Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants
Abstract
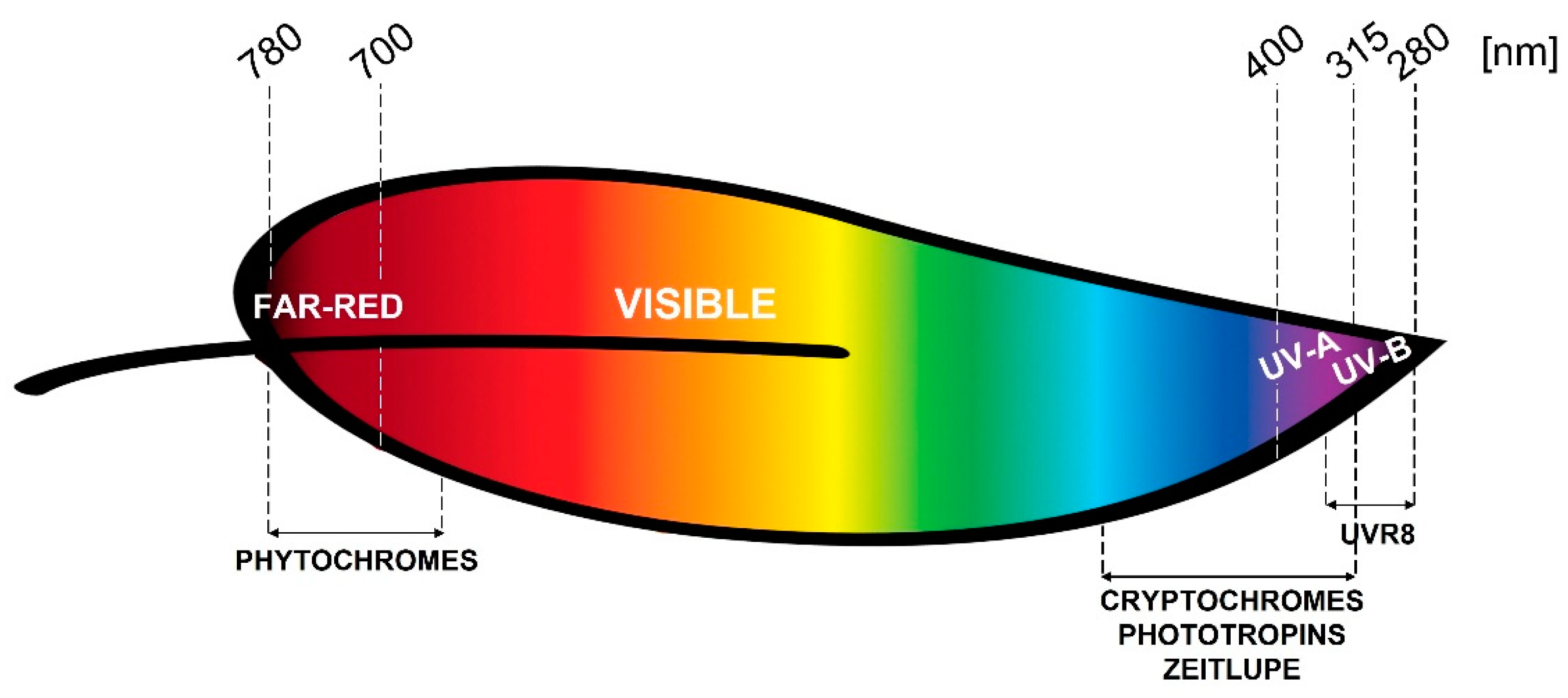
:1. Introduction
2. Photoreceptors
2.1. Phytochromes
2.2. Cryptochromes
2.3. Phototropins
2.4. Zeitlupe Family
2.5. UVR8
3. Signal Transduction Pathways
4. Plant Metabolism and Light
4.1. Phenolic Compounds
4.2. Terpenoids
4.3. Tocopherols and Tocotrienols
4.4. Ascorbic Acid
4.5. Glucosinolates
5. Red and Far-Red Light
5.1. Phenolics
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant | ||||||||
| Red leafy lettuce (Lactuca sativa. L.) | Sunmang | ↓ | ↑ | = | [129] | |||||
| Red Cross | ↑/= | =/↓ | =/↓ | = | [131] | |||||
| Red Fire | ↓/= | [132] | ||||||||
| Green leafy lettuce (Lactuca sativa. L.) | Grand Rapid TBR | = | = | = | [129,130] | |||||
| Thumper | ↓ | ↑ | [133] | |||||||
| Lamb’s lettuce (Valerianella locusta L.) | Noordhollandse | ↑ | [134] | |||||||
| Holländisher | ↓ | [119] | ||||||||
| Pea (Pisum sativum L.) | ↑ | =/↑ | [135] | |||||||
| Meteor | ↑ | ↑ | = | ↑ | [136] | |||||
| Amaranth (Amaranthus cruentus L.) | Red Army | ↓ | ↑ | = | ↑ | [136,137,138] | ||||
| Basil (Ocimum basilicum L.) | Genovese | ↑ | =/↑ | ↑ | ↓ | ↓ | ||||
| Kale (Brassica oleracea L.) | Red Russian | ↑ | ↑ | ↑ | ↑ | ↑ | [136,139] | |||
| Chinese kale (Brassica oleracea L. var. alboglabra Bailey) | DSCH | ↑ | [140] | |||||||
| DFZC | = | [141] | ||||||||
| Broccoli (Brassica oleracea L.) | ↑ | ↑ | = | ↑ | [136,142] | |||||
| Mustard (Brassica juncea L.) | Red Lion | ↑ | ↓ | ↑ | ↑ | |||||
| Tatsoi (Brassica rapa L.) | Rosularis | ↑ | ↑ | ↑ | = | |||||
| Orach (Atriplex hortensis L.) | ↑ | = | ||||||||
| Borage (Borago officinalis L.) | ↑ | ↓ | ↑ | ↓ | ||||||
| Beet (Beta vulgaris L.) | Bulls Blood | ↑ | ↓ | ↓ | = | |||||
| Parsley (Petroselinum crispum Mill.) | ↑ | ↑ | ↑ | = | ||||||
| Parsley (Petroselinum crispum Mill.) | ↑ | [137] | ||||||||
| Strawberry (Fragaria × Ananassa) | Elsinore | = | ↓ | = | [138] | |||||
| Cranberry (Vaccinium macrocarpon Ait) | Early Black | ↑ | [143] | |||||||
| Red clover (Trifolium pratense L.) | ↓/= | [144] | ||||||||
| Buckwheat (Fagopyrum esculentum) | Möench | ↑ | ↑ | [128] | ||||||
| Spinach (Spinacia oleracea L.) | Okame | ↓ | [132] | |||||||
| Wheat (Triticum aestivum L.) | = | [145] | ||||||||
| Tartary buckwheat (Fagopyrum tataricum Gaertn.) | Hokkai T8 | ↓ | [146] | |||||||
| Cowpea (Vigna unguiculata L. Walp.) | ↓ | [147] | ||||||||
| Bilberry (Vaccinium myrtillus L.) | ↑ | [148] | ||||||||
| Tomato (Solanum lycopersicum L.) | Red Ruby | ↑ | [47] | |||||||
| Tea leaves (Camellia sinensis) | Jinxuan | ↑ | [149] | |||||||
| Pak choi (Brassica rapa ssp. chinensis) | ↓ | [150] | ||||||||
| (Brassica rapa ssp. pekinensis) | Chiifu | ↑ | [151] | |||||||
| Satsuma mandarin fruit (Citrus unshiu Marc.) | ↓/↑ | [152] | ||||||||
5.2. Terpenoids and Chlorophylls
5.3. Other Secondary Metabolites
6. Green Light
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant | ||||||||
| Lettuce (Lactuca sativa. L.) | Youmaicai | ↑ | [162] | |||||||
| Butterhead | ↑ | [163] | ||||||||
| Tea leaves (Camellia sinensis L.O. Kuntze) | Zhonghuang 3 | ↑ | ↑ | [161] | ||||||
| Tomato plants (Solanum lycopersicum L.) | Komeett’ | =/↑ | [164] | |||||||
| Basil (Ocimum basilicum L.) | Improved Genovese Compact | =/↓ | =/↓ | =/↓ | =/↓ | [165] | ||||
| Red Rubin | =/↓ | =/↓ | =/↓ | =/↓ | ||||||
7. Blue Light
7.1. Phenolics
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant. | ||||||||
| Green leafy lettuce (Lactuca sativa. L.) | Thumper | ↓ | = | ↓/↑ | ↑ | [133] | ||||
| ↑ | [167] | |||||||||
| Grizzly | ↑ | [168] | ||||||||
