Novel Metrics to Characterize In Vitro Pollen Tube Growth Performance of Apple Cultivars
Abstract
:1. Introduction
2. Results
2.1. Effects of Incubation Time, Germination Media, and Techniques on Pollen Tube Growth
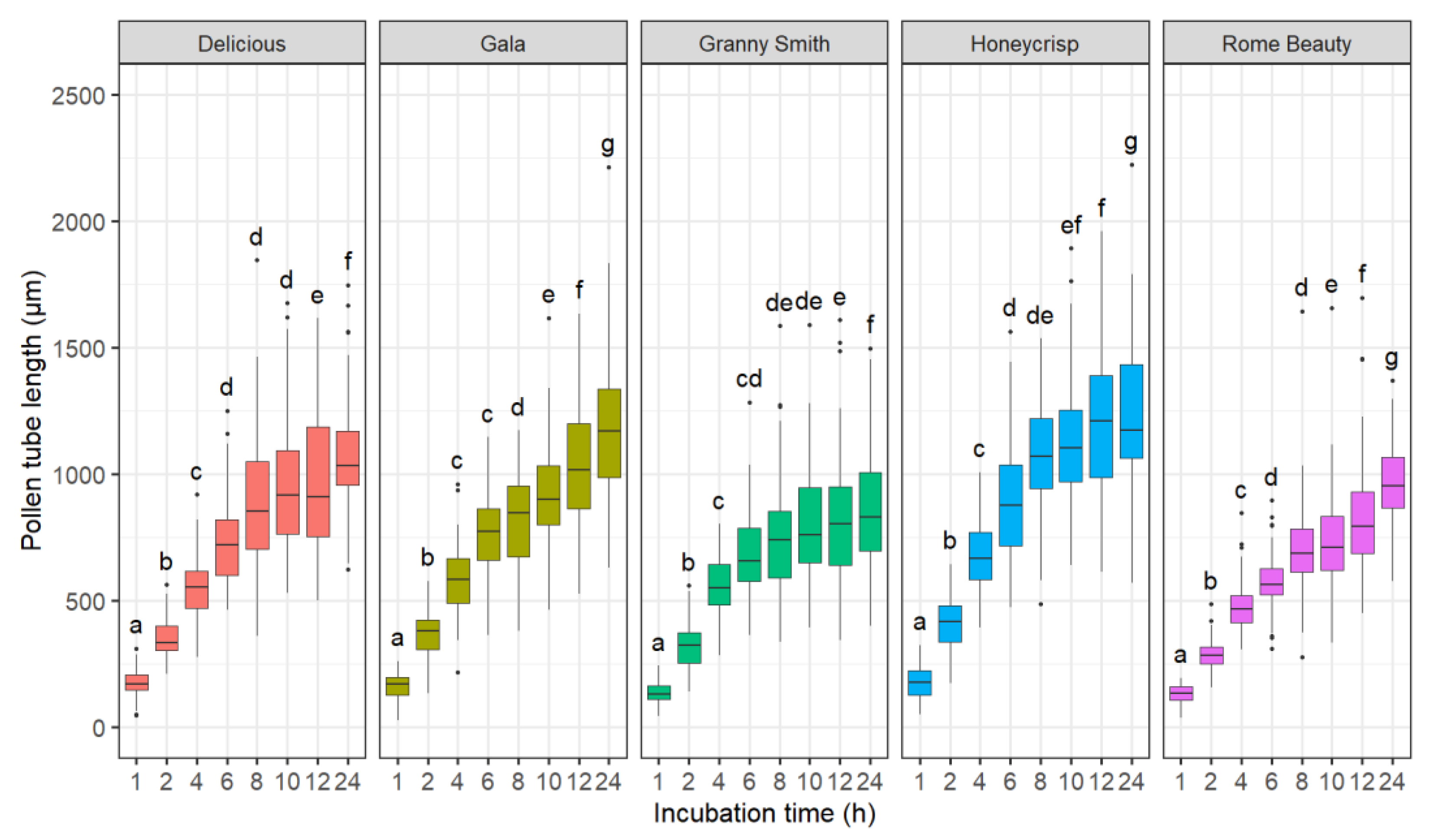
2.1.1. Effect of Incubation Time
2.1.2. Effect of Germination Medium
2.1.3. Effect of Germination Technique
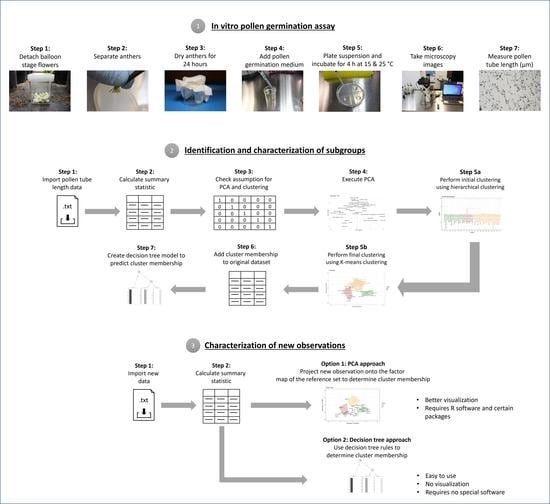
2.2. Identification and Characterization of Subgroups
2.2.1. Principal Component Analysis (PCA)
2.2.2. Hierarchical Clustering (HC) and K-Means Clustering
Pollen Tube Growth Performance between Years
Pollen Tube Growth Performance Within a Years
2.2.3. Determine Thresholds for Cluster Membership Using a Tree-Based Model
2.3. Using Predictive Modeling: A Case Study of 29 Apple Accessions
3. Discussion
3.1. Effects of Incubation Time, Germination Media, and Germination Technique on Pollen Tube Growth
3.2. Identification and Characterization of Subgroups
3.3. Using Predictive Modeling: A Case Studies of 29 Apple Accessions
4. Materials and Methods
4.1. Effects of Incubation Time, Germination Media, and Techniques on Pollen Tube Growth
4.1.1. Effect of Incubation Time
4.1.2. Effect of Germination Technique
4.1.3. Effect of Germination Media
4.2. Identification and Characterization of Subgroups
4.3. Using Predictive Modeling: A Case Study of 29 Apple Accessions
4.4. Microscopic Observation and Pollen Tube Measurements
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsumoto, S. Apple pollination biology for stable and novel fruit production: Search system for apple cultivar combination showing incompatibility, semicompatibility, and full-compatibility based on the S-RNase allele database. Int. J. Agron. 2014, 2014, 138271. [Google Scholar]
- Sassa, H. Molecular mechanism of the S-RNase-based gametophytic self-incompatibility in fruit trees of Rosaceae. Breed. Sci. 2016, 66, 116–121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bosch, J.; Kemp, W.P. Exceptional cherry production in an orchard pollinated with blue orchard bees. Bee World 1999, 80, 163–173. [Google Scholar] [CrossRef]
- Thomson, J.D.; Goodell, K. Pollen removal and deposition by honeybee and bumblebee visitors to apple and almond flowers. J. Appl. Ecol. 2001, 38, 1032–1044. [Google Scholar] [CrossRef]
- Gardner, K.; Ascher, J. Notes on the native bee pollinators in New York apple orchards. Entomol. Am. 2006, 114, 86–91. [Google Scholar] [CrossRef]
- Kwon, J.H.; Jun, J.H.; Nam, E.Y.; Chung, K.H.; Yoon, I.K.; Yun, S.K.; Kim, S.J. Selection of a Suitable Pollinizer for ‘Summer Fantasia’ Plum. HortScience 2017, 52, 1182. [Google Scholar] [CrossRef] [Green Version]
