Morpho-Physio-Biochemical and Molecular Responses of Maize Hybrids to Salinity and Waterlogging during Stress and Recovery Phase
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Plant Cultivation
2.2. Measurement and Physiological Analysis
2.3. Biochemical Parameters
2.3.1. Determination of Reactive Oxygen Species (ROS)
2.3.2. Antioxidant Enzyme Activities
2.4. Gene Expression Analysis
2.5. Experimental Design and Statistical Analysis
3. Results
3.1. Plant Growth and Development
3.1.1. Root and Shoot Length
3.1.2. Root and Shoot Fresh Weights
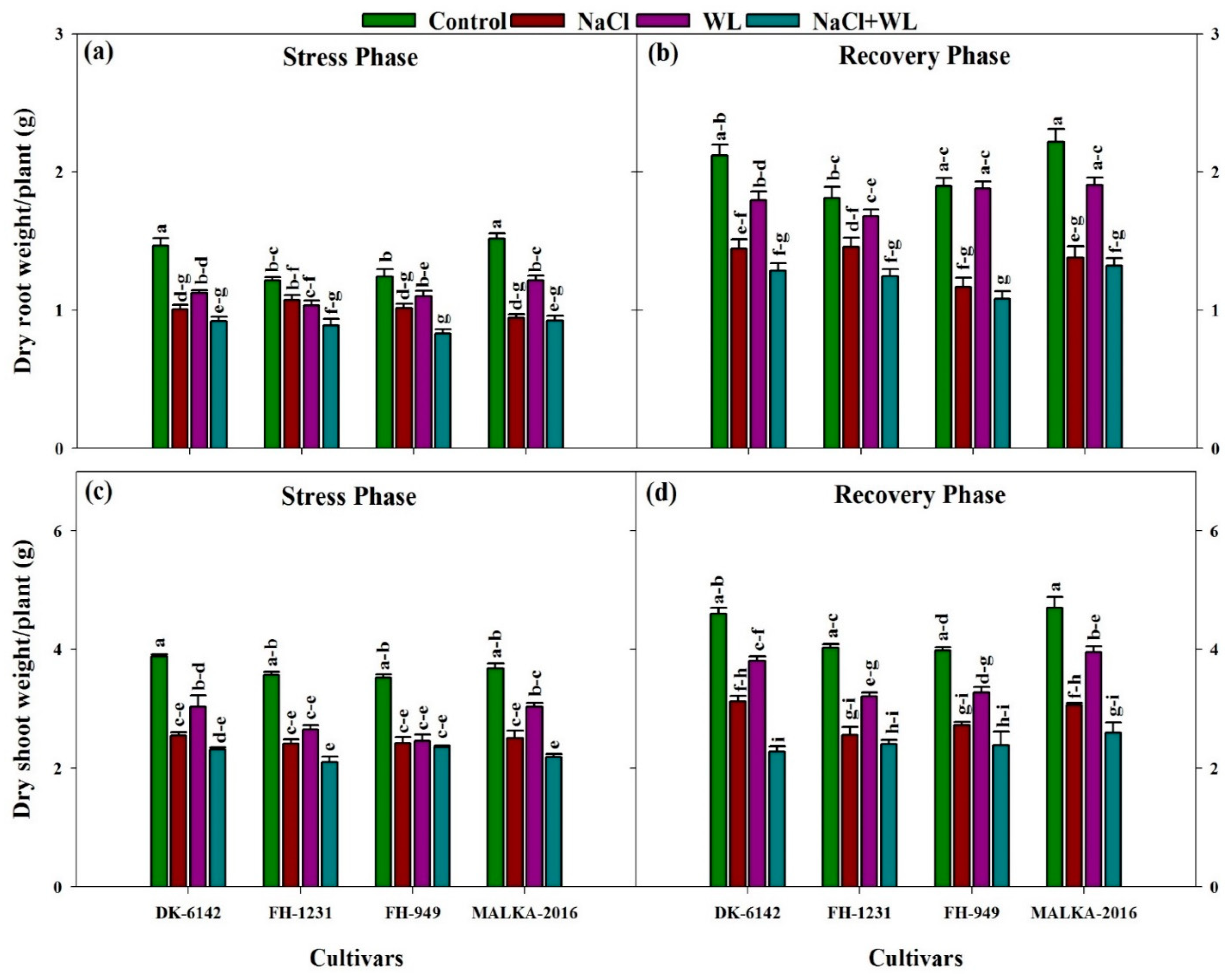
3.1.3. Dry Weight of Roots and Shoots
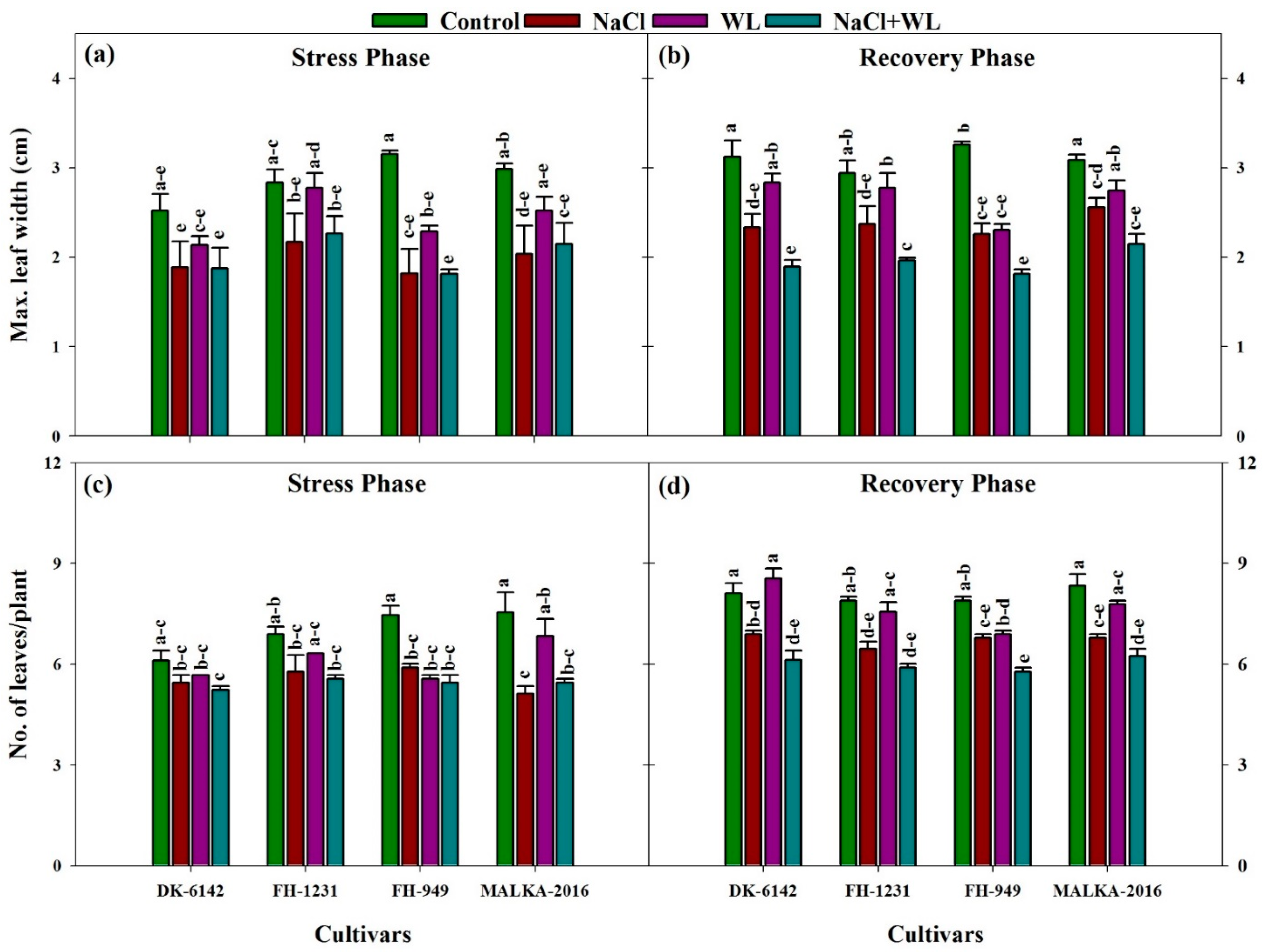
3.1.4. Leaf Width and Number of Leaves per Plant
3.2. Physiological Parameters
3.2.1. Chlorophyll and Carotenoids Contents
3.2.2. Hydrogen Peroxide Contents
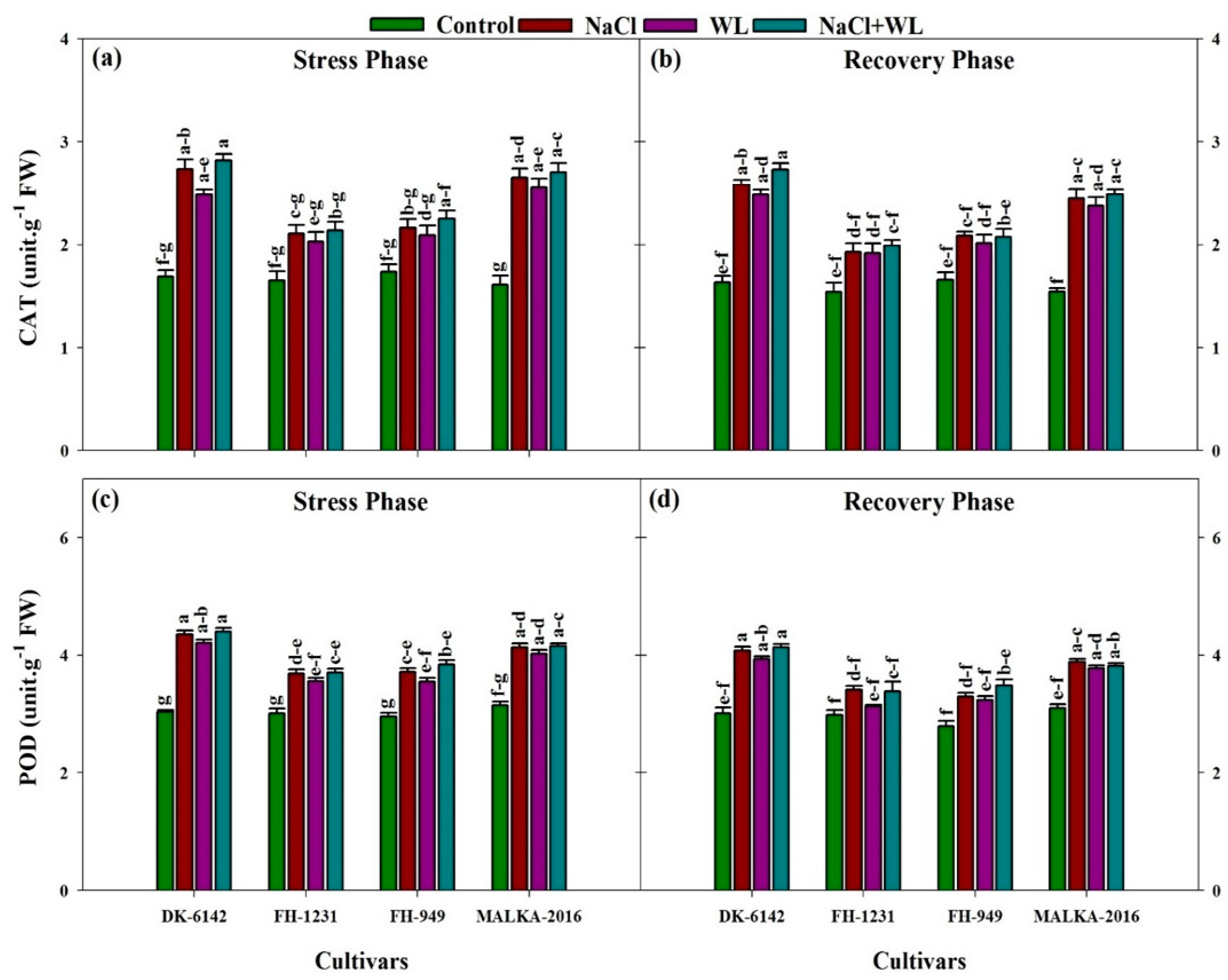
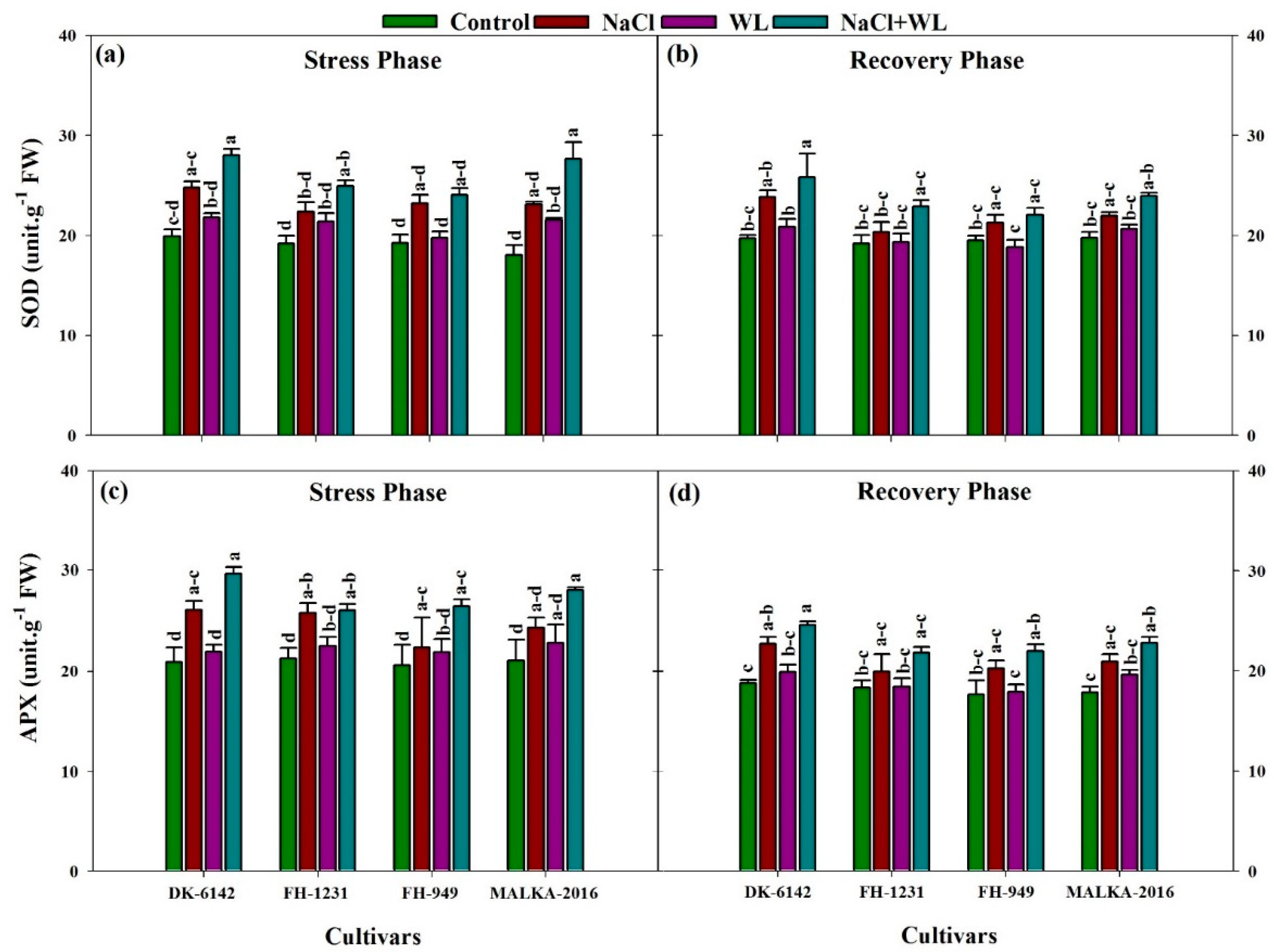
3.3. Antioxidant Enzymes
3.4. Gene Expression Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Qureshi, A.S.; McCornick, P.G.; Qadir, M.; Aslam, Z. Managing salinity and waterlogging in the Indus Basin of Pakistan. Agric. Water Manag. 2008, 95, 1–10. [Google Scholar] [CrossRef]
- Shah, T.R.; Prasad, K.; Kumar, P. Maize—A potential source of human nutrition and health: A review. Cogent Food Agric. 2016, 2, 1166995. [Google Scholar]
- Farooq, M.; Hussain, M.; Wakeel, A.; Siddique, K. Salt stress in maize: Effects, resistance mechanisms, and management. A review. Agron. Sustain. Dev. 2015, 35, 461–481. [Google Scholar] [CrossRef]
- Fukao, T.; Barrera-Figueroa, B.E.; Juntawong, P.; Peña-Castro, J.M. Submergence and waterlogging stress in plants: A review highlighting research opportunities and understudied aspects. Front. Plant Sci. 2019, 10, 340. [Google Scholar] [CrossRef] [PubMed]
- Sosa, L.; Llanes, A.; Reinoso, H.; Reginato, M.; Luna, V. Osmotic and specific ion effects on the germination of Prosopis strombulifera. Ann. Bot. 2005, 96, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Maiti, R.; Rodríguez, H.G.; Rajkumar, D.; Koushik, S.; Vidyasagar, P. Genotypic variability in salinity tolerance of maize pipe line hybrids at seedling stage. Inter. J. Bio-Resour. Stress Manag. 2012, 3, 427–432. [Google Scholar]