| Red leafy lettuce (Lactuca sativa. L.) | Red Cross | = | ↑ | [131] | ||||||
| Red clover (Trifolium pratense L.) | ↑ | [144] | ||||||||
| Chinese cabbage (Brassica campestris L.) | ↑ | [169] | ||||||||
| Mustard (Brassica juncea L.) | Red Lion | ↑ | ↑ | [170] | ||||||
| Beet (Beta vulgaris L.) | Bulls Blood | ↑ | ↑ | |||||||
| Parsley (Petroselinum crispum Mill.) | Plain Leaved or French | ↑ | ↑ | |||||||
| Buckwheat (Fagopyrum esculentum) | Möench | ↑ | ↑ | [128] | ||||||
| Wheat (Triticum aestivum L.) | ↑/↓ | [145] | ||||||||
| Soybean (Glycine max L.) | Dongnong 690 | ↑ | ↓ | [166] | ||||||
| Bilberry fruit (Vaccinium myrtillus L.) | ↑ | [148] | ||||||||
| Apple fruit (Malus domestica Borkh.) | Mishima Fuji | ↑ | [171] | |||||||
| Jonathan | ↑ | |||||||||
| Strawberry (Fragaria × Ananassa) | ↑ | [172,173] | ||||||||
| Fengguang | ↑ | [174] | ||||||||
| Cowpea (Vigna unguiculata L. Walp.) | ↓/↑/= | [147] | ||||||||
| Tartary buckwheat (Fagopyrum tataricum Gaertn.) | ↓ | [146] | ||||||||
| Pak choi (Brassica rapa ssp. chinensis) | ↓/= | [150] | ||||||||
| Tomato fruit (Solanum lycopersicum L.) | Micro-Tom | ↑ | [175] | |||||||
| Satsuma mandarin fruit (Citrus unshiu Marc.) | ↓/↑/= | [152] | ||||||||
| Tea leaves (Camellia sinensis) | Jinxuan | ↑ | [149] | |||||||
| Basil (Ocimum basilicum L.) | Genovese | ↑ | [176] | |||||||
| Satsuma mandarin fruit (Citrus unshiu Marc.) | ↑ | [177] | ||||||||
| Valencia orange fruit (Citrus sinensis Osbeck) | ↑ | |||||||||
| Lisbon lemon fruit (Citrus limon Burm.f.) | ↑ | |||||||||
| Canola (Brassica napus L.) | ↑/= | [178] | ||||||||
| Mustard (Brassica juncea L.) | ↓ | [179] | ||||||||
7.2. Terpenoids and Chlorophylls
7.3. Other Secondary Metabolites
8. UV-A Radiation
8.1. Phenolics
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant | ||||||||
| Pak-choi (Brassica rapa ssp. chinensis var. communis) | Red leaf cv. | ↑/= | ↑ | ↑ | ↑ | ↑/= | ↑/↓ | ↑ | ↑ | [183,188] |
| Green leaf cv. | ↑/= | ↑/= | ↑ | ↑ | = | ↓ | ↑ | |||
| Turnip (Brassica rapa subsp. rapa) | Tsuda | ↑ | [184] | |||||||
| Broccoli (Brassica oleracea L., var. italica) | Waltham 29 | =/↓ | =/↓ | [185] | ||||||
| Monopoly | =/↓ | = | [186] | |||||||
| Broccoli (Brassica oleracea L., var. gemmifera DC) | ↑/↓ | ↑/= | [187] | |||||||
| Lettuce (Lactuca sativa. L.) | Yanzhi | = | ↑ | ↑ | ↑ | ↓ | ↑ | [189] | ||
| Red butter | ↑ | ↑ | ↑ | = | ↓ | ↑ | ||||
| Klee | ↑/= | ↑/= | ↑ | ↑ | [190] | |||||
| Red leaf cvs. | = | [191] | ||||||||
| Green leaf cvs. | = | |||||||||
| Hongyeom | ↑/= | ↑/= | ↑/= | [192] | ||||||
| Tomato plant (Solanum lycopersicum L.) | Oxheart | ↓ | = | = | = | [193] | ||||
| Cherry | = | ↓ | ↓ | ↓/= | ||||||
| Roma | = | = | ↓ | ↑/= | ||||||
| MicroTom | ↑ | [194] | ||||||||
| Tomato fruit (Solanum lycopersicum L.) | Budenovka | ↑ | ↑ | ↑/= | [195] | |||||
| Bull Heart | ↑ | ↑ | ↑/= | |||||||
| Gina | ↑ | ↑ | ↑/= | |||||||
| Micro-Tom | ↑ | [194] | ||||||||
| Sowthistle (Ixeris dentata Nakai) | ↑/= | ↑/= | ↑/= | [196] | ||||||
| Grape berry (Vitis vinifera L.) | Cabernet Sauvignon | ↑ | [197] | |||||||
| Blueberry (Vaccinium corymbosum L.) | Duke | ↓ | = | [198] | ||||||
| Peach fruit (Prunus persica L. Batsch) | Hujingmilu | ↑ | [199] | |||||||
| Yulu | = | |||||||||
| Basil (Ocimum basilicum L.) | Genovese | ↑/= | ↑ | ↓ | ↑/↓ | [188,200,201,202,203] | ||||
| Beet (Beta vulgaris L.) | Bulls Blood | ↑/↓ | ↑ | [188] | ||||||
| Rice (Oryza sativa L.) | Kanchana | ↑ | ↑ | [204] | ||||||
| Mattatriveni | ↓/= | |||||||||
| Harsha | ↑/= | |||||||||
| Broccoli (Brassica oleracea L. var. italica) | Waltham 29 | = | ↑ | [205] | ||||||
| Wheat (Triticum aestivum L.) | Sumai188 | ↑ | [206] | |||||||
