Spatially Varying Relationships between Alien Plant Distributions and Environmental Factors in South Korea
Abstract
:1. Introduction
2. Results
3. Discussion
4. Materials and Methods
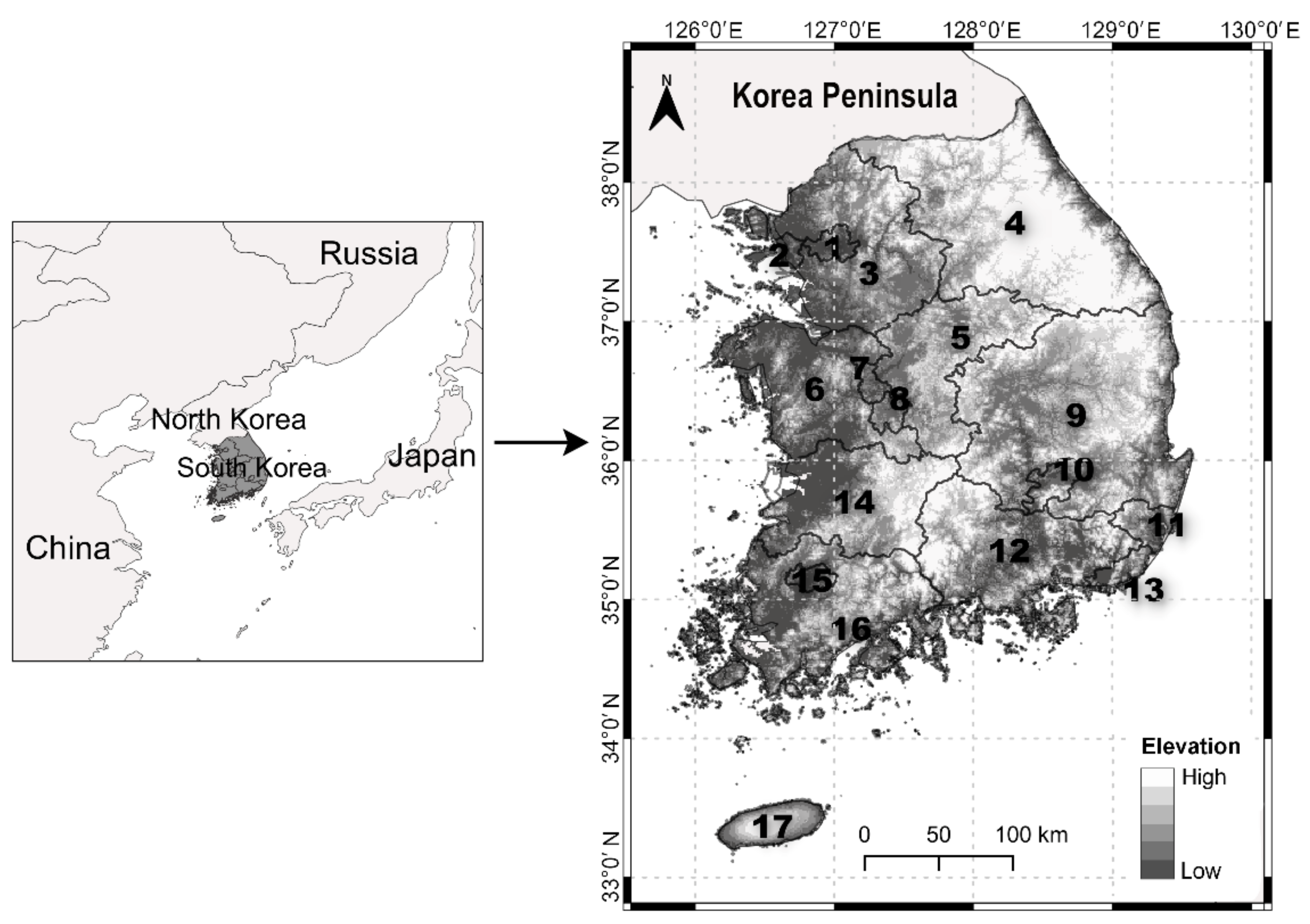
4.1. Study Area
4.2. Study Species
4.3. Data Preparation
4.4. Data Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hooftman, D.A.; Oostermeijer, J.G.; Den Nijs, J.C. Invasive behaviour of Lactuca serriola (Asteraceae) in the Netherlands: Spatial distribution and ecological amplitude. Basic Appl. Ecol. 2006, 7, 507–519. [Google Scholar] [CrossRef]
- Franklin, J. Mapping Species Distributions: Spatial Inference and Prediction; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Lebeda, A.; Doležalová, I.; Novotná, A. Wild and weedy Lactuca species, their distribution, ecogeography and ecobiology in USA and Canada. Genet. Resour. Crop Evol. 2012, 59, 1805–1822. [Google Scholar] [CrossRef]
- Novo, M.; Cunha, L.; Maced a-Veiga, A.; Talavera, J.A.; Hodson, M.E.; Spurgeon, D.; Bruford, M.W.; Morgan, A.J.; Kille, P. Multiple introductions and environmental factors affecting the establishment of invasive species on a volcanic island. Soil Biol. Biochem. 2015, 85, 89–100. [Google Scholar] [CrossRef] [Green Version]
- Park, J.S.; Yun, J.H.; Choi, J.Y.; Kim, J.C.; Lee, J.; Song, H.R. Multivariate associations between environmental variables and the invasion of alien plants in floodplain waterfront parklands along the Nakdong River. J. Plant Biol. 2019, 62, 400–409. [Google Scholar] [CrossRef]
- Pyšek, P.; Richardson, D.M. Invasive species, environmental change and management, and health. Annu. Rev. Environ. Resour. 2010, 21, 25–55. [Google Scholar] [CrossRef] [Green Version]
- Walther, G.R.; Roques, A.; Hulme, P.E.; Sykes, M.T.; Pyšek, P.; Kühn, I.; Zobel, M.; Bacher, S.; Botta-Dukat, Z.; Bugmann, H.; et al. Alien species in a warmer world: Risks and opportunities. Trends Ecol. Evol. 2009, 24, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Tripathi, P.; Behera, M.D.; Roy, P.S. Plant invasion correlation with climate anomaly: An Indian retrospect. Biodivers. Conserv. 2019, 28, 2049–2062. [Google Scholar] [CrossRef]
- Mou, Y.; He, Q.; Zhou, B. Detecting the spatially non-stationary relationships between housing price and its determinants in China: Guide for housing market sustainability. Sustainability 2017, 9, 1826. [Google Scholar] [CrossRef] [Green Version]