- Sulusoglu, M.; Cavusoglu, A. In Vitro pollen viability and pollen germination in cherry laurel (Prunus laurocerasus L.). Sci. World J. 2014, 2014, 657123. [Google Scholar] [CrossRef] [Green Version]
- Impe, D.; Reitz, J.; Köpnick, C.; Rolletschek, H.; Börner, A.; Senula, A.; Nagel, M. Assessment of pollen viability for wheat. Front. Plant Sci. 2020, 10, 1588. [Google Scholar] [CrossRef]
- Gaaliche, B.; Majdoub, A.; Trad, M.; Mars, M. Assessment of pollen viability, germination, and tube growth in eight tunisian caprifig (Ficus carica L.) cultivars. ISRN Agron. 2013, 2013, 207434. [Google Scholar]
- Rodriguez-Riano, T.; Dafni, A. A new procedure to asses pollen viability. Sex. Plant Reprod. 2000, 12, 241–244. [Google Scholar] [CrossRef]
- Huang, Z.; Zhu, J.; Mu, X.; Lin, J. Pollen dispersion, pollen viability and pistil receptivity in Leymus chinensis. Ann. Bot. 2004, 93, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calzoni, G.; Speranza, A.; Bagni, N. In vitro germination of apple pollens. Sci. Hortic. 1979, 10, 49–55. [Google Scholar] [CrossRef]
- Deckers, T.; Porreye, W. Influence of the temperature on pollen germination of different cultivars of apple and pear: Trials in vitro. Acta Hortic. 1984, 149, 123–130. [Google Scholar] [CrossRef]
- Imani, A.; Barzegar, K.; Piripireivatlou, S.; Masomi, S.H. Storage of apple pollen and in vitro germination. Afr. J. Agric. Res. 2011, 6, 624–629. [Google Scholar]
- Filiti, N.; Montalti, P. In vitro germination and tube growth of apple pollen as affected by cold storage. Riv. Ortoflorofruttic. Ital. 1982, 66, 361–368. [Google Scholar]
- Beltrán, R.; Valls, A.; Cebrián, N.; Zornoza, C.; Breijo, F.G.; Armiñana, J.R.; Garmendia, A.; Merle, H. Effect of temperature on pollen germination for several Rosaceae species: Influence of freezing conservation time on germination patterns. PeerJ 2019, 7, e8195. [Google Scholar] [CrossRef] [PubMed]
- Moshtagh, F.; Dadar, A.; Moghadam, E.; Asgharzadeh, A. Investigation on pollen viability, germination and tube growth in some apple cultivars in climate conditions of Shirvan. J. Appl. Environ. Biol. Sci. 2015, 4, 295–302. [Google Scholar]
- Rodriguez-Enriquez, M.; Mehdi, S.; Dickinson, H.; Grant-Downton, R. A novel method for efficient in vitro germination and tube growth of Arabidopsis thaliana pollen. New Phytol. 2013, 197, 668–679. [Google Scholar] [CrossRef]
- Florin, R. Pollen production and incompatibilities in apples and pears. Mem. Hort. N. Y. 1927, 3, 87–118. [Google Scholar]
- Kakani, V.; Prasad, P.; Craufurd, P.; Wheeler, T. Response of in vitro pollen germination and pollen tube growth of groundnut (Arachis hypogaea L.) genotypes to temperature. PlantCell Environ. 2002, 25, 1651–1661. [Google Scholar] [CrossRef] [Green Version]