- Cramer, G.; Alberico, G.; Schmidt, C. Salt tolerance is not associated with the sodium accumulation of two maize hybrids. Funct. Plant Biol. 1994, 21, 675–692. [Google Scholar] [CrossRef]
- Maiti, R.; Maiti, L.E.; Maiti, S.; Maiti, A.M.; Maiti, M.; Maiti, H. Genotypic variability in maize Cultivars Zea mays L.) for resistance to drought and salinity at the seedling stage. J. Plant Physiol. 1996, 148, 741–744. [Google Scholar] [CrossRef]
- Ashraf, M.; Wahid, S. Time-course changes in organic metabolites and mineral nutrients in germinating maize seeds under salt (NaCl) stress. Seed Sci. Technol. 2000, 28, 641–656. [Google Scholar]
- Mansour, M.; Salama, K.; Ali, F.; Abou Hadid, A. Cell and plant responses to NaCl in Zea mays L. cultivars differing in salt tolerance. Gen. Appl. Plant Physiol 2005, 31, 29–41. [Google Scholar]
- Qu, C.; Liu, C.; Gong, X.; Li, C.; Hong, M.; Wang, L.; Hong, F. Impairment of maize seedling photosynthesis caused by a combination of potassium deficiency and salt stress. Environ. Exp. Bot. 2012, 75, 134–141. [Google Scholar] [CrossRef]
- Sumer, A. Evidence of sodium toxicity for the vegetative growth of maize during the first phase of salt stress. J. Appl. Bot. 2004, 78, 135–139. [Google Scholar]
- Gong, X.; Chao, L.; Zhou, M.; Hong, M.; Luo, L.; Wang, L.; Ying, W.; Jingwei, C.; Songjie, G.; Fashui, H. Oxidative damages of maize seedlings caused by exposure to a combination of potassium deficiency and salt stress. Plant Soil 2011, 340, 443–452. [Google Scholar] [CrossRef]
- Tanou, G.; Molassiotis, A.; Diamantidis, G. Induction of reactive oxygen species and necrotic death-like destruction in strawberry leaves by salinity. Environ. Exp. Bot. 2009, 65, 270–281. [Google Scholar] [CrossRef]
- Hajer, A.; Malibari, A.A.; Al-Zahrani, H.S.; Almaghrabi, O.A. Responses of three tomato cultivars to sea water salinity 1. Effect of salinity on the seedling growth. Afr. J. Biotechnol. 2006, 5, 855–861. [Google Scholar]
- Li, X.; Liu, Z.; Li, T. An impact test study of the flood disasters on summer corn’s characters and yield. Sci. Meteorol. Sin. 2011, 31, 79–82. [Google Scholar]
- Ren, B.; Zhang, J.; Dong, S.; Liu, P.; Zhao, B. Effects of waterlogging on leaf mesophyll cell ultrastructure and photosynthetic characteristics of summer maize. PLoS ONE 2016, 11, e0161424. [Google Scholar] [CrossRef] [PubMed]