| Mung bean (Vigna radiata) | ↑/↓ | ↑ | ↑ | [207] | ||||||
| Peppermint (Mentha piperita L.) | Rubescens | ↑ | ↑/↓ | [208] | ||||||
8.2. Terpenoids and Chlorophylls
8.3. Other Secondary Metabolites
9. UV-B Radiation
9.1. Phenolics
| Species | Cultivar | Phenolics | AC | T | AA | TP | GSL | Ref. | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Tot | Flav | Ant | ||||||||
| Basil (Ocimum basilicum L.) | Genovese | ↑/= | ↑/= | [200,201,202,203] | ||||||
| Cinnamon | ↑/↓ | ↑/=/↓ | [211] | |||||||
| Rice (Oryza sativa L.) | Kanchana | ↑ | ↑ | [204] | ||||||
| Mattatriveni | ↓/= | |||||||||
| Harsha | ↑/= | |||||||||
| Broccoli (Brassica oleracea L. var. italica) | Waltham 29 | = | ↑/= | ↑ | [185,205,212] | |||||
| Broccoli (Brassica oleracea var. gemmifera DC) | = | [187] | ||||||||
| Wheat (Triticum aestivum L.) | Sumai188 | ↑ | [206] | |||||||
| Mung bean (Vigna radiata) | ↑/↓ | ↑ | ↑ | [207] | ||||||
| Peppermint (Mentha piperita L.) | Rubescens | ↑ | ↑/↓ | [208] | ||||||
| Lettuce (Lactuca sativa. L.) | Red leaf cvs. | ↑ | ↑ | ↑ | ↓ | [191,209] | ||||
| Green leaf cvs. | ↑ | ↑ | ↑ | |||||||
| Peach fruit (Prunus persica L.) | Suncrest | ↑/=/↓ | = | ↓/↑ | =/↓ | [213,214] | ||||
| Big Top | ↑/=/↓ | ↓/↑ | ↑ | |||||||
| Babygold 7 | ↓ | ↓/↑ | = | |||||||
| Fairtime | ↓/↑ | ↓/↑ | ↓/↑ | ↓ | [215,216] | |||||
| Yulu | ↑ | [199] | ||||||||
| Hujingmilu | ↑ | |||||||||
| Tomato fruit (Solanum lycopersicum L.) | Money Maker | ↑ | ↑ | [217,218] | ||||||
| Zhenfen 202 | ↓ | [219] | ||||||||
| Bell pepper fruit (Capsicum annum L.) | Angus | ↑ | [220] | |||||||
| Green lime fruit (Citrus latifolia Tan.) | ↑ | [221] | ||||||||
| Spinach (Spinacia oleracea L.) | Meridian | ↑ | [222] | |||||||
| Maize (Zea mays L.) | ↓ | [223] | ||||||||
| Cucumber (Cucumis sativus L.) | Long green | ↓ | [224] | |||||||
| Apple fruit (Malus domestica Borkh.) | Aroma | ↑ | [225] | |||||||
9.2. Terpenoids and Chlorophylls
9.3. Other Secondary Metabolites
10. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ashraf, M.A.; Iqbal, M.; Rasheed, R.; Hussain, I.; Riaz, M.; Arif, M.S. Environmental Stress and Secondary Metabolites in Plants: An Overview. In Plant Metabolites and Regulation under Environmental Stress; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–167. ISBN 9780128126905. [Google Scholar]
- Jenkins, G.I. The UV-B Photoreceptor UVR8: From Structure to Physiology. Plant Cell 2014, 26, 21–37. [Google Scholar] [CrossRef] [Green Version]
- Zoltowski, B.D.; Imaizumi, T. Structure and function of the ZTL/FKF1/LKP2 group proteins in arabidopsis. In Enzymes; Academic Press: Cambridge, MA, USA, 2014; Volume 35, pp. 213–239. [Google Scholar]
- Xu, X.; Paik, I.; Zhu, L.; Huq, E. Illuminating Progress in Phytochrome-Mediated Light Signaling Pathways. Trends Plant Sci. 2015, 20, 641–650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Liu, B.; Zhao, C.; Pepper, M.; Lin, C. The Action Mechanisms of Plant Cryptochromes. Trends Plant Sci. 2011, 16, 684–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, N.; O’Hara, A.; Farkas, D.; Safronov, O.; Ratanasopa, K.; Wang, F.; Lindfors, A.V.; Jenkins, G.I.; Lehto, T.; Salojärvi, J.; et al. The Photoreceptor UVR8 Mediates the Perception of Both UV-B and UV-A Wavelengths up to 350 Nm of Sunlight with Responsivity Moderated by Cryptochromes. Plant Cell Environ. 2020, 43, 1513–1527. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakuraba, Y.; Jeong, J.; Kang, M.Y.; Kim, J.; Paek, N.C.; Choi, G. Phytochrome-Interacting Transcription Factors PIF4 and PIF5 Induce Leaf Senescence in Arabidopsis. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [Green Version]
