Leaf Plasmodesmata Respond Differently to TMV, ToBRFV and TYLCV Infection
Abstract
:1. Introduction
2. Results
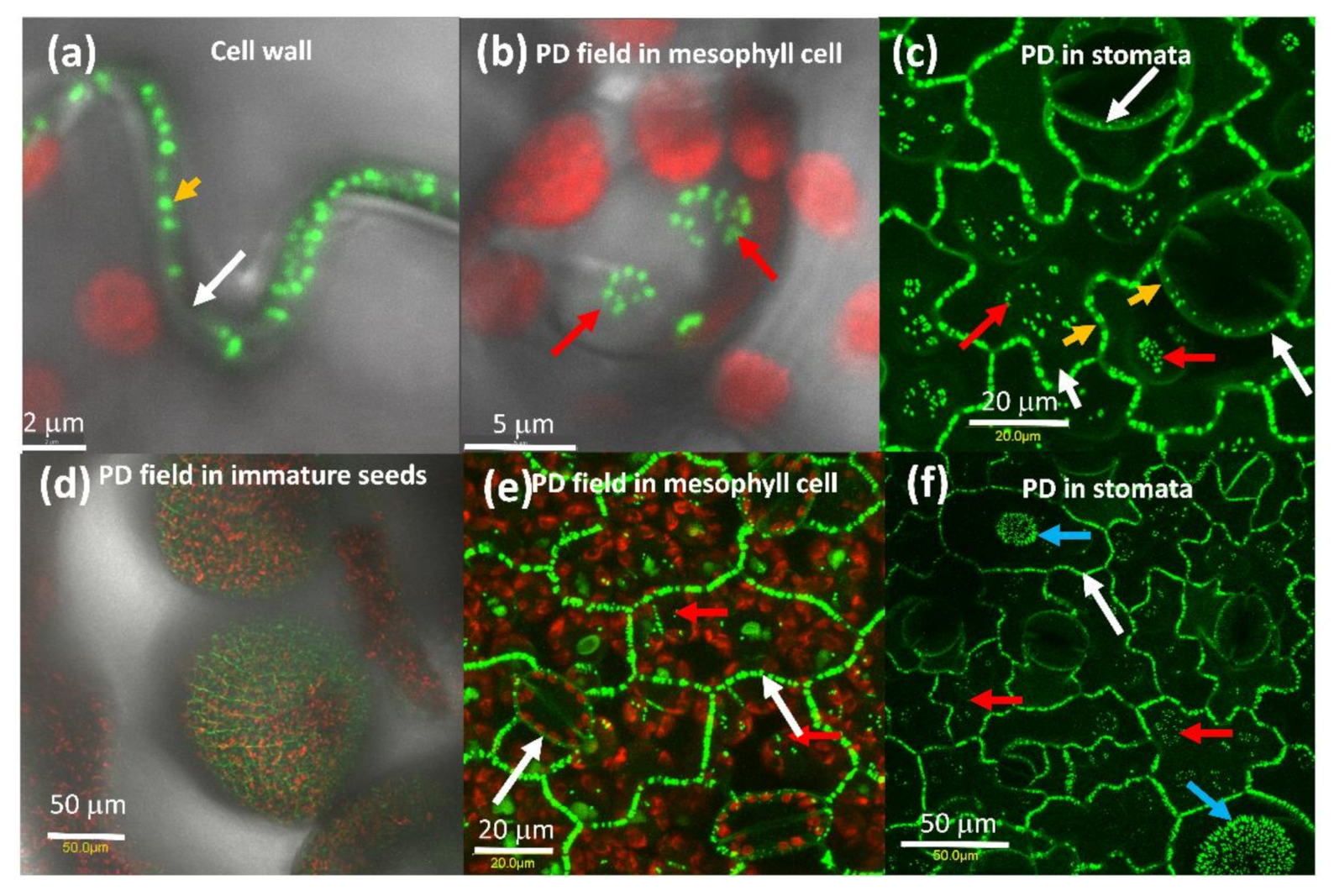
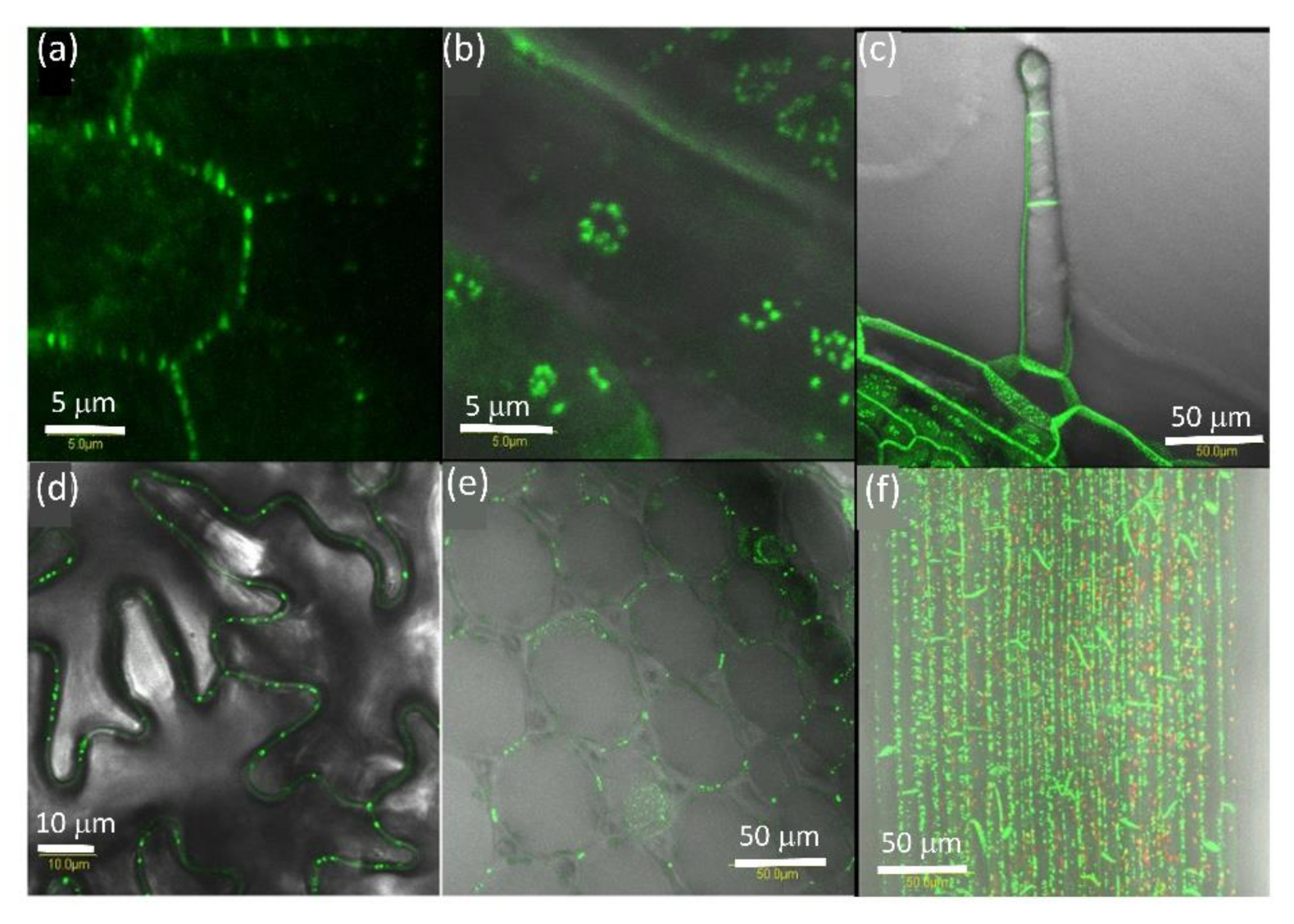
2.1. AtPDLP5 Is Present in Plasmodesmata
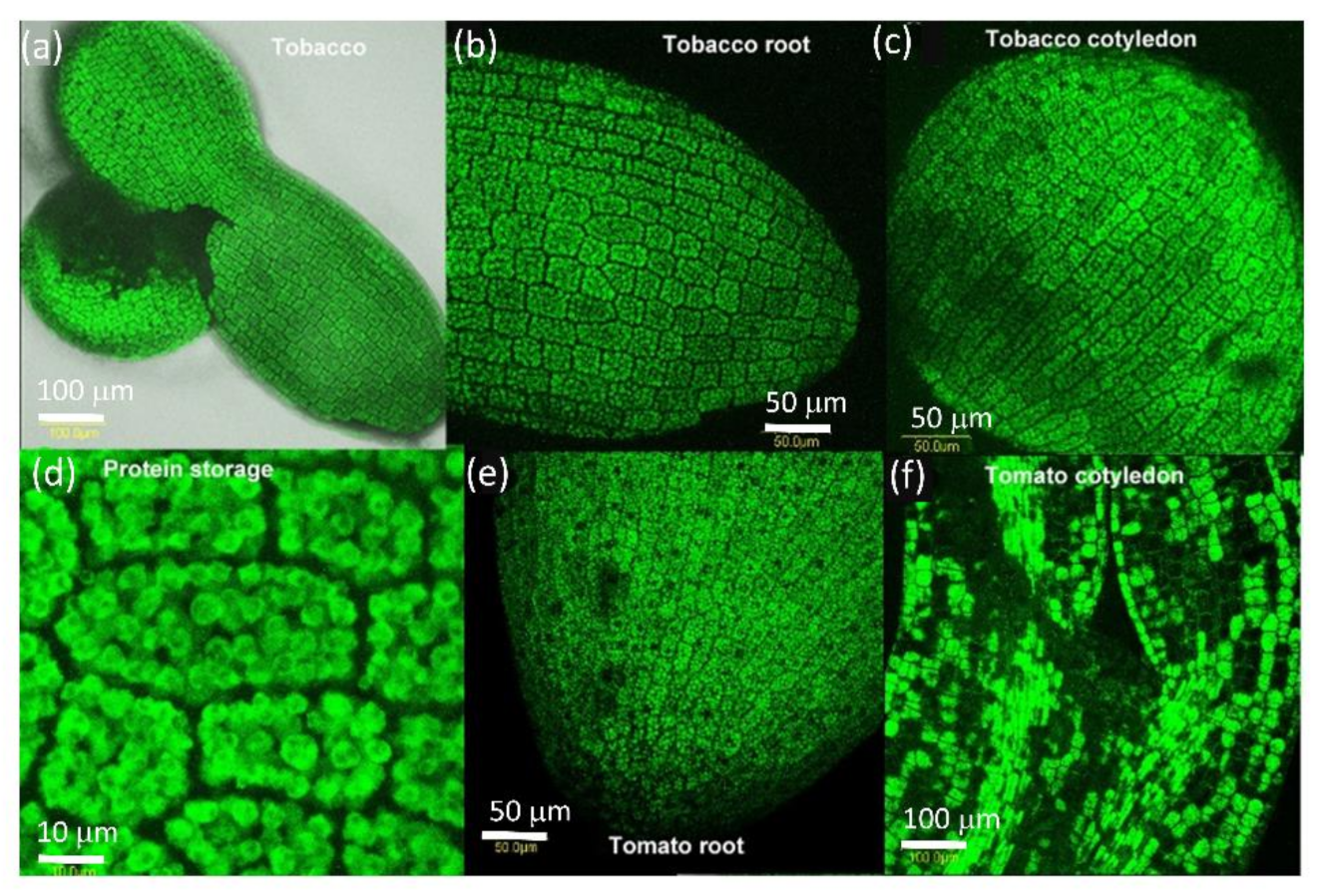
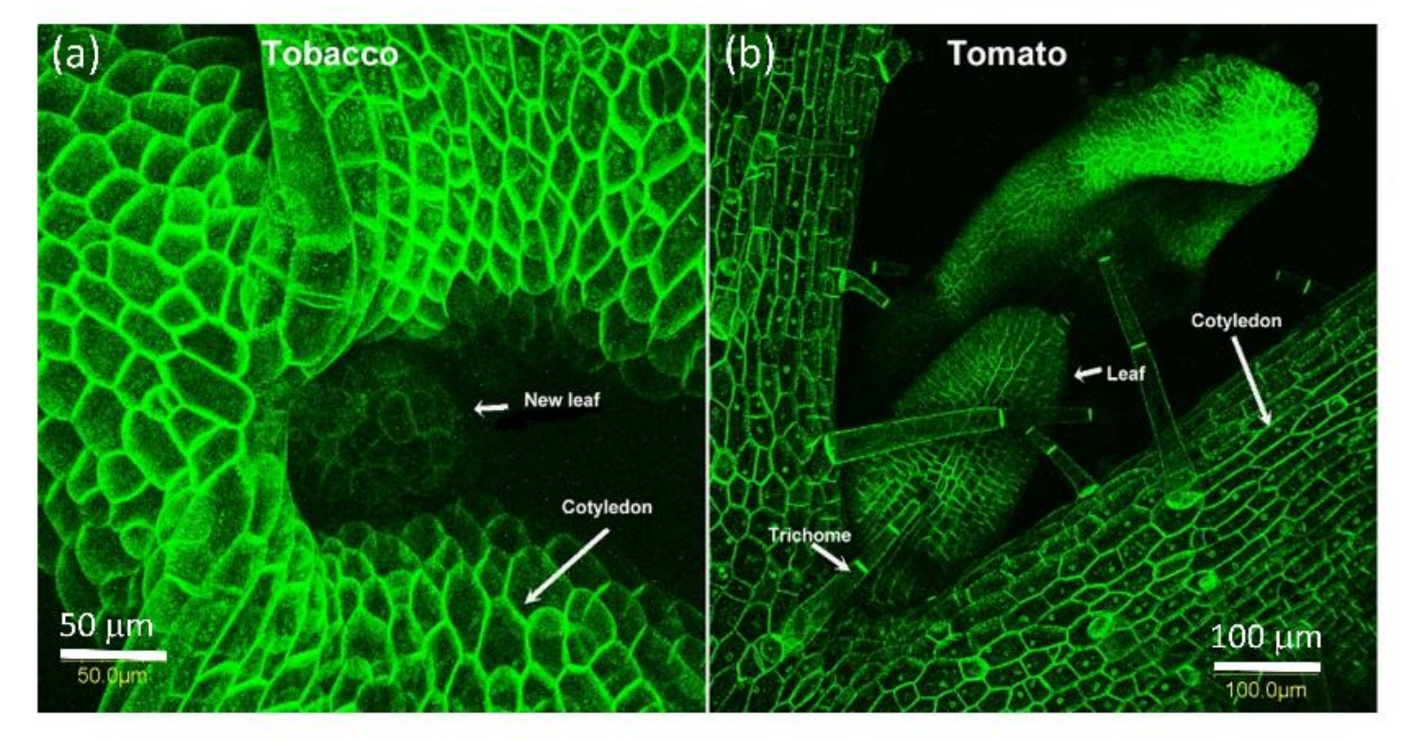
2.2. PDLP5 in Seed Germination
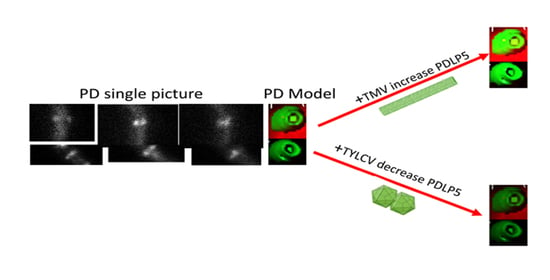
2.3. Effect of Plant Viruses on PDLP5 Deposition
3. Discussion
4. Methods
4.1. Virus Maintenance and Whitefly Rearing
4.2. Plant Material
4.3. TYLCV and Tobamoviruses Inoculation
4.4. TYLCV Detection in Plants
4.5. Microscopic and Chlorophyll Analysis of Leaf Tissue
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lucas, W.J. Plant viral movement proteins: Agents for cell-to-cell trafficking of viral genomes. Virology 2006, 344, 169–184. [Google Scholar] [CrossRef] [Green Version]
- Reuveni, M.; Lerner, H.R.; Poljakoff-Mayber, A. Changes in membrane potential as a demonstration of selective pore formation in the plasmalemma by poly-L-lysine treatment. Plant Physiol. 1985, 79, 406–410. [Google Scholar] [CrossRef] [Green Version]
- Heinlein, M. Plant virus replication and movement. Virology 2015, 479–480, 657–671. [Google Scholar] [CrossRef] [Green Version]
- Lucas, W.J.; Lee, J.Y. Plasmodesmata as a supracellular control network in plants. Nat. Rev. Mol. Cell Biol. 2004, 5, 712–726. [Google Scholar] [CrossRef]