- Brunsdon, C.; Fotheringham, S.; Charlton, M. Geographically weighted regression. J. R. Stat. Soc. Ser. D Stat. 1998, 47, 431–443. [Google Scholar] [CrossRef]
- Nakaya, T.; Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically weighted Poisson regression for disease association mapping. Stat. Med. 2005, 24, 2695–2717. [Google Scholar] [CrossRef] [Green Version]
- Jensen, T.; Deller, S. Spatial modeling of the migration of older people with a focus on amenities. Rev. Reg. Stud. 2007, 37, 303–343. [Google Scholar]
- Park, J.; Choi, B.; Lee, J. Spatial distribution characteristics of species diversity using geographically weighted regression model. Sens. Mat. 2019, 31, 3197–3213. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.H.; Kil, J.H.; Hwang, S.M.; Lee, C.W. Spreading and distribution of Lactuca scariola, invasive alien plant, by habitat types in Korea. Weed Turfgrass Sci. 2013, 2, 138–151. [Google Scholar] [CrossRef]
- Kim, C.G.; Kil, J. Alien flora of the Korean Peninsula. Biol. Invasions 2016, 18, 1843–1852. [Google Scholar] [CrossRef]
- Lebeda, A.; Doležalová, I.; Křístková, E.; Mieslerová, B. Biodiversity and ecogeography of wild Lactuca spp. in some European countries. Genet. Resour. Crop Evol. 2001, 48, 153–164. [Google Scholar] [CrossRef]
- Weaver, S.E.; Downs, M.P. The biology of Canadian weeds. 122. Lactuca serriola L. Can. J. Plant Sci. 2003, 83, 619–628. [Google Scholar] [CrossRef] [Green Version]
- Chmielewski, J.G.; Semple, J.C. The biology of Canadian weeds. 114. Symphyotrichum pilosum (Willd.) Nesom (Aster pilosus Willd.). Can. J. Plant Sci. 2001, 81, 851–865. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.J.; Ni, J.; Tenhunen, J. Application of a geographically weighted regression analysis to estimate net primary production of Chinese forest ecosystems. Glob. Ecol. Biolgeogr. 2005, 14, 379–393. [Google Scholar] [CrossRef]
- Kupfer, J.A.; Farris, C.A. Incorporating spatial non-stationarity of regression coefficients into predictive vegetation models. Landsc. Ecol. 2007, 22, 837–852. [Google Scholar] [CrossRef]
- Gutterman, Y. Maturation dates affecting the germinability of Lactuca serriola L. achenes collected from a natural population in the Negev desert highlands. Germination under constant temperatures. J. Arid Environ. 1992, 22, 353–362. [Google Scholar] [CrossRef]
