Phosphite Reduces the Predation Impact of Poterioochromonas malhamensis on Cyanobacterial Culture
Abstract
:1. Introduction
2. Results and Discussion
2.1. Detection of Chrysophyceae from Environmental Freshwater
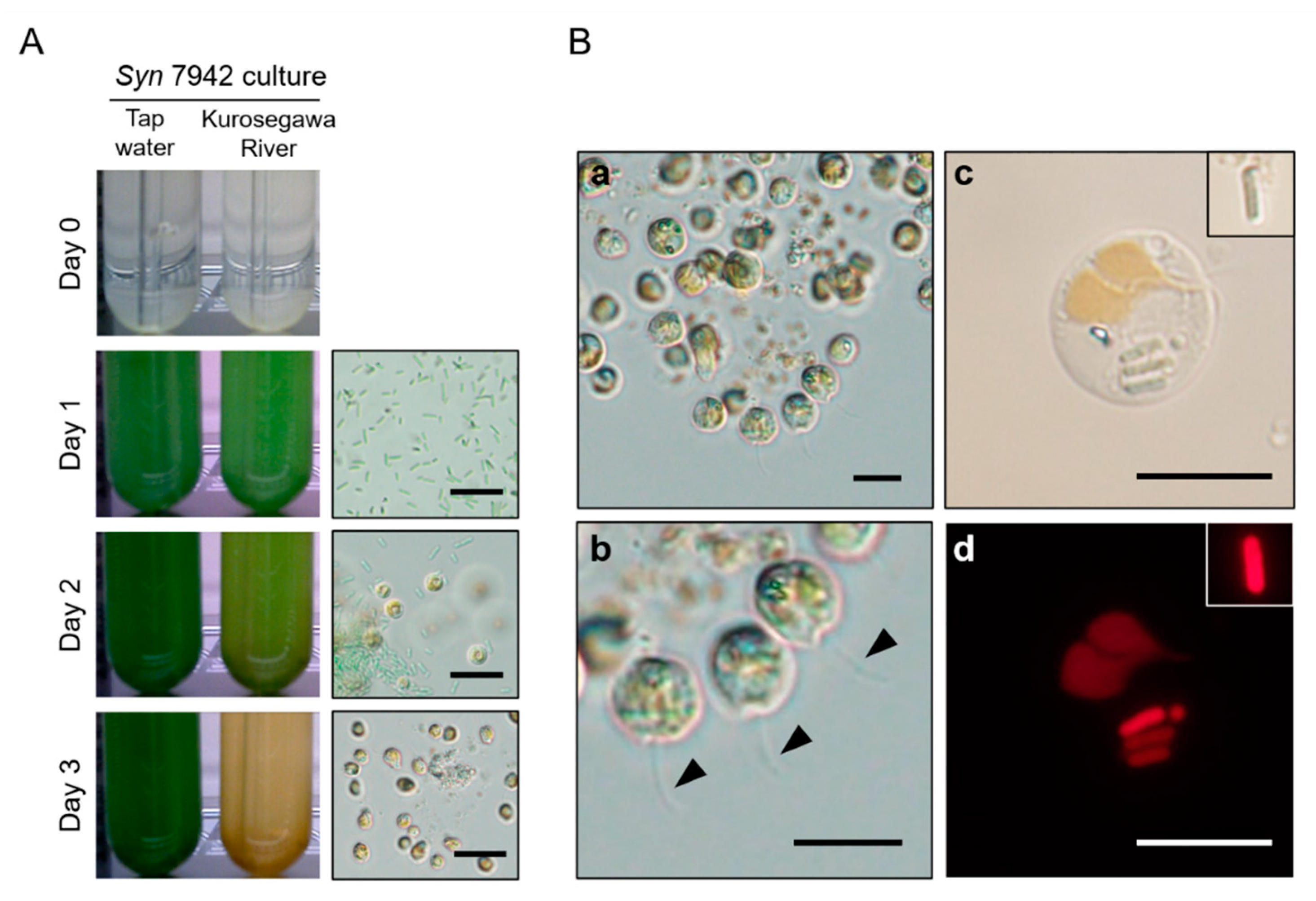
2.2. Isolation, Identification, and Characterization of Chrysophyceae Emerged in Syn 7942 Cultures
2.3. Pt Reduces Predation Impacts of P. malhamensis on Syn 7942 Cultivation
2.4. The Effect of Pt on Syn 7942 Culture Prepared with Environmental Freshwater
2.5. Eukaryotic Microbial Composition of the Culture Using Environmental Water Was Drastically Changed Depending on Applied P Sources on Syn 7942 Culture
3. Materials and Methods
3.1. Bacterial Strains and Culture Conditions
3.2. Cultivation Using Environmental Freshwater Samples
3.3. Identification of Protists in Environmental Freshwater
3.4. Isolation of P. malhamensis from Environmental Freshwater Samples
3.5. Co-Culture Experiment of Synechococcus with Isolated P. malhamensis
3.6. Phylogenetic Analysis
3.7. Microbial Community Analysis
3.8. Nucleotide Sequence Accession Numbers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Williams, P.J.L.B.; Laurens, L.M.L. Microalgae as biodiesel & biomass feedstocks: Review & analysis of the biochemistry, energetics & economics. Energy Environ. Sci. 2010, 3, 554–590. [Google Scholar] [CrossRef]
- Bilanovic, D.; Andargatchew, A.; Kroeger, T.; Shelef, G. Freshwater and marine microalgae sequestering of CO2 at different C and N concentrations—Response surface methodology analysis. Energy Convers. Manag. 2009, 50, 262–267. [Google Scholar] [CrossRef]
- Borowitzka, M.A. High-value products from microalgae—Their development and commercialisation. J. Appl. Phycol. 2013, 25, 743–756. [Google Scholar] [CrossRef]
- Deore, P.; Beardall, J.; Noronha, S. A perspective on the current status of approaches for early detection of microalgal grazing. J. Appl. Phycol. 2020, 32, 3723–3733. [Google Scholar] [CrossRef]
- McBride, R.C.; Lopez, S.; Meenach, C.; Burnett, M.; Lee, P.A.; Nohilly, F.; Behnke, C. Contamination Management in Low Cost Open Algae Ponds for Biofuels Production. Ind. Biotechnol. 2014, 10, 221–227. [Google Scholar] [CrossRef]
- Lee, Y.K. Microalgal mass culture systems and methods: Their limitation and potential. J. Appl. Phycol. 2001, 13, 307–315. [Google Scholar] [CrossRef]