- Savita, S.; Sheela, K.; Sunanda, S.; Shankar, A.; Ramakrishna, P. Stevia rebaudiana—A functional component for food industry. J. Hum. Ecol. 2004, 15, 261–264. [Google Scholar] [CrossRef]
- Grzesiak, M.T.; Ostrowska, A.; Hura, K.; Rut, G.; Janowiak, F.; Rzepka, A.; Hura, T.; Grzesiak, S. Interspecific differences in root architecture among maize and triticale genotypes grown under drought, waterlogging and soil compaction. Acta Physiol. Plant 2014, 36, 3249–3261. [Google Scholar] [CrossRef]
- Yu, M.; Zhou, Z.; Deng, X.; Li, J.; Mei, F.; Qi, Y. Physiological mechanism of programmed cell death aggravation and acceleration in wheat endosperm cells caused by waterlogging. Acta Physiol. Plant 2017, 39, 23. [Google Scholar] [CrossRef]
- Jackson, M.B.; Ram, P.C. Physiological and molecular basis of susceptibility and tolerance of rice plants to complete submergence. Ann. Bot. 2003, 91, 227–241. [Google Scholar] [CrossRef]
- Steffens, D.; Hutsch, B.; Eschholz, T.; Losak, T.; Schubert, S. Water logging may inhibit plant growth primarily by nutrient deficiency rather than nutrient toxicity. Plant Soil Environ. 2005, 51, 545. [Google Scholar] [CrossRef]
- Subbaiah, C.C.; Sachs, M.M. Molecular and cellular adaptations of maize to flooding stress. Ann. Bot. 2003, 91, 119–127. [Google Scholar] [CrossRef]
- Jackson, M.; Colmer, T. Response and adaptation by plants to flooding stress. Ann. Bot. 2005, 96, 501–505. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; de Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Hussain, S.; Khan, F.; Cao, W.; Wu, L.; Geng, M. Seed priming alters the production and detoxification of reactive oxygen intermediates in rice seedlings grown under sub-optimal temperature and nutrient supply. Front. Plant Sci. 2016, 7, 439. [Google Scholar] [CrossRef]
- Parveen, A.; Liu, W.; Hussain, S.; Asghar, J.; Perveen, S.; Xiong, Y. Silicon priming regulates morpho-physiological growth and oxidative metabolism in maize under drought stress. Plants 2019, 8, 431. [Google Scholar] [CrossRef]
- Ashraf, M.; Athar, H.; Harris, P.; Kwon, T. Some prospective strategies for improving crop salt tolerance. Adv. Agron. 2008, 97, 45–110. [Google Scholar]
- Setter, T.; Waters, I. Review of prospects for germplasm improvement for waterlogging tolerance in wheat, barley and oats. Plant Soil 2003, 253, 1–34. [Google Scholar] [CrossRef]