- Prince, S.D.; Carter, R.N. The geographical distribution of prickly lettuce (Lactuca serriola): III. Its performance in transplant sites beyond its distribution limit in Britain. J. Ecol. 1985, 1, 49–64. [Google Scholar] [CrossRef]
- Pitelka, L.F.; Plant Migration Workshop Group. Plant migration and climate change: A more realistic portrait of plant migration is essential to predicting biological responses to global warming in a world drastically altered by human activity. Am. Sci. 1997, 85, 464–473. [Google Scholar]
- D’Andrea, L.; Broennimann, O.; Kozlowski, G.; Guisan, A.; Morin, X.; Keller-Senften, J.; Felber, F. Climate change, anthropogenic disturbance and the northward range expansion of Lactuca serriola (Asteraceae). J. Biogeogr. 2009, 36, 1573–1587. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D. Plant Strategies and the Dynamics and Structure of Plant Communities. (MPB-26); Princeton University Press: Princeton, NJ, USA, 2020; Volume 26. [Google Scholar]
- Prince, S.D.; Marks, M.K.; Carter, R.N. Induction of flowering in wild lettuce (Lactuca serriola L.) vernalization. New Phytol. 1978, 81, 265–277. [Google Scholar] [CrossRef]
- Peterson, D.L.; Bazzaz, F.A. Life cycle characteristics of Aster pilosus in early successional habitats. Ecology 1978, 59, 1005–1013. [Google Scholar] [CrossRef]
- [KOSIS] Korean Statistical Information Service. Available online: https://kosis.kr (accessed on 1 May 2021).
- Yun, K.S.; Heo, K.Y.; Chu, J.E.; Ha, K.J.; Lee, E.J.; Choi, Y.; Kitoh, A. Changes in climate classification and extreme climate indices from a high-resolution future projection in Korea. Asia Pac. J. Atmos. Sci. 2012, 48, 213–226. [Google Scholar] [CrossRef]
- [KMA] Korea Meteorological Administration. Available online: https://www.weather.go.kr (accessed on 1 May 2021).
- KewScience: Plants of the World Online. Available online: http://plantsoftheworldonline.org/taxon/ (accessed on 20 April 2021).
- Wu, H.; Asaduzzaman, M.; Shephard, A.; Hopwood, M.; Ma, X. Germination and emergence characteristics of prickly lettuce (Lactuca serriola L.). Crop Prot. 2020, 136, 105222. [Google Scholar] [CrossRef]
- Weaver, S.; Cluney, K.; Downs, M.; Page, E. Prickly lettuce (Lactuca serriola) interference and seed production in soybeans and winter wheat. Weed Sci. 2006, 54, 496–503. [Google Scholar] [CrossRef]
- Public Data Portal. Available online: https://www.data.go.kr (accessed on 10 January 2021).
- WorldClim. Available online: https://worldclim.org (accessed on 10 January 2021).