- Carney, L.T.; Wilkenfeld, J.S.; Lane, P.D.; Solberg, O.D.; Fuqua, Z.B.; Cornelius, N.G.; Gillespie, S.; Williams, K.P.; Samocha, T.M.; Lane, T.W. Pond Crash Forensics: Presumptive identification of pond crash agents by next generation sequencing in replicate raceway mass cultures of Nannochloropsis salina. Algal Res. 2016, 17, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M.W.; Hofle, M.G. Grazing of protozoa and its effect on populations of aquatic bacteria. FEMS Microbiol. Ecol. 2001, 35, 113–121. [Google Scholar] [CrossRef]
- Day, J.G.; Gong, Y.; Hu, Q. Microzooplanktonic grazers—A potentially devastating threat to the commercial success of microalgal mass culture. Algal Res. 2017, 27, 356–365. [Google Scholar] [CrossRef]
- Ma, M.Y.; Yuan, D.N.; He, Y.; Park, M.; Gong, Y.C.; Hu, Q. Effective control of Poterioochromonas malhamensis in pilot-scale culture of Chlorella sorokiniana GT-1 by maintaining CO2-mediated low culture pH. Algal Res. Biomass Biofuels Bioprod. 2017, 26, 436–444. [Google Scholar] [CrossRef]
- Ma, M.; Gong, Y.; Hu, Q. Identification and feeding characteristics of the mixotrophic flagellate Poterioochromonas malhamensis, a microalgal predator isolated from outdoor massive Chlorella culture. Algal Res. 2018, 29, 142–153. [Google Scholar] [CrossRef]
- Zhang, X.M.; Watanabe, M.M. Grazing and growth of the mixotrophic chrysomonad Poterioochromonas malhamensis (Chrysophyceae) feeding on algae. J. Phycol. 2001, 37, 738–743. [Google Scholar] [CrossRef]
- Touloupakis, E.; Cicchi, B.; Benavides, A.M.S.; Torzillo, G. Effect of high pH on growth of Synechocystis sp. PCC 6803 cultures and their contamination by golden algae (Poterioochromonas sp.). Appl. Microbiol. Biotechnol. 2016, 100, 1333–1341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Wei, C.; Wang, H.; Sha, C.; Chen, M.; Gong, Y.; Hu, Q. Isolation and evaluation of a novel strain of Chlorella sorokiniana that resists grazing by the predator Poterioochromonas malhamensis. Algal Res. 2019, 38, 101429. [Google Scholar] [CrossRef]
- White, A.K.; Metcalf, W.W. Microbial metabolism of reduced phosphorus compounds. Ann. Rev. Microbiol. 2007, 61, 379–400. [Google Scholar] [CrossRef] [PubMed]
- Costas, A.M.; White, A.K.; Metcalf, W.W. Purification and characterization of a novel phosphorus-oxidizing enzyme from Pseudomonas stutzeri WM88. J. Biol. Chem. 2001, 276, 17429–17436. [Google Scholar] [CrossRef] [Green Version]
- Hirota, R.; Motomura, K.; Kuroda, A. Biological Phosphite Oxidation and Its Application to Phosphorus Recycling. In Phosphorus Recovery and Recycling; Springer: Singapore, 2019; pp. 499–513. [Google Scholar]