- Leivar, P.; Monte, E. PIFs: Systems Integrators in Plant Development. Plant Cell 2014, 26, 56–78. [Google Scholar] [CrossRef] [Green Version]
- Rösler, J.; Klein, I.; Zeidler, M. Arabidopsis Fhl/Fhy1 Double Mutant Reveals a Distinct Cytoplasmic Action of Phytochrome A. Proc. Natl. Acad. Sci. USA 2007, 104, 10737–10742. [Google Scholar] [CrossRef] [Green Version]
- Matsushita, T.; Mochizuki, N.; Nagatani, A. Dimers of the N-Terminal Domain of Phytochrome B Are Functional in the Nucleus. Nature 2003, 424, 571–574. [Google Scholar] [CrossRef]
- Smith, H. Phytochromes and Light Signal Perception by Plants—an Emerging Synthesis. Nature 2000, 407, 585–591. [Google Scholar] [CrossRef]
- Casal, J.J.; Candia, A.N.; Sellaro, R. Light Perception and Signalling by Phytochrome A. J. Exp. Bot. 2014, 65, 2835–2845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burgie, E.S.; Vierstra, R.D. Phytochromes: An Atomic Perspective on Photoactivation and Signaling. Plant Cell 2014, 26, 4568–4583. [Google Scholar] [CrossRef] [Green Version]
- Kircher, S.; Kozma-Bognar, L.; Kim, L.; Adam, E.; Harter, K.; Schäfer, E.; Nagy, F. Light Quality-Dependent Nuclear Import of the Plant Photoreceptors Phytochrome A and B. Plant Cell 1999, 11, 1445–1456. [Google Scholar] [PubMed] [Green Version]
- Nagy, F.; Kircher, S.; Schäfer, E. Nucleo-Cytoplasmic Partitioning of the Plant Photoreceptors Phytochromes. Semin. Cell Dev. Biol. 2000, 11, 505–510. [Google Scholar] [CrossRef] [Green Version]
- Hoang, Q.T.N.; Han, Y.J.; Kim, J. Plant Phytochromes and Their Phosphorylation. Int. J. Mol. Sci. 2019, 20, 3450. [Google Scholar] [CrossRef] [Green Version]
- Yang, Z.; Liu, B.; Su, J.; Liao, J.; Lin, C.; Oka, Y. Cryptochromes Orchestrate Transcription Regulation of Diverse Blue Light Responses in Plants. Photochem. Photobiol. 2017, 93, 112–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christie, J.M.; Blackwood, L.; Petersen, J.; Sullivan, S. Plant Flavoprotein Photoreceptors. Plant Cell Physiol. 2015, 56, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Chory, J.; Fankhauser, C. Light Signal Transduction in Higher Plants. Annu. Rev. Genet. 2004, 38, 87–117. [Google Scholar] [CrossRef] [Green Version]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; van der Straeten, D.; Bakrim, N.; Meier, S.; Batschauer, A.; Galland, P.; Bittl, R.; et al. Cryptochrome Blue Light Photoreceptors Are Activated through Interconversion of Flavin Redox States. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mishra, S.; Khurana, J.P. Emerging Roles and New Paradigms in Signaling Mechanisms of Plant Cryptochromes. Crit. Rev. Plant Sci. 2017, 36, 89–115. [Google Scholar] [CrossRef]
- Wang, Q.; Lin, C. Mechanisms of Cryptochrome-Mediated Photoresponses in Plants. Annu. Rev. Plant Biol. 2020, 71, 103–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Klejnot, J.; Zhao, X.; Shalitin, D.; Maymon, M.; Yang, H.; Lee, J.; Liu, X.; Lopez, J.; Lina, C. Arabidopsis Cryptochrome 2 Completes Its Posttranslational Life Cycle in the Nucleus. Plant Cell 2007, 19, 3146–3156. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Yang, H.; Guo, H.; Mockler, T.; Chen, J.; Cashmore, A.R. Enhancement of Blue-Light Sensitivity of Arabidopsis Seedlings by a Blue Light Receptor Cryptochrome 2. Proc. Natl. Acad. Sci. USA 1998, 95, 2686–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swartz, T.E.; Corchnoy, S.B.; Christie, J.M.; Lewis, J.W.; Szundi, I.; Briggs, W.R.; Bogomolni, R.A. The Photocycle of a Flavin-Binding Domain of the Blue Light Photoreceptor Phototropin*. J. Biol. Chem. 2001, 276, 36493–36500. [Google Scholar] [CrossRef] [Green Version]