- Graham, M.H. Confronting multicollinearity in ecological multiple regression. Ecology 2003, 84, 2809–2815. [Google Scholar] [CrossRef] [Green Version]
- Fotheringham, A.S.; Charlton, M.; Brunsdon, C. Measuring spatial variations in relationships with geographically weighted regression. In Recent Developments in Spatial Analysis; Springer: Berlin/Heidelberg, Germany, 1997; pp. 60–82. [Google Scholar]
- Fotheringham, A.S.; Brunsdon, C.; Charlton, M. Geographically Weighted Regression: The Analysis of Spatially Varying Relationships; John Wiley & Sons: New York, NY, USA, 2003. [Google Scholar]
- Mennis, J. Mapping the results of geographically weighted regression. Cartogr. J. 2006, 43, 171–179. [Google Scholar] [CrossRef] [Green Version]
- Brunsdon, C.; Fotheringham, A.S.; Charlton, M.E. Geographically weighted regression: A method for exploring spatial nonstationarity. Geogr. Anal. 1996, 28, 281–298. [Google Scholar] [CrossRef]
- Moran, P.A. The interpretation of statistical maps. J. R. Stat. Soc. Ser. B Methodol. 1948, 10, 243–251. [Google Scholar] [CrossRef]

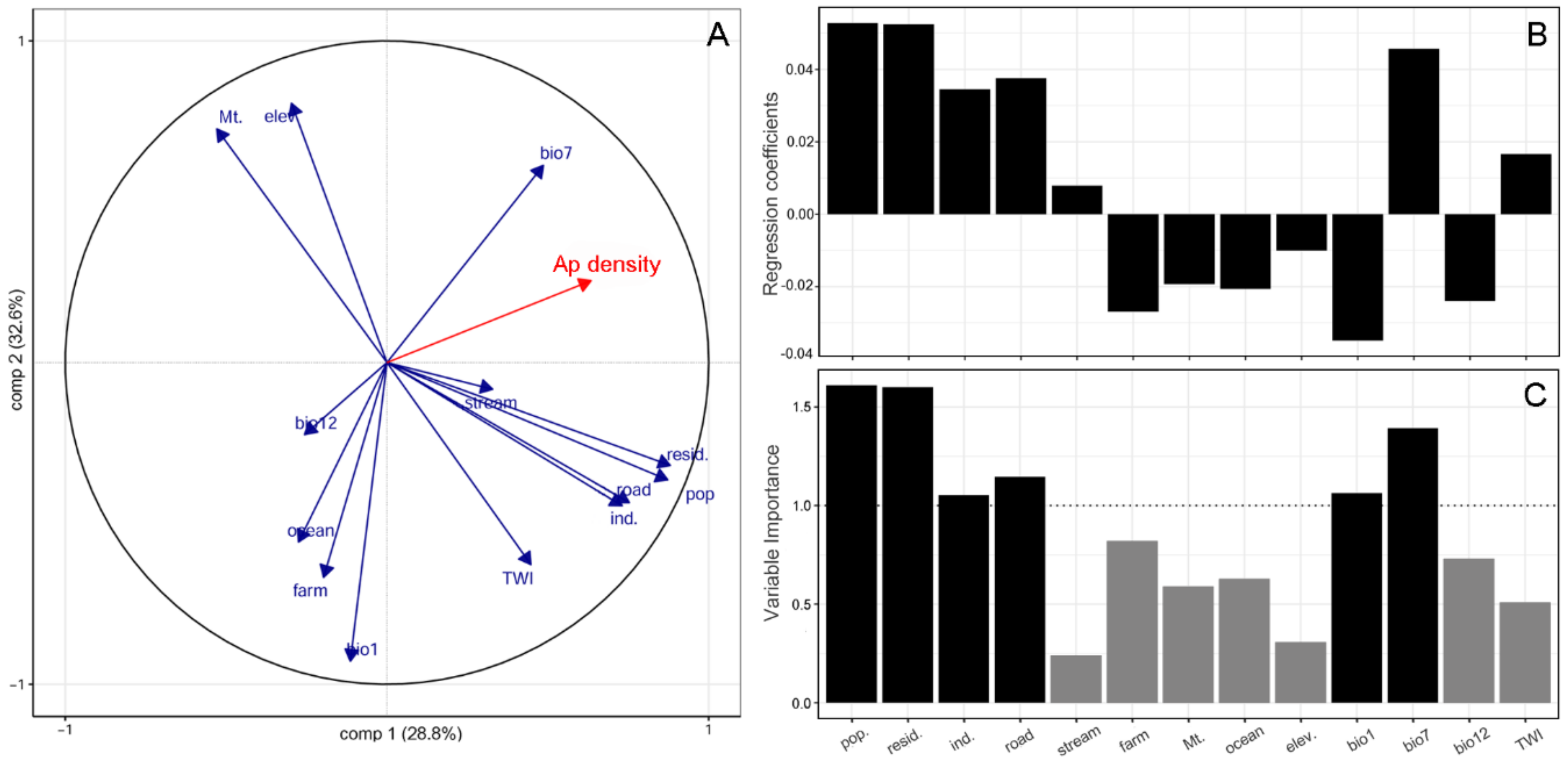
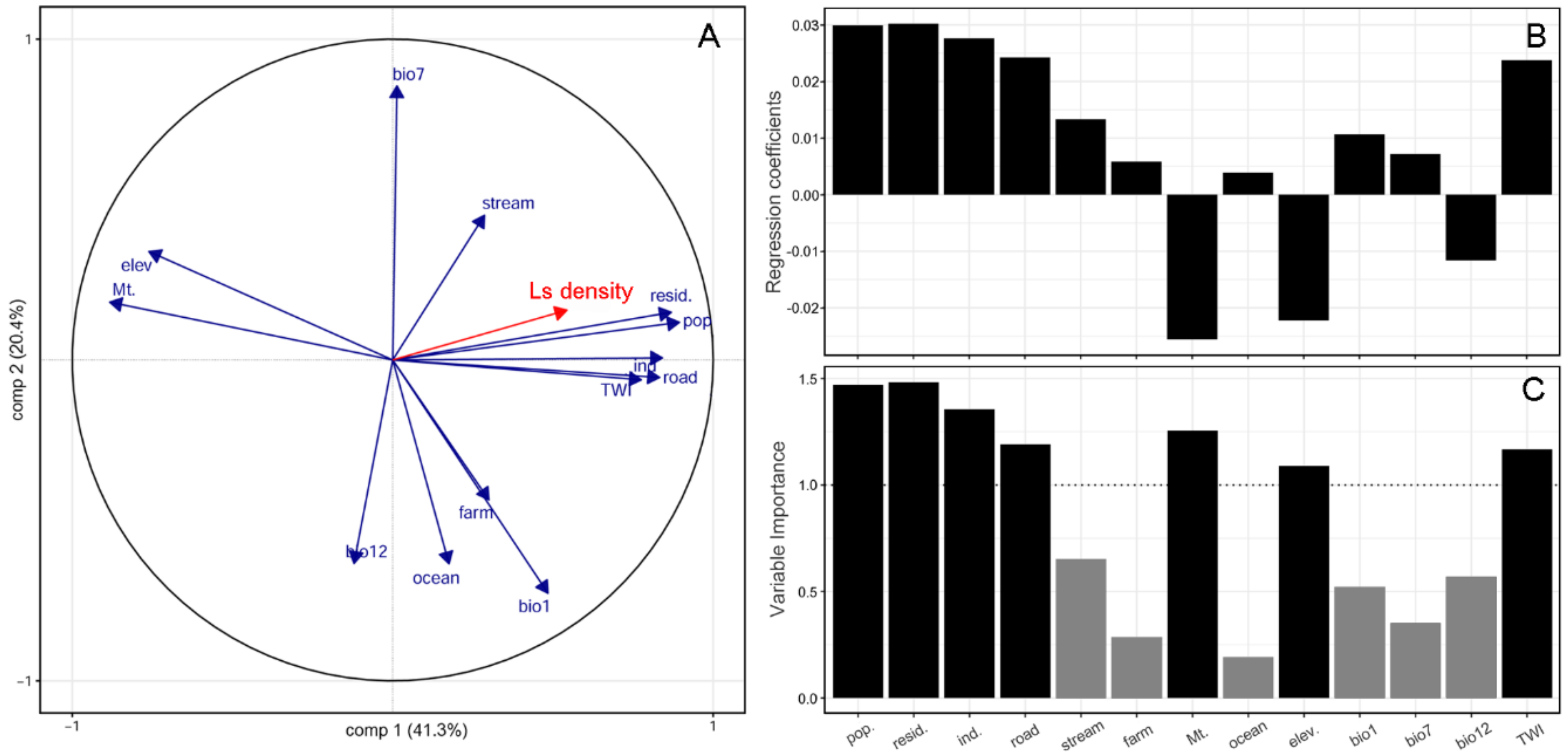


| Category | OLS | GWR | ||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | t-Value a | 2.5% | 50% | 97.5% | |||
| A. pilosus | Coefficient | Intercept | 0.000 | 0.059 | 0.000 | −0.839 | −0.141 | 0.894 |