- Kanda, K.; Ishida, T.; Hirota, R.; Ono, S.; Motomura, K.; Ikeda, T.; Kitamura, K.; Kuroda, A. Application of a phosphite dehydrogenase gene as a novel dominant selection marker for yeasts. J. Biotechnol. 2014, 182–183, 68–73. [Google Scholar] [CrossRef]
- Guo, Z.-W.; Ou, X.-Y.; Xu, P.; Gao, H.-F.; Zhang, L.-Y.; Zong, M.-H.; Lou, W.-Y. Energy- and cost-effective non-sterilized fermentation of 2,3-butanediol by an engineered Klebsiella pneumoniae OU7 with an anti-microbial contamination system. Green Chem. 2020, 22, 8584–8593. [Google Scholar] [CrossRef]
- Guo, Z.W.; Ou, X.Y.; Liang, S.; Gao, H.F.; Zhang, L.Y.; Zong, M.H.; Lou, W.Y. Recruiting a Phosphite Dehydrogenase/Formamidase-Driven Antimicrobial Contamination System in Bacillus subtilis for Nonsterilized Fermentation of Acetoin. ACS Synth. Biol. 2020, 9, 2537–2545. [Google Scholar] [CrossRef]
- Shaw, A.J.; Lam, F.H.; Hamilton, M.; Consiglio, A.; MacEwen, K.; Brevnova, E.E.; Greenhagen, E.; LaTouf, W.G.; South, C.R.; van Dijken, H.; et al. Metabolic engineering of microbial competitive advantage for industrial fermentation processes. Science 2016, 353, 583–586. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Morales, S.I.; Pacheco-Gutierrez, N.B.; Ramirez-Rodriguez, C.A.; Brito-Bello, A.A.; Estrella-Hernandez, P.; Herrera-Estrella, L.; Lopez-Arredondo, D.L. Metabolic engineering of phosphite metabolism in Synechococcus elongatus PCC 7942 as an effective measure to control biological contaminants in outdoor raceway ponds. Biotechnol. Biofuels 2020, 13, 119. [Google Scholar] [CrossRef]
- Motomura, K.; Sano, K.; Watanabe, S.; Kanbara, A.; Gamal Nasser, A.H.; Ikeda, T.; Ishida, T.; Funabashi, H.; Kuroda, A.; Hirota, R. Synthetic Phosphorus Metabolic Pathway for Biosafety and Contamination Management of Cyanobacterial Cultivation. ACS Synth. Biol. 2018, 7, 2189–2198. [Google Scholar] [CrossRef] [PubMed]
- Selao, T.T.; Wlodarczyk, A.; Nixon, P.J.; Norling, B. Growth and selection of the cyanobacterium Synechococcus sp. PCC 7002 using alternative nitrogen and phosphorus sources. Metab. Eng. 2019, 54, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Changko, S.; Rajakumar, P.D.; Young, R.E.B.; Purton, S. The phosphite oxidoreductase gene, ptxD as a bio-contained chloroplast marker and crop-protection tool for algal biotechnology using Chlamydomonas. Appl. Microbiol. Biotechnol. 2020, 104, 675–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, R.A.; Graf, L.; Malakhov, Y.; Yoon, H.S. Rediscovery of the Ochromonas type species Ochromonas triangulata (Chrysophyceae) from its type locality (Lake Veysove, Donetsk region, Ukraine). Phycologia 2019, 56, 591–604. [Google Scholar] [CrossRef]
