content, C/N and Total Biomass
4.5. Total Soluble Sugar Assays
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hussner, A.; Stiers, I.; Verhofstad, M.J.J.M.; Bakker, E.S.; Grutters, B.M.C.; Haury, J.; van Valkenburg, J.L.C.H.; Brundu, G.; Newman, J.; Clayton, J.S.; et al. Management and control methods of invasive alien freshwater aquatic plants: A review. Aquat. Bot. 2017, 136, 112–137. [Google Scholar] [CrossRef]
- Havel, J.E.; Kovalenko, K.E.; Thomaz, S.M.; Amalfitano, S.; Kats, L.B. Aquatic invasive species: Challenges for the future. Hydrobiologia 2015, 147–170. [Google Scholar] [CrossRef] [PubMed]
- Madani, N.; Kimball, J.S.; Ballantyne, A.P.; Affleck, D.L.R.; van Bodegom, P.M.; Reich, P.B.; Kattge, J.; Sala, A.; Nazeri, M.; Jones, M.O.; et al. Future global productivity will be affected by plant trait response to climate. Sci. Rep. 2018, 8, 2870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B Biol. 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Atkin, O.K.; Tjoelker, M.G. Thermal acclimation and the dynamic response of plant respiration to temperature. Trends Plant Sci. 2003, 8, 343–351. [Google Scholar] [CrossRef]
- Marx, J.M.; Rall, B.C.; Phillips, H.R.P.; Brose, U. Opening the black box of plant nutrient uptake under warming predicts global patterns in community biomass and biological carbon storage. Oikos 2019, 128, 1503–1514. [Google Scholar] [CrossRef]
- IPCC Long-term climate change: Projections, commitments and irreversibility. In Climate Change 2013—The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136.
- Violle, C.; Navas, M.-L.; Vile, D.; Kazakou, E.; Fortunel, C.; Hummel, I.; Garnier, E. Let the concept of trait be functional! Oikos 2007, 116, 882–892. [Google Scholar] [CrossRef]
- Funk, J.L.; Zachary, V.A. Physiological responses to short-term water and light stress in native and invasive plant species in southern California. Biol. Invasions 2010, 12, 1685–1694. [Google Scholar] [CrossRef]
- León-Chan, R.G.; López-Meyer, M.; Osuna-Enciso, T.; Sañudo-Barajas, J.A.; Heredia, J.B.; León-Félix, J. Low temperature and ultraviolet-B radiation affect chlorophyll content and induce the accumulation of UV-B-absorbing and antioxidant compounds in bell pepper (Capsicum annuum) plants. Environ. Exp. Bot. 2017, 139, 143–151. [Google Scholar] [CrossRef]
- Ahammed, G.J.; Xu, W.; Liu, A.; Chen, S. COMT1 Silencing Aggravates Heat Stress-Induced Reduction in Photosynthesis by Decreasing Chlorophyll Content, Photosystem II Activity, and Electron Transport Efficiency in Tomato. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef] [PubMed]
- Croft, H.; Chen, J.M.; Luo, X.; Bartlett, P.; Chen, B.; Staebler, R.M. Leaf chlorophyll content as a proxy for leaf photosynthetic capacity. Glob. Chang. Biol. 2017, 23, 3513–3524. [Google Scholar] [CrossRef]
- Olsen, K.M.; Slimestad, R.; Lea, U.S.; Brede, C.; Løvdal, T.; Ruoff, P.; Verheul, M.; Lillo, C. Temperature and nitrogen effects on regulators and products of the flavonoid pathway: Experimental and kinetic model studies. Plant Cell Environ. 2009, 32, 286–299. [Google Scholar] [CrossRef]
- Shao, L.; Shu, Z.; Sun, S.-L.; Peng, C.-L.; Wang, X.-J.; Lin, Z.-F. Antioxidation of Anthocyanins in Photosynthesis Under High Temperature Stress. J. Integr. Plant Biol. 2007, 49, 1341–1351. [Google Scholar] [CrossRef]