| Residential area | 0.492 | 0.059 | 8.376 ** | 0.134 | 0.416 | 0.602 | ||
| Bio7 | 0.421 | 0.059 | 7.171 ** | −0.310 | 0.333 | 0.704 | ||
| Performance | Adjusted R2 | 0.44 | 0.66 | |||||
| AIC | 379.8 | 304.2 | ||||||
| Moran’s I for residuals | 0.098 (p = 0.110) | 0.078 (p = 0.078) | ||||||
| L. scariola | Coefficient | Intercept | 0.000 | 0.067 | 0.000 | −0.365 | 0.030 | 0.335 |
| Residential area | 0.392 | 0.085 | 4.604 ** | −0.133 | 0.279 | 0.675 | ||
| Mountain | −0.186 | 0.085 | −2.186 * | −0.486 | −0.278 | −0.013 | ||
| Performance | Adjusted R2 | 0.27 | 0.48 | |||||
| AIC | 422.1 | 379.6 | ||||||
| Moran’s I for residuals | 0.121 (p = 0.023) | 0.047 (p = 0.184) | ||||||
| Category | Variables | Mean | Minimum | Maximum | SD | Moran’s I |
|---|---|---|---|---|---|---|
| Dependent variables | A. pilosus population/100 km2 | 3.2 | 0 | 32.8 | 5.3 | 0.632 * |
| L. scariola population/100 km2 | 2 | 0 | 25.7 | 3.4 | 0.316 * | |
| Anthropogenic activity | Population (people/km2) | 1101 | 19 | 16,074 | 2457 | 0.571 * |
| Residential area (%) | 4.9 | 0.04 | 53.7 | 8.4 | 0.505 * | |
| Industrial area (%) | 2.4 | 0.02 | 20.1 | 3.7 | 0.338 * | |
| Road (km/km2) | 1.6 | 0.3 | 13.7 | 1.9 | 0.397 * | |
| Land use | Stream area (%) | 3 | 0.002 | 13.1 | 2.1 | 0.285 * |
| Farm (%) | 20.1 | 2.6 | 50 | 9.9 | 0.607 * | |