- Christie, J.M. Phototropin Blue-Light Receptors. Annu. Rev. Plant Biol. 2007, 58, 21–45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suetsugu, N.; Wada, M. Signalling Mechanism of Phototropin-Mediated Chloroplast Movement in Arabidopsis. J. Plant Biochem. Biotechnol. 2020, 29, 580–589. [Google Scholar] [CrossRef]
- Kim, W.-Y.; Fujiwara, S.; Suh, S.-S.; Kim, J.; Kim, Y.; Han, L.; David, K.; Putterill, J.; Gil Nam, H.; Somers, D.E. ZEITLUPE Is a Circadian Photoreceptor Stabilized by GIGANTEA in Blue Light. Nature 2007, 449. [Google Scholar] [CrossRef]
- Rizzini, L.; Favory, J.J.; Cloix, C.; Faggionato, D.; O’Hara, A.; Kaiserli, E.; Baumeister, R.; Schäfer, E.; Nagy, F.; Jenkins, G.I.; et al. Perception of UV-B by the Arabidopsis UVR8 Protein. Science 2011, 332, 103–106. [Google Scholar] [CrossRef] [Green Version]
- Favory, J.J.; Stec, A.; Gruber, H.; Rizzini, L.; Oravecz, A.; Funk, M.; Albert, A.; Cloix, C.; Jenkins, G.I.; Oakeley, E.J.; et al. Interaction of COP1 and UVR8 Regulates UV-B-Induced Photomorphogenesis and Stress Acclimation in Arabidopsis. Embo J. 2009, 28, 591–601. [Google Scholar] [CrossRef]
- Ulm, R.; Baumann, A.; Oravecz, A.; Máté, Z.; Ádám, É.; Oakeley, E.J.; Schäfer, E.; Nagy, F. Genome-Wide Analysis of Gene Expression Reveals Function of the BZIP Transcription Factor HY5 in the UV-B Response of Arabidopsis. Proc. Natl. Acad. Sci. USA 2004, 101, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Ouyang, X.; Yang, P.; Lau, O.S.; Li, G.; Li, J.; Chen, H.; Deng, X.W. Arabidopsis FHY3 and HY5 Positively Mediate Induction of COP1 Transcription in Response to Photomorphogenic UV-B Light. Plant Cell 2012, 24, 4590–4607. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Yang, P.; Ouyang, X.; Chen, L.; Deng, X.W. Photoactivated UVR8-COP1 Module Determines Photomorphogenic UV-B Signaling Output in Arabidopsis. PLoS Genet. 2014, 10. [Google Scholar] [CrossRef]
- Wargent, J.J.; Gegas, V.C.; Jenkins, G.I.; Doonan, J.H.; Paul, N.D. UVR8 in Arabidopsis Thaliana Regulates Multiple Aspects of Cellular Differentiation during Leaf Development in Response to Ultraviolet B Radiation. J. Compil. New Phytol. 2009, 183, 315–326. [Google Scholar] [CrossRef]
- Hayes, S.; Velanis, C.N.; Jenkins, G.I.; Franklin, K.A. UV-B Detected by the UVR8 Photoreceptor Antagonizes Auxin Signaling and Plant Shade Avoidance. Proc. Natl. Acad. Sci. USA 2014, 111, 11894–11899. [Google Scholar] [CrossRef] [Green Version]
- Heijde, M.; Ulm, R. Reversion of the Arabidopsis UV-B Photoreceptor UVR8 to the Homodimeric Ground State. Proc. Natl. Acad. Sci. USA 2013, 110, 1113–1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teixeira, R.T. Distinct Responses to Light in Plants. Plants 2020, 9, 894. [Google Scholar] [CrossRef]
- Pierik, R.; Ballaré, C.L. Control of Plant Growth and Defense by Photoreceptors: From Mechanisms to Opportunities in Agriculture. Mol. Plant 2021, 14, 61–76. [Google Scholar] [CrossRef]
- Han, X.; Huang, X.; Deng, X.W. The Photomorphogenic Central Repressor COP1: Conservation and Functional Diversification during Evolution. Plant Commun. 2020, 1, 1–13. [Google Scholar] [CrossRef]
- Jing, Y.; Lin, R. Transcriptional Regulatory Network of the Light Signaling Pathways. New Phytol. 2020, 227, 683–697. [Google Scholar] [CrossRef] [Green Version]
- Bhatnagar, A.; Singh, S.; Khurana, J.P.; Burman, N. HY5-COP1: The Central Module of Light Signaling Pathway. J. Plant Biochem. Biotechnol. 2020, 29, 590–610. [Google Scholar] [CrossRef]
- Ulm, R.; Jenkins, G.I. Q&A: How Do Plants Sense and Respond to UV-B Radiation? BMC Biol. 2015, 13, 45. [Google Scholar] [CrossRef] [Green Version]