- Peizhou, X.; Yun, L.; Shu, Y. Studies of photosystem complexes and chlorophyll synthesis in chlorophyll-deficient rice mutant W1. Sci. Agric. Sin. 2006, 39, 1299–1305. [Google Scholar]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Aebi, H. Catalase. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Pütter, J. Peroxidases. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: New York, NY, USA, 1974; pp. 685–690. [Google Scholar]
- Dhindsa, R.S.; Plumb-Dhindsa, P.; Thorpe, T.A. Leaf senescence: Correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Nakano, Y.; Asada, K. Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol. 1981, 22, 867–880. [Google Scholar]
- Huang, H.; Lv, L.; Wang, D.; Guo, B.; Lv, J.; Luo, L.; Wen, B.; Kang, Y. Biochemical and molecular responses of maize (Zea mays L.) to 1, 2-dibromo-4-(1, 2 dibromoethyl) cyclohexane (TBECH) diastereomers: Oxidative stress, DNA damage, antioxidant enzyme gene expression and diversity of root exudates. Sci. Total Environ. 2021, 753, 141872. [Google Scholar] [CrossRef]
- Zheng, C.; Jiang, D.; Liu, F.; Dai, T.; Jing, Q.; Cao, W. Effects of salt and waterlogging stresses and their combination on leaf photosynthesis, chloroplast ATP synthesis, and antioxidant capacity in wheat. Plant Sci. 2009, 176, 575–582. [Google Scholar] [CrossRef]
- Falakboland, Z. Understanding the Physiology of Combined Salinity and Waterlogging Tolerance in Barley. Ph.D. Thesis, University of Tasmania, Tasmania, Australia, 2016. Available online: https://eprints.utas.edu.au/23432/ (accessed on 10 May 2021).
- Hussain, M.; Park, H.-W.; Farooq, M.; Jabran, K.; Lee, D.-J. Morphological and physiological basis of salt resistance in different rice genotypes. Int. J. Agric. Biol. 2013, 15, 113–118. [Google Scholar]
- Zeng, F.; Shabala, L.; Zhou, M.; Zhang, G.; Shabala, S. Barley responses to combined waterlogging and salinity stress: Separating effects of oxygen deprivation and elemental toxicity. Front. Plant Sci. 2013, 4, 313. [Google Scholar] [CrossRef]
- Millaleo, R.; Reyes-Díaz, M.; Alberdi, M.; Ivanov, A.; Krol, M.; Hüner, N. Excess manganese differentially inhibits photosystem I versus II in Arabidopsis thaliana. J. Exp. Bot. 2013, 64, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Führs, H.; Behrens, C.; Gallien, S.; Heintz, D.; Van Dorsselaer, A.; Braun, H.-P.; Horst, W.J. Physiological and proteomic characterization of manganese sensitivity and tolerance in rice (Oryza sativa) in comparison with barley (Hordeum vulgare). Ann. Bot. 2010, 105, 1129–1140. [Google Scholar] [CrossRef]