| Mountain (%) | 58.5 | 15.2 | 89.4 | 17.7 | 0.664 * | |
| Topographic properties | Elevation (m) | 175.3 | 7.0 | 894 | 158.3 | 0.686 * |
| Normalized TWI | 0.42 | 0 | 1 | 0.22 | 0.613 * | |
| Climate properties | Bio1 (°C) | 11.6 | 7.9 | 14.1 | 1.3 | 0.836 * |
| Bio7 (°C) | 35.3 | 27.3 | 38.4 | 2.1 | 0.847 * | |
| Bio12 (mm) | 1296 | 1100 | 1869 | 104 | 0.869 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.-S.; Lee, H.; Choi, D.; Kim, Y. Spatially Varying Relationships between Alien Plant Distributions and Environmental Factors in South Korea. Plants 2021, 10, 1377. https://doi.org/10.3390/plants10071377
Park J-S, Lee H, Choi D, Kim Y. Spatially Varying Relationships between Alien Plant Distributions and Environmental Factors in South Korea. Plants. 2021; 10(7):1377. https://doi.org/10.3390/plants10071377
Chicago/Turabian StylePark, Jeong-Soo, Hyohyemi Lee, Donghui Choi, and Youngha Kim. 2021. "Spatially Varying Relationships between Alien Plant Distributions and Environmental Factors in South Korea" Plants 10, no. 7: 1377. https://doi.org/10.3390/plants10071377
APA StylePark, J.-S., Lee, H., Choi, D., & Kim, Y. (2021). Spatially Varying Relationships between Alien Plant Distributions and Environmental Factors in South Korea. Plants, 10(7), 1377. https://doi.org/10.3390/plants10071377