- Gunn, S.; Farrar, J.F. Effects of a 4 °C increase in temperature on partitioning of leaf area and dry mass, root respiration and carbohydrates. Funct. Ecol. 1999, 13, 12–20. [Google Scholar] [CrossRef]
- Couée, I.; Sulmon, C.; Gouesbet, G.; El Amrani, A. Involvement of soluble sugars in reactive oxygen species balance and responses to oxidative stress in plants. J. Exp. Bot. 2006, 57, 449–459. [Google Scholar] [CrossRef]
- Silveira, M.J.; Thiébaut, G. Impact of climate warming on plant growth varied according to the season. Limnologica 2017, 65, 4–9. [Google Scholar] [CrossRef]
- Gillard, M.; Thiébaut, G.; Rossignol, N.; Berardocco, S.; Deleu, C. Impact of climate warming on carbon metabolism and on morphology of invasive and native aquatic plant species varies between spring and summer. Environ. Exp. Bot. 2017, 144, 1–10. [Google Scholar] [CrossRef]
- Zhang, P.; Bakker, E.S.; Zhang, M.; Xu, J. Effects of warming on Potamogeton crispus growth and tissue stoichiometry in the growing season. Aquat. Bot. 2016, 128, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Rooney, N.; Kalff, J. Inter-annual variation in submerged macrophyte community biomass and distribution: The influence of temperature and lake morphometry. Aquat. Bot. 2000, 68, 321–335. [Google Scholar] [CrossRef]
- Calero, S.; Rodrigo, M.A. Reproductive phenology of submerged macrophytes: A tracker of year-to-year environmental variations. J. Veg. Sci. 2019, 30, 1217–1227. [Google Scholar] [CrossRef]
- Li, Z.; He, L.; Zhang, H.; Urrutia-Cordero, P.; Ekvall, M.K.; Hollander, J.; Hansson, L.-A. Climate warming and heat waves affect reproductive strategies and interactions between submerged macrophytes. Glob. Chang. Biol. 2017, 23, 108–116. [Google Scholar] [CrossRef]
- Yu, H.; Shen, N.; Yu, S.; Yu, D.; Liu, C. Responses of the native species Sparganium angustifolium and the invasive species Egeria densa to warming and interspecific competition. PLoS ONE 2018, 13, e0199478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netten, J.J.C.; Arts, G.H.P.; Gylstra, R.; van Nes, E.H.; Scheffer, M.; Roijackers, R.M.M. Effect of temperature and nutrients on the competition between free-floating Salvinia natans and submerged Elodea nuttalii in mesocosms. Fundam. Appl. Limnol. Arch. für Hydrobiol. 2010, 177, 125–132. [Google Scholar] [CrossRef]
- Davidson, A.M.; Jennions, M.; Nicotra, A.B. Do invasive species show higher phenotypic plasticity than native species and, if so, is it adaptive? A meta-analysis. Ecol. Lett. 2011, 14, 419–431. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Ribas, L.G.; de Cássia-Silva, C.; Petsch, D.K.; Silveira, M.J.; Lima-Ribeiro, M.S. The potential invasiveness of an aquatic macrophyte reflects founder effects from native niche. Biol. Invasions 2018, 20, 3347–3355. [Google Scholar] [CrossRef]
- Rodríguez-Merino, A.; Fernández-Zamudio, R.; García-Murillo, P.; Muñoz, J. Climatic Niche Shift during Azolla filiculoides Invasion and Its Potential Distribution under Future Scenarios. Plants 2019, 8, 424. [Google Scholar] [CrossRef] [Green Version]
- He, Z.-S.; He, W.-M. Asymmetric climate warming does not benefit plant invaders more than natives. Sci. Total Environ. 2020, 742, 140624. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, M.; Nijs, I. Alien plant species favoured over congeneric natives under experimental climate warming in temperate Belgian climate. Biol. Invasions 2010, 12, 2777–2787. [Google Scholar] [CrossRef]