- Kakani, V.; Reddy, K.; Koti, S.; Wallace, T.; Prasad, P.; Reddy, V.; Zhao, D. Differences in in vitro pollen germination and pollen tube growth of cotton cultivars in response to high temperature. Ann. Bot. 2005, 96, 59–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sorkheh, K.; Shiran, B.; Rouhi, V.; Khodambashi, M. Influence of temperature on the in vitro pollen germination and pollen tube growth of various native Iranian almonds (Prunus L. spp.) species. Trees 2011, 25, 809–822. [Google Scholar] [CrossRef]
- Ranasinghe, C.; Kumarathunge, M.; Kiriwandeniya, K. Genotypic differences in cardinal temperatures for in vitro pollen germination and pollen tube growth of coconut hybrids. Exp. Agr. 2018, 54, 731–743. [Google Scholar] [CrossRef]
- Akšić, M.F.; Rakonjac, V.; Nikolic, D.; Zec, G. Reproductive biology traits affecting productivity of sour cherry. Pesqui. Agropecuária Bras. 2013, 48, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Rokach, L.; Maimon, O. Clustering methods. In Data Mining and Knowledge Discovery Handbook; Springer: Boston, MA, USA, 2005; pp. 321–352. [Google Scholar]
- Rodriguez, M.Z.; Comin, C.H.; Casanova, D.; Bruno, O.M.; Amancio, D.R.; Costa, L.d.F.; Rodrigues, F.A. Clustering algorithms: A comparative approach. PLoS ONE 2019, 14, e0210236. [Google Scholar] [CrossRef]
- Kostick, S.A.; Norelli, J.L.; Evans, K.M. Novel metrics to classify fire blight resistance of 94 apple cultivars. Plant Pathol. 2019, 68, 985–996. [Google Scholar] [CrossRef]
- Sochor, J.; Skutkova, H.; Babula, P.; Zitka, O.; Cernei, N.; Rop, O.; Krska, B.; Adam, V.; Provazník, I.; Kizek, R. Mathematical evaluation of the amino acid and polyphenol content and antioxidant activities of fruits from different apricot cultivars. Molecules 2011, 16, 7428–7457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Banerjee, H.; Samanta, S.; Sarkar, S.; Garai, S.; Pal, S.; Brahmachari, K. Growth, productivity and nutrient uptake of different rice cultivars under coastal eco-system of West Bengal. J. Indian Soc. Coast. Agric. Res. 2018, 36, 115–121. [Google Scholar]
- Gregorio, G.; Antonio, D.; Rocco, Z. Comparative study of some fruit quality characteristics of two of Annona cherimola Mill. grown in southern Italy. AIMS Agric. Food 2019, 4, 658–671. [Google Scholar] [CrossRef]
- Říhová, L.; Hrabětová, E.; Tupý, J. Optimization of conditions for in vitro pollen germination and tube growth in potatoes. Int. J. Plant Sci. 1996, 157, 561–566. [Google Scholar] [CrossRef]