- Keunen, E.; Remans, T.; Bohler, S.; Vangronsveld, J.; Cuypers, A. Metal-induced oxidative stress and plant mitochondria. Int. J. Mol. Sci. 2011, 12, 6894–6918. [Google Scholar] [CrossRef] [PubMed]
- Katerji, N.; Van Hoorn, J.; Hamdy, A.; Mastrorilli, M. Comparison of corn yield response to plant water stress caused by salinity and by drought. Agric. Water Manag. 2004, 65, 95–101. [Google Scholar] [CrossRef]
- Giaveno, C.D.; Ribeiro, R.V.; Souza, G.M.; de Oliveira, R.F. Screening of tropical maize for salt stress tolerance. Crop. Breed. Appl. Biotechnol. 2007, 7, 304–313. [Google Scholar] [CrossRef][Green Version]
- Shahzad, M.; Witzel, K.; Zörb, C.; Mühling, K. Growth-related changes in subcellular ion patterns in maize leaves (Zea mays L.) under salt stress. J. Agron. Crop. Sci. 2012, 198, 46–56. [Google Scholar] [CrossRef]
- Rios-Gonzalez, K.; Erdei, L.; Lips, S.H. The activity of antioxidant enzymes in maize and sunflower seedlings as affected by salinity and different nitrogen sources. Plant Sci. 2002, 162, 923–930. [Google Scholar] [CrossRef]
- Akram, M.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Iqbal, J.; Mohsan, M. Screening for salt tolerance in maize (Zea mays L.) hybrids at an early seedling stage. Pak. J. Bot. 2010, 42, 141–154. [Google Scholar]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Schortemeyer, M. Changes in physiological and morphological traits of roots and shoots of wheat in response to different depths of waterlogging. Funct. Plant Biol. 2001, 28, 1121–1131. [Google Scholar] [CrossRef]
- Zubairi, Z.; Saeed, Z.; Nazir, A.; Saddique, S.; Chaudhary, F.; Saeed, S. Water Logging a serious problem for the growth of maize (Zea mays L.). Int. J. Water Resour. Environ. Sci. 2012, 1, 109–112. [Google Scholar]
- Barrett-Lennard, E. The interaction between waterlogging and salinity in higher plants: Causes, consequences and implications. Plant Soil 2003, 253, 35–54. [Google Scholar] [CrossRef]