- Heinlein, M.; Epel, B.L. Macromolecular transport and signaling through plasmodesmata. Int. Rev. Cytol. 2004, 235, 93–164. [Google Scholar]
- Rybicki, E.P. Top Ten list for economically important plant viruses. Arch. Virol. 2015, 160, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, K.B.; Adkins, S.; Czosnek, H.; Palukaitis, P.; Jacquot, E.; Hohn, T.; Hohn, B.; Saunders, K.; Candresse, T.; Ahlquist, P.; et al. Top 10 plant viruses in molecular plant pathology. Mol. Plant Pathol. 2011, 12, 938–954. [Google Scholar] [CrossRef] [PubMed]
- Ding, B.; Haudenshield, J.S.; Hull, R.J.; Wolf, S.; Beachy, R.N.; Lucas, W.J. Secondary plasmodesmata are specific sites of localization of the Tobacco mosaic virus movement protein in transgenic tobacco plants. Plant Cell 1992, 4, 915–928. [Google Scholar] [PubMed] [Green Version]
- Ritzenthaler, C.; Hofmann, C. Tubule-guided movement of plant viruses. In Plant Cell Monographs; Waigmann, E., Heinlein, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 63–83. [Google Scholar]
- Sanchez-Navarro, J.; Fajardo, T.; Zicca, S.; Pallas, V.; Stavolone, L. Caulimoviridae tubule-guided transport is dictated by movement protein properties. J. Virol. 