- Amengual, J. Bioactive Properties of Carotenoids in Human Health. Nutrients 2019, 11, 2388. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Miekus, N.; Marszałek, K.; Podlacha, M.; Iqbal, A.; Puchalski, C.; Swiergiel, A.H. Health Benefits of Plant-Derived Sulfur Compounds, Glucosinolates, and Organosulfur Compounds. Molecules 2020, 25, 3804. [Google Scholar] [CrossRef]
- Szewczyk, K.; Chojnacka, A.; Górnicka, M. Tocopherols and Tocotrienols—Bioactive Dietary Compounds; What Is Certain, What Is Doubt? Int. J. Mol. Sci. 2021, 22, 6222. [Google Scholar] [CrossRef]
- Liu, L.H.; Zabaras, D.; Bennett, L.E.; Aguas, P.; Woonton, B.W. Effects of UV-C, Red Light and Sun Light on the Carotenoid Content and Physical Qualities of Tomatoes during Post-Harvest Storage. Food Chem. 2009, 115, 495–500. [Google Scholar] [CrossRef]
- Hernandez-Aguilar, C.; Dominguez-Pacheco, A.; Tenango, M.P.; Valderrama-Bravo, C.; Hernández, M.S.; Cruz-Orea, A.; Ordonez-Miranda, J. Characterization of Bean Seeds, Germination, and Phenolic Compounds of Seedlings by UV-C Radiation. J. Plant Growth Regul. 2021, 40, 642–655. [Google Scholar] [CrossRef]
- Zhu, T.; Yang, J.; Zhang, D.; Cai, Q.; Zhou, D.; Tu, S.; Liu, Q.; Tu, K. Effects of White LED Light and UV-C Radiation on Stilbene Biosynthesis and Phytochemicals Accumulation Identified by UHPLC-MS/MS during Peanut (Arachis Hypogaea L.) Germination. J. Agric. Food Chem. 2020, 68, 5900–5909. [Google Scholar] [CrossRef]
- Cheynier, V.; Comte, G.; Davies, K.M.; Lattanzio, V.; Martens, S. Plant Phenolics: Recent Advances on Their Biosynthesis, Genetics, Andecophysiology. Plant Physiol. Biochem. 2013, 72, 1–20. [Google Scholar] [CrossRef]
- Lattanzio, V.; Lattanzio, V.M.T.; Cardinali, A.; Amendola, V. Role of Phenolics in the Resistance Mechanisms of Plants against Fungal Pathogens and Insects; Research Signpost: Kerala, India, 2006; Volume 661, ISBN 8130800349. [Google Scholar]
- Randhir, R.; Lin, Y.T.; Shetty, K. Phenolics, Their Antioxidant and Antimicrobial Activity in Dark Germinated Fenugreek Sprouts in Response to Peptide and Phytochemical Elicitors. Asia Pac. J. Clin. Nutr. 2004, 13, 295–307. [Google Scholar] [CrossRef]
- Martens, S.; Mithöfer, A. Flavones and Flavone Synthases. Phytochemistry 2005, 66, 2399–2407. [Google Scholar] [CrossRef]
- Balasundram, N.; Sundram, K.; Samman, S. Phenolic Compounds in Plants and Agri-Industrial by-Products: Antioxidant Activity, Occurrence, and Potential Uses. Food Chem. 2006, 99, 191–203. [Google Scholar] [CrossRef]
- Sies, H. Polyphenols and Health: Update and Perspectives. Arch. Biochem. Biophys. 2010, 501, 2–5. [Google Scholar] [CrossRef]
- Samoylenko, A.; Hossain, J.; Mennerich, D.; Kellokumpu, S.; Hiltunen, J.K.; Kietzmann, T. Nutritional Countermeasures Targeting Reactive Oxygen Species in Cancer: From Mechanisms to Biomarkers and Clinical Evidence. Antioxid. Redox Signal. 2013, 19, 2157–2196. [Google Scholar] [CrossRef] [Green Version]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of Antioxidants Supplementation on Aging and Longevity. Biomed Res. Int. 2014, 2014. [Google Scholar] [CrossRef]
- Dimitrios, B. Sources of Natural Phenolic Antioxidants. Trends Food Sci. Technol. 2006, 17, 505–512. [Google Scholar] [CrossRef]
- Călinoiu, L.F.; Vodnar, D.C. Whole Grains and Phenolic Acids: A Review on Bioactivity, Functionality, Health Benefits and Bioavailability. Nutrients 2018, 10, 1615. [Google Scholar] [CrossRef] [Green Version]
- Singh, B.; Singh, J.P.; Kaur, A.; Singh, N. Phenolic Composition and Antioxidant Potential of Grain Legume Seeds: A Review. Food Res. Int. 2017, 101, 1–16. [Google Scholar] [CrossRef]