- Conner, P.J. Optimization of in vitro pecan pollen germination. Hortscience 2011, 46, 571–576. [Google Scholar] [CrossRef] [Green Version]
- Hine, A.; Rojas, A.; Suarez, L.; Murillo, O.; Espinoza, M. Optimization of Pollen Germination in Tectona grandis (Teak) for Breeding Programs. Forests 2019, 10, 908. [Google Scholar] [CrossRef] [Green Version]
- Dancs, Z.; Kiss, A. Effect of fungicides applied at the time of flowering on the pollen germination and fruit set of Jonathan apple trees. Acta Agron. Hung. 1970, 19, 313–320. [Google Scholar]
- Brewbaker, J.L.; Kwack, B.H. The essential role of calcium ion in pollen germination and pollen tube growth. Am. J. Bot. 1963, 50, 859–865. [Google Scholar] [CrossRef]
- Demeke, T.; Hughes, H. Germination and storage of pollen of Phytolacca dodecandra L. (endod). Ann. Bot. 1991, 68, 13–15. [Google Scholar] [CrossRef]
- El Mardi, M.O.; Bakheit, C.S. Effect of Storage Conditions and Methods of Pollen Germination on Viability of Three Date Palm Cultivars. Emir. J. Food Agric. 1996, 8, 146–154. [Google Scholar] [CrossRef]
- Jayaprakash, P.; Sarla, N. Development of an improved medium for germination of Cajanus cajan (L.) Millsp. pollen in vitro. J. Exp. Bot. 2001, 52, 851–855. [Google Scholar] [CrossRef]
- De Sousa, V.A.; Schemberg, E.A.; Aguiar, A.V. In vitro pollen germination of jerivá (Syagrus romanzoffiana (S.) Cham). Sci. For. 2010, 38, 147–151. [Google Scholar]
- Jayaprakash, P.; Sheeba, D.; Vikas, V.; Sivasamy, M.; Sabesan, T. Development of pollen germination medium to test pollen viability of eggplant and its wild species. Indian J. Hortic. 2018, 75, 237–244. [Google Scholar] [CrossRef]
- Chen, W.; Liu, D.; Chen, M. Effects of high level of sucrose on the moisture content, water activity, protein denaturation and sensory properties in Chinese-style pork jerky. Asian-Australas. J. Anim. Sci. 2002, 15, 585–590. [Google Scholar] [CrossRef]
- Hirsche, J.; García Fernández, J.M.; Stabentheiner, E.; Großkinsky, D.K.; Roitsch, T. Differential effects of carbohydrates on Arabidopsis pollen germination. Plant Cell Physiol. 2017, 58, 691–701. [Google Scholar] [CrossRef]
- Obermeyer, G.; Kriechbaumer, R.; Strasser, D.; Maschessnig, A.; Bentrup, F.W. Boric acid stimulates the plasma membrane H+-ATPase of ungerminated lily pollen grains. Physiol Plant. 1996, 98, 281–290. [Google Scholar] [CrossRef]
- Nyomora, A.; Brown, P.; Pinney, K.; Polito, V. Foliar application of boron to almond trees affects pollen quality. J. Am. Soc. Hortic. Sci. 2000, 125, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Wee, Y.; Rao, A. Ananas pollen germination. Grana 1979, 18, 33–39. [Google Scholar] [CrossRef]