2010, 84, 4109–4112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Lent, J.W.M.; Schmitt-Keichinger, C. Viral movement proteins induce tubule formation in plant and insect cells. In Cell-Cell Channels; Baluska, F., Volkmann, D., Barlow, P.W., Eds.; Landes Bioscience: Austin, TX, USA, 2006; pp. 160–174. [Google Scholar]
- Lapidot, M.; Gafny, R.; Ding, B.; Wolf, S.; Lucas, W.J.; Beachy, R.N. A dysfunctional movement protein of tobacco mosaic virus that partially modifies the plasmodesmata and limits virus Spread in transgenic plants. Plant J. 1993, 4, 959–970. [Google Scholar] [CrossRef]
- Cooper, B.; Lapidot, M.; Heick, J.A.; Dodds, J.A.; Beach, R.N. Defective movement protein of TMV in transgenic plants confers resistance to multiple viruses whereas the functional analog increases susceptibility. Virology 1995, 20, 307–313. [Google Scholar] [CrossRef]
- Kim, I.; Kobayashi, K.; Cho, E.; Zambryski, P.C. Subdomains for transport via plasmodesmata corresponding to the apical-basal axis are established during Arabidopsis embryogenesis. Proc. Natl. Acad. Sci. USA 2005, 102, 11945–11950. [Google Scholar] [CrossRef] [Green Version]
- Kozieradzka-Kiszkurno, M.; Płachno, B.J. Are there symplastic connections between the endosperm and embryo in some angiosperms? A lesson from the Crassulaceae family. Protoplasma 2012, 24, 1081–1089. [Google Scholar] [CrossRef] [Green Version]
- Robarts, A.W. Plasmodesmata. Ann. Rev. Plant Physiol. 1990, 26, 13–29. [Google Scholar] [CrossRef]