- Armstrong, W. Root growth and metabolism under oxygen deficiency. In Plant Roots: The Hidden Half; Waisel, E.A., Kafkafi, Y., Eds.; Marcel Dekker: New York, NY, USA, 2002; pp. 729–761. [Google Scholar]
- Malik, A.I.; Colmer, T.D.; Lambers, H.; Setter, T.L.; Schortemeyer, M. Short-term waterlogging has long-term effects on the growth and physiology of wheat. New Phytol. 2002, 153, 225–236. [Google Scholar] [CrossRef]
- Gu, X.; Li, Y.; Du, Y. Compensative impact of winter oilseed rape (Brassica napus L.) affected by water stress at re-greening stage under different nitrogen rates. Chin. J. Eco-Agric. 2016, 24, 572–581. [Google Scholar]
- Duan, H.; Ma, Y.; Liu, R.; Li, Q.; Yang, Y.; Song, J. Effect of combined waterlogging and salinity stresses on euhalophyte Suaeda glauca. Plant Physiol. Biochem. 2018, 127, 231–237. [Google Scholar] [CrossRef]
- Bailey-Serres, J.; Voesenek, L. Flooding stress: Acclimations and genetic diversity. Ann. Rev. Plant Biol. 2008, 59, 313–339. [Google Scholar] [CrossRef] [PubMed]
- Barrett-Lennard, E.G.; Shabala, S.N. The waterlogging/salinity interaction in higher plants revisited–focusing on the hypoxia-induced disturbance to K+ homeostasis. Funct. Plant Biol. 2013, 40, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Aranda, A.; Pascual, A. Nuclear hormone receptors and gene expression. Physiol. Rev. 2001, 81, 1269–1304. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Burucs, Z.; von Tucher, S.; Schmidhalter, U. Short-term effects of drought and salinity on mineral nutrient distribution along growing leaves of maize seedlings. Environ. Exp. Bot. 2007, 60, 268–275. [Google Scholar] [CrossRef]
- Ashraf, M.A. Waterlogging stress in plants: A review. Afr. J. Agric. Res. 2012, 7, 1976–1981. [Google Scholar]
- Ashraf, M.A.; Ahmad, M.S.A.; Ashraf, M.; Al-Qurainy, F.; Ashraf, M.Y. Alleviation of waterlogging stress in upland cotton (Gossypium hirsutum L.) by exogenous application of potassium in soil and as a foliar spray. Crop. Pasture Sci. 2011, 62, 25–38. [Google Scholar] [CrossRef]
- Lim, J.-H.; Park, K.-J.; Kim, B.-K.; Jeong, J.-W.; Kim, H.-J. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012, 135, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Knox, J.P.; Dodge, A.D. Singlet oxygen and plants. Phytochemistry 1985, 24, 889–896. [Google Scholar] [CrossRef]