- Thompson, A.; Batjer, L. The effect of boron in the germinating medium on pollen germination and pollen tube growth for several deciduous tree fruits. Proc. Am. Soc. Hortic. Sci. 1950, 56, 227–230. [Google Scholar]
- Sotomayor, C.; Ruiz, R.; García-Huidobro, J. Boron concentration affects in vitro pollen germination and tube growth in kiwifruit (Actinidia deliciosa) cultivar ‘Matua’. Acta Hortic. 2012, 932, 517–521. [Google Scholar] [CrossRef]
- Matsuda, H.; Higuchi, H. Effects of temperature and medium composition on pollen germination of ‘Bengal’ and ‘Chakrapat’ lychee (Litchi chinensis Sonn.) in vitro. Trop. Agric. Dev. 2013, 57, 120–125. [Google Scholar]
- de Wet, E.; Robbertse, P.; Groeneveld, H. The influence of temperature and boron on pollen germination in Mangifera indica L. S. Afr. J. Plant Soil 1989, 6, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Korkmaz, N.; Güneri, M. Effect of different boron levels on pollen germination of hicaz nar pomegranate (Punica granatum L.) cultivar. Int. J. Agric. For. Life Sci. 2019, 3, 151–156. [Google Scholar]
- Mehri, S.; Piri, S.; Imani, A. Optimization of Apple Pollen Culture and Its Maintenance of Pollen Germination Capacity. Int. J. Adv. Agric. Environ. Engg. 2015, 2, 54–56. [Google Scholar]
- Giulivo, C.; Ramina, A. Effetto di massa ed azione del calcio sulla germinazione del polline di alcune specie arboree da frutto. Riv. Ortoflorofruttic. Ital. 1974, 5, 3–13. [Google Scholar]
- Hebbar, K.; Rose, H.M.; Nair, A.R.; Kannan, S.; Niral, V.; Arivalagan, M.; Gupta, A.; Samsudeen, K.; Chandran, K.; Chowdappa, P. Differences in in vitro pollen germination and pollen tube growth of coconut (Cocos nucifera L.) cultivars in response to high temperature stress. Environ. Exp. Bot. 2018, 153, 35–44. [Google Scholar] [CrossRef]
- Radovic, A.; Nikolic, D.; Milatovic, D.; Živkovic, B.; Stevanovic, N. The effect of plant hormones on pollen germination and pollen tube growth of almond cultivars. Acta Hortic. 2016, 1139, 375–380. [Google Scholar] [CrossRef]
- Beck-Pay, S. The effect of temperature and relative humidity on Acacia mearnsii polyad viability and pollen tube development. S. Afr. J. Bot. 2012, 83, 165–171. [Google Scholar] [CrossRef]
- Beyhan, N.; Serdar, U. Assessment of pollen viability and germinability in some European chestnut genotypes (Castanea sativa L.). Hortic. Sci. 2008, 35, 171–178. [Google Scholar] [CrossRef] [Green Version]
- Nikkanen, T.; Aronen, T.; Häggman, H.; Venäläinen, M. Variation in pollen viability among Picea abies genotypes–potential for unequal paternal success. Theor. Appl. Genet. 2000, 101, 511–518. [Google Scholar] [CrossRef]