- Short, F.T.; Kosten, S.; Morgan, P.A.; Malone, S.; Moore, G.E. Impacts of climate change on submerged and emergent wetland plants. Aquat. Bot. 2016, 135, 3–17. [Google Scholar] [CrossRef]
- Chimney, M.J.; Wenkert, L.; Pietro, K.C. Patterns of vertical stratification in a subtropical constructed wetland in south Florida (USA). Ecol. Eng. 2006, 27, 322–330. [Google Scholar] [CrossRef]
- Reeder, B.C. Assessing constructed wetland functional success using diel changes in dissolved oxygen, pH, and temperature in submerged, emergent, and open-water habitats in the Beaver Creek Wetlands Complex, Kentucky (USA). Ecol. Eng. 2011, 37, 1772–1778. [Google Scholar] [CrossRef]
- Cook, C.D.K.; Urmi-König, K. A revision of the genus Egeria (Hydrocharitaceaea). Aquat. Bot. 1984, 19, 73–96. [Google Scholar] [CrossRef]
- Thiébaut, G.; Gillard, M.; Deleu, C. Growth, regeneration and colonisation of Egeria densa fragments: The effect of autumn temperature increases. Aquat. Ecol. 2016, 50, 175–185. [Google Scholar] [CrossRef]
- Lambert, E.; Dutartre, A.; Coudreuse, J.; Haury, J. Relationships between the biomass production of invasive Ludwigia species and physical properties of habitats in France. Hydrobiologia 2010, 656, 173–186. [Google Scholar] [CrossRef]
- Hussner, A. Growth response and root system development of the invasive Ludwigia grandiflora and Ludwigia peploides to nutrient availability and water level. Fundam. Appl. Limnol. Arch. Für Hydrobiol. 2010, 177, 189–196. [Google Scholar] [CrossRef]
- Thiébaut, G.; Tarayre, M.; Jambon, O.; Le Bris, N.; Colinet, H.; Renault, D. Variation of thermal plasticity for functional traits between populations of an invasive aquatic plant from two climatic regions. Hydrobiologia 2020. [Google Scholar] [CrossRef]
- Reich, P.B.; Oleksyn, J. Global patterns of plant leaf N and P in relation to temperature and latitude. Proc. Natl. Acad. Sci. USA 2004, 101, 11001–11006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Grutters, B.M.C.; van Leeuwen, C.H.A.; Xu, J.; Petruzzella, A.; van den Berg, R.F.; Bakker, E.S. Effects of Rising Temperature on the Growth, Stoichiometry, and Palatability of Aquatic Plants. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef]
- Rivero, R.M.; Ruiz, J.M.; García, P.C.; López-Lefebre, L.R.; Sánchez, E.; Romero, L. Resistance to cold and heat stress: Accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001, 160, 315–321. [Google Scholar] [CrossRef]
- Renner, S.S.; Zohner, C.M. The occurrence of red and yellow autumn leaves explained by regional differences in insolation and temperature. New Phytol. 2019, 224, 1464–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Wang, T.; García Molinos, J.; Li, C.; Hu, B.; Pan, M.; Zhang, M. Effects of warming, climate extremes and phosphorus enrichment on the growth, sexual reproduction and propagule carbon and nitrogen stoichiometry of Potamogeton crispus L. Environ. Int. 2020, 137, 105502. [Google Scholar] [CrossRef] [PubMed]
- Santamaría, L.; van Vierssen, W. Photosynthetic temperature responses of fresh- and brackish-water macrophytes: A review. Aquat. Bot. 1997, 58, 135–150. [Google Scholar] [CrossRef]
- Khodorova, N.; Boitel-Conti, M. The Role of Temperature in the Growth and Flowering of Geophytes. Plants 2013, 2, 699–711. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, G.; Biemelt, S.; Baumgartner, S. Accumulation of fructans following oxygen deficiency stress in related plant species with different flooding tolerances. New Phytol. 1997, 136, 137–144. [Google Scholar] [CrossRef]