- Duhan, S.; Kumari, A.; Sheokand, S. Effect of waterlogging and salinity on antioxidative system in pigeonpea plant leaves at different stages of development. Res. Crop. 2017, 18, 559–568. [Google Scholar] [CrossRef]
- Hernandez, J.A.; Jiménez, A.; Mullineaux, P.; Sevilia, F. Tolerance of pea (Pisum sativum L.) to long-term salt stress is associated with induction of antioxidant defences. Plant Cell Environ. 2000, 23, 853–862. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Sheokand, S.; Kumari, A. Nitric oxide and abiotic stress-induced oxidative stress. In Nitric Oxide Action in Abiotic Stress Responses in Plants; Khan, M.N., Mobin, M., Mohammad, F., Corpas, F.J., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 43–63. [Google Scholar]
- Haddadi, B.S.; Hassanpour, H.; Niknam, V. Effect of salinity and waterlogging on growth, anatomical and antioxidative responses in Mentha aquatica L. Acta Physiol. Plant 2016, 38, 119. [Google Scholar] [CrossRef]
- Mubeen, K.; AH, S. Effect of water logging and salinity stress on physiological and biochemical changes in tolerant and susceptible varieties of Triticum aestivum L. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 975–981. [Google Scholar]
- Noctor, G.; Foyer, C.H. Ascorbate and glutathione: Keeping active oxygen under control. Ann. Rev. Plant Biol. 1998, 49, 249–279. [Google Scholar] [CrossRef]
- Sharma, I.; Ching, E.; Saini, S.; Bhardwaj, R.; Pati, P.K. Exogenous application of brassinosteroid offers tolerance to salinity by altering stress responses in rice variety Pusa Basmati-1. Plant Physiol. Biochem. 2013, 69, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Alhaithloul, H.A.; Hakeem, K.R.; Alharbi, B.M.; El-Esawi, M.; Elkelish, A. Exogenous nitric oxide mitigates nickel-induced oxidative damage in eggplant by upregulating antioxidants, osmolyte metabolism, and glyoxalase systems. Plants 2019, 8, 562. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.; Glascoe, A.; Engle, D.; Ritz, T.; Link, J.; Ahmad, M. Cellular metabolites modulate in vivo signaling of Arabidopsis cryptochrome-1. Plant Signal. Behav. 2015, 10, e1063758. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Al-Ghamdi, A.A.; Ali, H.M.; Alayafi, A.A.; Witczak, J.; Ahmad, M. Analysis of Genetic Variation and Enhancement of Salt Tolerance in French Pea (Pisum Sativum L.). Int. J. Mol. Sci. 2018, 19, 2433. [Google Scholar] [CrossRef]