- Adolfsson, A.; Ackerman, M.; Brownstein, N.C. To cluster, or not to cluster: An analysis of clusterability methods. Pattern Recognit. 2019, 88, 13–26. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.H.; Holmes, S. Ten quick tips for effective dimensionality reduction. PLoS Comput. Biol. 2019, 15, e1006907. [Google Scholar] [CrossRef] [Green Version]
- DeLong, C.N.; Yoder, K.S.; Combs, L.; Veilleux, R.E.; Peck, G.M. Apple pollen tube growth rates are regulated by parentage and environment. J. Am. Soc. Hortic. Sci. 2016, 141, 548–554. [Google Scholar] [CrossRef] [Green Version]
- Marcucci, M.; Filiti, N. Germination of pear [Pyrus] and apple [Malus] pollen as influenced by fungicides. Gartenbauwissenschaft 1984, 49, 28–32. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Project: Vienna, Italy, 2021; Available online: https://www.R-project.org/ (accessed on 10 December 2020).
- Mendiburu, F. agricolae: Statistical Procedures for Agricultural Research. R Package Version 1.3-5. 2021. Available online: https://CRAN.R-project.org/package=agricolae (accessed on 10 December 2020).
- Peters, G. userfriendlyscience: Quantitative Analysis Made Accessible. R Package Version 0.2-2. 2018. Available online: http://CRAN.R-project.org/package=userfriendlyscience (accessed on 10 December 2020).
- Graves, S.; Piepho, H.P.; Selzer, L. With Help from Sundar Dorai-Raj. Multcompview: Visualizations of Paired Comparisons. R Package Version 0.1-8. 2019. Available online: https://CRAN.R-project.org/package=multcompView (accessed on 10 December 2020).
- Wickham, H. The Split-Apply-Combine Strategy for Data Analysis. J. Stat. Softw. 2011, 40, 1–29. [Google Scholar] [CrossRef] [Green Version]
- Le, S.; Josse, J.; Husson, F. FactoMineR: An R Package for Multivariate Analysis. J. Stat. Softw. 2008, 25, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Revelle, W. psych: Procedures for Personality and Psychological Research; Northwestern University: Evanston, IL, USA, 2020; Version = 2.1.3; Available online: https://CRAN.R-project.org/package=psych (accessed on 10 December 2020).
- Kassambara, A.; Mundt, F. factoextra: Extract and Visualize the Results of Multivariate Data Analyses. R Package Version 1.0.7. 2020. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 10 December 2020).
- Hothorn, T.; Hornik, K.; Zeileis, A. Unbiased Recursive Partitioning: A Conditional Inference Framework. J. Comput. Graph. Stat. 2006, 15, 651–674. [Google Scholar] [CrossRef] [Green Version]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Kassambara, A. ggpubr: ‘ggplot2’ Based Publication Ready Plots. R Package Version 0.4.0. 2020. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 10 December 2020).