- Machinandiarena, M.F.; Lobato, M.C.; Feldman, M.L.; Daleo, G.R.; Andreu, A.B. Potassium phosphite primes defense responses in potato against Phytophthora infestans. J. Plant Physiol. 2012, 169, 1417–1424. [Google Scholar] [CrossRef] [PubMed]
- Shade, A.; Jones, S.E.; McMahon, K.D. The influence of habitat heterogeneity on freshwater bacterial community composition and dynamics. Environ. Microbiol. 2008, 10, 1057–1067. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, L.F.; Margis, R. The source of the river as a nursery for microbial diversity. PLoS ONE 2015, 10, e0120608. [Google Scholar] [CrossRef]
- Scoble, J.M.; Cavalier-Smith, T. Scale evolution in Paraphysomonadida (Chrysophyceae): Sequence phylogeny and revised taxonomy of Paraphysomonas, new genus Clathromonas, and 25 new species. Eur. J. Protistol. 2014, 50, 551–592. [Google Scholar] [CrossRef] [Green Version]
- Medlin, L.; Elwood, H.J.; Stickel, S.; Sogin, M.L. The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene 1988, 71, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef] [Green Version]
- Talavera, G.; Castresana, J. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 2007, 56, 564–577. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular Evolutionary Genetics Analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Tian, R.M.; Gao, Z.M.; Bougouffa, S.; Qian, P.Y. Optimal eukaryotic 18S and universal 16S/18S ribosomal RNA primers and their application in a study of symbiosis. PLoS ONE 2014, 9, e90053. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toda, N.; Murakami, H.; Kanbara, A.; Kuroda, A.; Hirota, R. Phosphite Reduces the Predation Impact of Poterioochromonas malhamensis on Cyanobacterial Culture. Plants 2021, 10, 1361. https://doi.org/10.3390/plants10071361
Toda N, Murakami H, Kanbara A, Kuroda A, Hirota R. Phosphite Reduces the Predation Impact of Poterioochromonas malhamensis on Cyanobacterial Culture. Plants. 2021; 10(7):1361. https://doi.org/10.3390/plants10071361
Chicago/Turabian StyleToda, Narumi, Hiroki Murakami, Akihiro Kanbara, Akio Kuroda, and Ryuichi Hirota. 2021. "Phosphite Reduces the Predation Impact of Poterioochromonas malhamensis on Cyanobacterial Culture" Plants 10, no. 7: 1361. https://doi.org/10.3390/plants10071361