- Amari, K.; Lerich, A.; Schmitt-Keichinger, C.; Dolja, V.V.; Ritzenthaler, C. Tubule-guided cell-to-cell movement of a plant virus requires class XI myosin motors. PLoS Pathog. 2011, 7, e1002327. [Google Scholar] [CrossRef]
- den Hollander, P.W.; Kieper, S.N.; Borst, J.W.; van Lent, J.W.M. The role of plasmodesma-located proteins in tubule-guided virus transport is limited to the plasmodesmata. Arch. Virol. 2016, 161, 2431–2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.Y.; Wang, X.; Cui, W.; Sager, R.; Modla, S.; Czymmek, K.; Zybaliov, B.; van Wijk, K.; Zhang, C.; Lu, H.; et al. A Plasmodesmata-Localized Protein Mediates Crosstalk between Cell-to-Cell Communication and Innate Immunity in Arabidopsis. Plant Cell 2011, 23, 3353–3373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLean, B.G.; Hempel, F.D.; Zambryski, P.C. Plant intercellular communication via plasmodesmata. Plant Cell 1997, 9, 1043–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, A.; Epel, B.L. Cytology of the (1-3)-β-glucan (Callose) in plasmodesmata and sieve plate pores. In Chemistry, Biochemistry, and Biology of 1-3 Beta Glucans and Related Polysaccharides; Academic Press: Cambridge, MA, USA, 2009; pp. 439–463. [Google Scholar]
- Brecknock, S.; Dibbayawan, T.P.; Vesk, M.V.; Vesk, P.A.; Faulkner, C.; Barton, D.A.; Overall, R.L. High resolution scanning electron microscopy of plasmodesmata. Planta 2011, 234, 749–758. [Google Scholar] [CrossRef]