- Alhaithloul, H.A.; Soliman, M.H.; Ameta, K.L.; El-Esawi, M.A.; Elkelish, A. Changes in Ecophysiology, Osmolytes, and Secondary Metabolites of the Medicinal Plants of Mentha piperita and Catharanthus roseus Subjected to Drought and Heat Stress. Biomolecules 2020, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Elkelish, A.; Qari, S.H.; Mazrou, Y.S.A.; Abdelaal, K.A.A.; Hafez, Y.M.; Abu-Elsaoud, A.M.; Batiha, G.E.-S.; El-Esawi, M.A.; El Nahhas, N. Exogenous Ascorbic Acid Induced Chilling Tolerance in Tomato Plants through Modulating Metabolism, Osmolytes, Antioxidants, and Transcriptional Regulation of Catalase and Heat Shock Proteins. Plants 2020, 9, 431. [Google Scholar] [CrossRef] [PubMed]
- El-Esawi, M.A.; Alayafi, A.A. Overexpression of StDREB2 Transcription Factor Enhances Drought Stress Tolerance in Cotton (Gossypium barbadense L.). Genes 2019, 10, 142. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmood, U.; Hussain, S.; Hussain, S.; Ali, B.; Ashraf, U.; Zamir, S.; Al-Robai, S.A.; Alzahrani, F.O.; Hano, C.; El-Esawi, M.A. Morpho-Physio-Biochemical and Molecular Responses of Maize Hybrids to Salinity and Waterlogging during Stress and Recovery Phase. Plants 2021, 10, 1345. https://doi.org/10.3390/plants10071345
Mahmood U, Hussain S, Hussain S, Ali B, Ashraf U, Zamir S, Al-Robai SA, Alzahrani FO, Hano C, El-Esawi MA. Morpho-Physio-Biochemical and Molecular Responses of Maize Hybrids to Salinity and Waterlogging during Stress and Recovery Phase. Plants. 2021; 10(7):1345. https://doi.org/10.3390/plants10071345
Chicago/Turabian StyleMahmood, Umer, Saddam Hussain, Sadam Hussain, Basharat Ali, Umair Ashraf, Shahid Zamir, Sami Asir Al-Robai, Fatima Omari Alzahrani, Christophe Hano, and Mohamed A. El-Esawi. 2021. "Morpho-Physio-Biochemical and Molecular Responses of Maize Hybrids to Salinity and Waterlogging during Stress and Recovery Phase" Plants 10, no. 7: 1345. https://doi.org/10.3390/plants10071345
APA StyleMahmood, U., Hussain, S., Hussain, S., Ali, B., Ashraf, U., Zamir, S., Al-Robai, S. A., Alzahrani, F. O., Hano, C., & El-Esawi, M. A. (2021). Morpho-Physio-Biochemical and Molecular Responses of Maize Hybrids to Salinity and Waterlogging during Stress and Recovery Phase. Plants, 10(7), 1345. https://doi.org/10.3390/plants10071345