- Ofosu, F.K.; Elahi, F.; Daliri, E.B.M.; Chelliah, R.; Ham, H.J.; Kim, J.H.; Han, S.I.; Hur, J.H.; Oh, D.H. Phenolic Profile, Antioxidant, and Antidiabetic Potential Exerted by Millet Grain Varieties. Antioxidants 2020, 9, 254. [Google Scholar] [CrossRef] [Green Version]
- Domínguez-Perles, R.; Baenas, N.; García-Viguera, C. New Insights in (Poly)Phenolic Compounds: From Dietary Sources to Health Evidence. Foods 2020, 9, 543. [Google Scholar] [CrossRef]
- Thilakarathna, S.H.; Vasantha Rupasinghe, H.P. Flavonoid Bioavailability and Attempts for Bioavailability Enhancement. Nutrients 2013, 5, 3367–3387. [Google Scholar] [CrossRef]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef]
- Arct, J.; Pytkowska, K. Flavonoids as Components of Biologically Active Cosmeceuticals. Clin. Dermatol. 2008, 26, 347–357. [Google Scholar] [CrossRef]
- Abdallah, I.I.; Quax, W.J. A Glimpse into the Biosynthesis of Terpenoids. Kne Life Sci. 2017, 3, 81. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. The 1-Deoxy-D-Xilulose-5-Phosphate Pathway of Isoprenoid Biosynthesis in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 47–65. [Google Scholar] [CrossRef]
- Newman, J.D.; Chappell, J. Isoprenoid Biosynthesis in Plants: Carbon Partitioning within the Cytoplasmic Pathway. Crit. Rev. Biochem. Mol. Biol. 1999, 34, 95–106. [Google Scholar] [CrossRef]
- Abbas, F.; Ke, Y.; Yu, R.; Yue, Y.; Amanullah, S.; Jahangir, M.M.; Fan, Y. Volatile Terpenoids: Multiple Functions, Biosynthesis, Modulation and Manipulation by Genetic Engineering. Planta 2017, 246, 803–816. [Google Scholar] [CrossRef]
- Dudareva, N.; Klempien, A.; Muhlemann, J.K.; Kaplan, I. Biosynthesis, Function and Metabolic Engineering of Plant Volatile Organic Compounds. New Phytol. 2013, 198, 16–32. [Google Scholar] [CrossRef]
- Yu, F.; Utsumi, R. Diversity, Regulation, and Genetic Manipulation of Plant Mono- and Sesquiterpenoid Biosynthesis. Cell. Mol. Life Sci. 2009, 66, 3043–3052. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive Profile of Pumpkin: An Overview on Terpenoids and Their Health-Promoting Properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Wang, C.Y.; Chen, Y.W.; Hou, C.Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.A.; Khundmiri, S.U.K.; Khundmiri, S.R.; Al-Sanea, M.M.; Mok, P.L. Fruit-Derived Polysaccharides and Terpenoids: Recent Update on the Gastroprotective Effects and Mechanisms. Front. Pharmacol. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Yan, J.W.; Ban, Z.J.; Lu, H.Y.; Li, D.; Poverenov, E.; Luo, Z.S.; Li, L. The Aroma Volatile Repertoire in Strawberry Fruit: A Review. J. Sci. Food Agric. 2018, 98, 4395–4402. [Google Scholar] [CrossRef]
- Liu, F.; Li, S.; Gao, J.; Cheng, K.; Yuan, F. Changes of Terpenoids and Other Volatiles during Alcoholic Fermentation of Blueberry Wines Made from Two Southern Highbush Cultivars. LWT 2019, 109, 233–240. [Google Scholar] [CrossRef]
- Ma, Y.; Li, S.; Yin, X.; Xing, Y.; Lin, H.; Xu, Q.; Bi, X.; Chen, C. Effects of Controlled Atmosphere on the Storage Quality and Aroma Compounds of Lemon Fruits Using the Designed Automatic Control Apparatus. Biomed Res. Int. 2019, 2019. [Google Scholar] [CrossRef]
- Mele, M.A.; Kang, H.M.; Lee, Y.T.; Islam, M.Z. Grape Terpenoids: Flavor Importance, Genetic Regulation, and Future Potential. Crit. Rev. Food Sci. Nutr. 2021, 61, 1429–1447. [Google Scholar] [CrossRef]
- Deng, H.; Chen, S.; Zhou, Z.; Li, X.; Chen, S.; Hu, J.; Lai, Z.; Sun, Y. Transcriptome Analysis Reveals the Effect of Short-Term Sunlight on Aroma Metabolism in Postharvest Leaves of Oolong Tea(Camellia Sinensis). Food Res. Int. 2020, 137. [Google Scholar] [CrossRef]