- Dandelot, S.; Verlaque, R.; Dutartre, A.; Cazaubon, A. Ecological, Dynamic and Taxonomic Problems Due to Ludwigia (Onagraceae) in France. Hydrobiologia 2005, 551, 131–136. [Google Scholar] [CrossRef]
- Dorji, T.; Hopping, K.A.; Meng, F.; Wang, S.; Jiang, L.; Klein, J.A. Impacts of climate change on flowering phenology and production in alpine plants: The importance of end of flowering. Agric. Ecosyst. Environ. 2020, 291, 106795. [Google Scholar] [CrossRef]
- Gillard, M.; Grewell, B.J.; Deleu, C.; Thiébaut, G. Climate warming and water primroses: Germination responses of populations from two invaded ranges. Aquat. Bot. 2017, 136, 155–163. [Google Scholar] [CrossRef]
- Gillard, M.; Grewell, B.J.; Futrell, C.J.; Deleu, C.; Thiébaut, G. Germination and Seedling Growth of Water Primroses: A Cross Experiment between Two Invaded Ranges with Contrasting Climates. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef]
- Kehrberger, S.; Holzschuh, A. Warmer temperatures advance flowering in a spring plant more strongly than emergence of two solitary spring bee species. PLoS ONE 2019, 14, e0218824. [Google Scholar] [CrossRef]
- Bock, A.; Sparks, T.H.; Estrella, N.; Jee, N.; Casebow, A.; Schunk, C.; Leuchner, M.; Menzel, A. Changes in first flowering dates and flowering duration of 232 plant species on the island of Guernsey. Glob. Chang. Biol. 2014, 20, 3508–3519. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Escudero, A.; Iriondo, J.M.; Martínez-Vilalta, J.; Valladares, F. Extreme climatic events and vegetation: The role of stabilizing processes. Glob. Chang. Biol. 2012, 18, 797–805. [Google Scholar] [CrossRef]
- Hegland, S.J.; Nielsen, A.; Lázaro, A.; Bjerknes, A.-L.; Totland, Ø. How does climate warming affect plant-pollinator interactions? Ecol. Lett. 2009, 12, 184–195. [Google Scholar] [CrossRef]
- Koutecká, E.; Lepš, J. Effect of light and moisture conditions and seed age on germination of three closely related myosotis species. Folia Geobot. 2009, 44, 109–130. [Google Scholar] [CrossRef]
- Thouvenot, L.; Haury, J.; Thiebaut, G. A success story: Water primroses, aquatic plant pests. Aquat. Conserv. Mar. Freshw. Ecosyst. 2013, 23, 790–803. [Google Scholar] [CrossRef]
- Thiébaut, G.; Thouvenot, L.; Rodríguez-Pérez, H. Allelopathic Effect of the Invasive Ludwigia hexapetala on Growth of Three Macrophyte Species. Front. Plant Sci. 2018, 9, 1–10. [Google Scholar] [CrossRef]
- Thiébaut, G.; Tarayre, M.; Rodríguez-Pérez, H. Allelopathic effects of native versus invasive plants on one major invader. Front. Plant Sci. 2019, 10, 854. [Google Scholar] [CrossRef] [PubMed]
- Barko, J.W.; Smart, R.M. Comparative influences of light and temperature on the growth and metabolism of selected submersed freswater macrophytes. Ecol. Monogr. 1981, 51, 219–235. [Google Scholar] [CrossRef]
- Drexler, J.Z.; Khanna, S.; Lacy, J.R. Carbon storage and sediment trapping by Egeria densa Planch., a globally invasive, freshwater macrophyte. Sci. Total Environ. 2021, 755, 142602. [Google Scholar] [CrossRef]
- Yarrow, M.; Marin, V.H.; Finlayson, M.; Tironi, A.; Delgado, L.E.; Fisher, F. The ecology of Egeria densa Planchon (Liliopsida: Alismatales): A wetland ecosystem engineer? Rev. Chil. Hist. Nat. 2009, 82, 299–313. [Google Scholar] [CrossRef] [Green Version]