- Czosnek, H. (Ed.) Tomato Yellow Leaf Curl Virus Disease: Management, Molecular Biology, Breeding for Resistance; Springer: Dordrecht, The Netherlands, 2007; pp. 157–170. [Google Scholar]
- Waigmann, E.; Chen, M.H.; Bachmaier, R.; Ghoshroy, S.; Citovsky, V. Regulation of plasmodesmal transport by phosphorylation of tobacco mosaic virus cell-to-cell movement protein. EMBO J. 2000, 19, 4875–4884. [Google Scholar] [CrossRef] [Green Version]
- Zambryski, P.C. Plasmodesmata. Curr. Biol. 2008, 18, 324–325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Zhang, X.; Hong, Y.; Liu, Y. Chloroplast in Plant-Virus Interaction. Front. Microbiol. 2016, 27, 1565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luria, N.; Smith, E.; Reingold, V.; Bekelman, I.; Lapidot, M.; Levin, I.; Elad, N.; Tam, Y.; Sela, N.; Abu-Ras, A.; et al. A New Israeli Tobamovirus Isolate Infects Tomato Plants Harboring Tm-22 Resistance Genes. PLoS ONE 2017, 12, e0170429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreiber, G.; Reuveni, M.; Evenor, D.; Oren-Shamir, M.; Ovadia, R.; Sapir-Mir, M.; Bootbool-Man, A.; Nahon, S.; Shlomo, H.; Chen, L.; et al. ANTHOCYANIN1 from Solanum chilense is more efficient in accumulating anthocyanin metabolites than its Solanum lycopersicum counterpart in association with the ANTHOCYANIN FRUIT phenotype of tomato. Theor. Appl. Genet. 