| Temperature (°C) | Variable | Pollen Tube Length (µm) | F (2, 38) | p | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cluster 1 (n = 11) | Cluster 2 (n = 22) | Cluster 3 (n = 8) | ||||||||||
| Mean | 95% CI | Group | Mean | 95% CI | Group | Mean | 95% CI | Group | ||||
| 15 | Minimum | 119 | 97, 141 | b | 145 | 129, 160 | b | 289 | 263, 315 | a | 59.4 | <0.001 |
| Average | 283 | 242, 325 | b | 326 | 297, 356 | b | 589 | 540, 638 | a | 54.5 | <0.001 | |
| Maximum | 544 | 445, 642 | b | 567 | 497, 637 | b | 968 | 853, 1084 | a | 20.8 | <0.001 | |
| Q1 (1) | 218 | 182, 254 | b | 267 | 241, 292 | b | 487 | 445, 529 | a | 53.7 | <0.001 | |
| Q2 (2) | 273 | 233, 314 | b | 318 | 290, 347 | b | 577 | 530, 624 | a | 56.8 | <0.001 | |
| Q3 (3) | 334 | 286, 383 | b | 381 | 346, 415 | b | 681 | 623, 738 | a | 51.5 | <0.001 | |
| 25 | Minimum | 339 | 284, 395 | b | 545 | 506, 584 | a | 490 | 425, 455 | a | 18.8 | <0.001 |
| Average | 619 | 578, 660 | b | 879 | 850, 908 | a | 902 | 854, 950 | a | 63.3 | <0.001 | |
| Maximum | 1002 | 911, 1094 | b | 1264 | 1199, 1329 | a | 1399 | 1292, 1506 | a | 18.3 | <0.001 | |
| Q1 (1) | 531 | 485, 576 | b | 788 | 756, 821 | a | 778 | 725, 832 | a | 47.0 | <0.001 | |
| Q2 (2) | 611 | 569, 654 | b | 874 | 843, 904 | a | 895 | 845, 945 | a | 59.5 | <0.001 | |
| Q3 (3) | 697 | 657, 738 | b | 969 | 964, 1059 | a | 1012 | 964, 1059 | a | 73.6 | <0.001 | |
| Media ID | Sucrose (g L−1) | Boric Acid (mg L−1) | Calcium Nitrate (mg L−1) | Reference |
|---|---|---|---|---|
| Medium 1 | 0 | 0 | 0 | - |
| Medium 2 | 68 | 20 | 300 | [12] |
| Medium 3 | 100 | 10 | 0 | [60] |
| Medium 4 | 150 | 100 | 300 | [14] |
| Medium 5 | 125 | 22.5 | 250 | [61] 1 |
| Medium 6 | 150 | 200 | 300 | [17] |
| Medium 7 | 100 | 25 | 0 | Roeder et al. (2021)—current study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roeder, S.; Serra, S.; Musacchi, S. Novel Metrics to Characterize In Vitro Pollen Tube Growth Performance of Apple Cultivars. Plants 2021, 10, 1460. https://doi.org/10.3390/plants10071460
Roeder S, Serra S, Musacchi S. Novel Metrics to Characterize In Vitro Pollen Tube Growth Performance of Apple Cultivars. Plants. 2021; 10(7):1460. https://doi.org/10.3390/plants10071460
Chicago/Turabian StyleRoeder, Stefan, Sara Serra, and Stefano Musacchi. 2021. "Novel Metrics to Characterize In Vitro Pollen Tube Growth Performance of Apple Cultivars" Plants 10, no. 7: 1460. https://doi.org/10.3390/plants10071460
APA StyleRoeder, S., Serra, S., & Musacchi, S. (2021). Novel Metrics to Characterize In Vitro Pollen Tube Growth Performance of Apple Cultivars. Plants, 10(7), 1460. https://doi.org/10.3390/plants10071460