- Schuyler, A.E. Classification of Life Forms and Growth Forms of Aquatic Macrophytes. Bartonia 1984, 50, 8–11. [Google Scholar]
- Gillard, M.; Thiébaut, G.; Deleu, C.; Leroy, B. Present and future distribution of three aquatic plants taxa across the world: Decrease in native and increase in invasive ranges. Biol. Invasions 2017, 19, 2159–2170. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Masdoumier, G.; Ghozlen, N.B.; Latouche, G. A new optical leaf-clip meter for simultaneous non-destructive assessment of leaf chlorophyll and epidermal flavonoids. Physiol. Plant. 2012, 146, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef] [Green Version]
- R Core Team R: A Language and Environment for Statistical Computing 2020. Available online: https://www.r-project.org/index.html (accessed on 20 May 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar]
- Fox, J.; Weisberg, S. An R Companion to Applied Regression, 3rd ed.; Sage Publications: Thousand Oaks, CA, USA, 2019. [Google Scholar]
- Nakagawa, S.; Schielzeth, H. A general and simple method for obtaining R 2 from generalized linear mixed-effects models. Methods Ecol. Evol. 2013, 4, 133–142. [Google Scholar] [CrossRef]
- Barton, K. MuMIn: Multi-Model Inference; R Package Version 1.43.17, 2020. Available online: https://cran.r-project.org/web/packages/MuMIn/index.html (accessed on 15 April 2020).
- de Mendiburu, F. Agricolae: Statistical Procedures for Agricultural Research; R Foundation: Vienna, Austria, 2013. [Google Scholar]
- Benaglia, T.; Chauveau, D.; Hunter, D.R.; Young, D. Mixtools: An R Package for Analyzing Finite Mixture Models. J. Stat. Softw. 2009, 32, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]


| % Sum Square | df | p | |
|---|---|---|---|
| Species | 36.3 | 5 | <0.001 |
| Temperature | <0.1 | 1 | 0.91 |
| Time | 3.8 | 1 | 0.01 |
| Species: Temperature | 1.2 | 5 | 0.83 |
| Species: Time | 2.6 | 5 | 0.48 |
| Temperature: Time | <0.1 | 1 | 0.85 |
| Species: Temperature: Time | 0.2 | 5 | 0.99 |
| Residuals | 55.9 | 96 |
| Percent Cover | Maximum Canopy Height | |||||
|---|---|---|---|---|---|---|
| Chi Square | df | p | Chi Square | df | p | |
| Time | 484.51 | 1 | <0.001 | 78.99 | 1 | <0.001 |
| Species | 402.21 | 5 | <0.001 | 1188.36 | 5 | <0.001 |
| Temperature | 0.22 | 1 | 0.64 | 0.38 | 1 | 0.54 |
| Time: Species | 103.38 | 5 | <0.001 | 302.93 | 5 | <0.001 |
| Time: Temperature | 5.91 | 1 | 0.015 | 4.22 | 1 | 0.039 |
| Species: Temperature | 71.47 | 5 | <0.001 | 34.78 | 5 | <0.001 |
| Time: Species: Temperature | 47.38 | 5 | <0.001 | 20.40 | 5 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillard, M.B.; Caudal, J.-P.; Deleu, C.; Thiébaut, G. Heterogeneous Impact of Water Warming on Exotic and Native Submerged and Emergent Plants in Outdoor Mesocosms. Plants 2021, 10, 1324. https://doi.org/10.3390/plants10071324
Gillard MB, Caudal J-P, Deleu C, Thiébaut G. Heterogeneous Impact of Water Warming on Exotic and Native Submerged and Emergent Plants in Outdoor Mesocosms. Plants. 2021; 10(7):1324. https://doi.org/10.3390/plants10071324
Chicago/Turabian StyleGillard, Morgane B., Jean-Pierre Caudal, Carole Deleu, and Gabrielle Thiébaut. 2021. "Heterogeneous Impact of Water Warming on Exotic and Native Submerged and Emergent Plants in Outdoor Mesocosms" Plants 10, no. 7: 1324. https://doi.org/10.3390/plants10071324