2011, 124, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Shaya, F.; Gaiduk, S.; Keren, I.; Shevtsov, S.; Zemah, H.; Belausov, E.; Evenor, D.; Reuveni, M. Ostersetzer-Biran, O. Expression of mitochondrial gene fragments within the tapetum induce male-sterility by limiting the biogenesis of the respiratory machinery in transgenic tobacco plants. J. Integr. Plant Biol. 2012, 54, 115–130. [Google Scholar] [CrossRef] [PubMed]
- Polston, J.E.; Sherwood, T.A.; Cohen, L.; Ben-Joseph, R.; Lapidot, M. Capsicum Species: Symptomless Hosts and Reservoirs of Tomato Yellow Leaf Curl Virus (TYLCV). Phytopathology 2006, 96, 447–452. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, M.; Friedmann, M.; Lachman, O.; Yehezkel, A.; Nahon, S.; Cohen, S.; Pilowsky, M. Comparison of resistance level to tomato yellow leaf curl virus among commercial cultivars and breeding lines. Plant Dis. 1997, 81, 1425–1428. [Google Scholar] [CrossRef] [Green Version]
- Lapidot, M. Screening for TYLCV-resistant plants using whitefly-mediated inoculation. In Tomato Yellow Leaf Curl Virus Disease; Czosnek, H., Ed.; Springer: Dordrecht, The Netherlands, 2007; pp. 329–342. [Google Scholar]
- Reuveni, M.; Debbi, A.; Kutsher, Y.; Gelbart, D.; Zemach, H.; Belausov, E.; Levin, I.; Lapidot, M. Tomato yellow leaf curl virus effects on chloroplast biogenesis and cellular structure. Physiol. Mol. Plant Pathol. 2015, 92, 51–58. [Google Scholar] [CrossRef]
- Teper-Bamnolker, P.; Buskila, Y.; Belausov, E.; Wolf, D.; Doron-Faigenboim, A.; Ben-Dor, S.; Van der Hoorn, R.A.L.; Lers, A.; Eshel, D. Vacuolar processing enzyme activates programmed cell death in the apical meristem inducing loss of apical dominance. Plant Cell Environ. 2017, 40, 2381–2392. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kutsher, Y.; Evenor, D.; Belausov, E.; Lapidot, M.; Reuveni, M. Leaf Plasmodesmata Respond Differently to TMV, ToBRFV and TYLCV Infection. Plants 2021, 10, 1442. https://doi.org/10.3390/plants10071442
Kutsher Y, Evenor D, Belausov E, Lapidot M, Reuveni M. Leaf Plasmodesmata Respond Differently to TMV, ToBRFV and TYLCV Infection. Plants. 2021; 10(7):1442. https://doi.org/10.3390/plants10071442
Chicago/Turabian StyleKutsher, Yaarit, Dalia Evenor, Eduard Belausov, Moshe Lapidot, and Moshe Reuveni. 2021. "Leaf Plasmodesmata Respond Differently to TMV, ToBRFV and TYLCV Infection" Plants 10, no. 7: 1442. https://doi.org/10.3390/plants10071442
APA StyleKutsher, Y., Evenor, D., Belausov, E., Lapidot, M., & Reuveni, M. (2021). Leaf Plasmodesmata Respond Differently to TMV, ToBRFV and TYLCV Infection. Plants, 10(7), 1442. https://doi.org/10.3390/plants10071442