- Eggersdorfer, M.; Wyss, A. Carotenoids in Human Nutrition and Health. Arch. Biochem. Biophys. 2018, 652, 18–26. [Google Scholar] [CrossRef]
- Young, A.J.; Lowe, G.L. Carotenoids—Antioxidant Properties. Antioxidants 2018, 7, 28. [Google Scholar] [CrossRef] [Green Version]
- Bolhassani, A.; Milani, A.; Basirnejad, M.; Shahbazi, S. Carotenoids: Biochemistry, Pharmacology and Treatment. Br. J. Pharmacol. 2017, 174, 1290. [Google Scholar] [CrossRef]
- Rodriguez-Concepcion, M.; Avalos, J.; Bonet, M.L.; Boronat, A.; Gomez-Gomez, L.; Hornero-Mendez, D.; Limon, M.C.; Meléndez-Martínez, A.J.; Olmedilla-Alonso, B.; Palou, A.; et al. A Global Perspective on Carotenoids: Metabolism, Biotechnology, and Benefits for Nutrition and Health. Prog. Lipid Res. 2018, 70, 62–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zimmer, J.P.; Hammond, B.R. Possible Influences of Lutein and Zeaxanthin on the Developing Retina. Clin. Opthalmol. 2007, 1, 25–35. [Google Scholar]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.P.; Ferris, F.L.; Elman, M.J.; Antoszyk, A.N.; Ruby, A.J.; Orth, D.; Bressler, S.B.; et al. Secondary Analyses of the Effects of Lutein/Zeaxanthin on Age-Related Macular Degeneration Progression AREDS2 Report No. 3. JAMA Ophthalmol. 2014, 132, 142–149. [Google Scholar] [CrossRef]
- Walk, A.M.; Khan, N.A.; Barnett, S.M.; Raine, L.B.; Kramer, A.F.; Cohen, N.J.; Moulton, C.J.; Renzi-Hammond, L.M.; Hammond, B.R.; Hillman, C.H. From Neuro-Pigments to Neural Efficiency: The Relationship between Retinal Carotenoids and Behavioral and Neuroelectric Indices of Cognitive Control in Childhood. Int. J. Psychophysiol. 2017, 118, 1–8. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and Tomato and Risk of Cardiovascular Diseases: A Systematic Review and Meta-Analysis of Epidemiological Evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Tan, H.L.; Thomas-Ahner, J.M.; Pearl, D.K.; Erdman, J.W.; Moran, N.E.; Clinton, S.K. Dietary Tomato and Lycopene Impact Androgen Signaling- and Carcinogenesis-Related Gene Expression during Early TRAMP Prostate Carcinogenesis. Chest 2014, 146, 1228–1239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mínguez-Alarcón, L.; Mendiola, J.; López-Espín, J.J.; Sarabia-Cos, L.; Vivero-Salmerón, G.; Vioque, J.; Navarrete-Muñoz, E.M.; Torres-Cantero, A.M. Dietary Intake of Antioxidant Nutrients Is Associated with Semen Quality in Young University Students. Hum. Reprod. 2012, 27, 2807–2814. [Google Scholar] [CrossRef] [Green Version]
- Palmer, A.C.; West, K.P.; Dalmiya, N.; Schultink, W. The Use and Interpretation of Serum Retinol Distributions in Evaluating the Public Health Impact of Vitamin A Programmes. Public Health Nutr. 2012, 15, 1201–1215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saini, R.K.; Keum, Y.S. Tocopherols and Tocotrienols in Plants and Their Products: A Review on Methods of Extraction, Chromatographic Separation, and Detection. Food Res. Int. 2016, 82, 59–70. [Google Scholar] [CrossRef]
- Shahidi, F.; de Camargo, A.C. Tocopherols and Tocotrienols in Common and Emerging Dietary Sources: Occurrence, Applications, and Health Benefits. Int. J. Mol. Sci. 2016, 17, 1745. [Google Scholar] [CrossRef]
- Mocchegiani, E.; Costarelli, L.; Giacconi, R.; Malavolta, M.; Basso, A.; Piacenza, F.; Ostan, R.; Cevenini, E.; Gonos, E.S.; Franceschi, C.; et al. Vitamin E-Gene Interactions in Aging and Inflammatory Age-Related Diseases: Implications for Treatment. A Systematic Review. Ageing Res. Rev. 2014, 14, 81–101. [Google Scholar] [CrossRef] [Green Version]
- Rashid Khan, M.; Ahsan, H.; Siddiqui, S.; Siddiqui, W.A. Tocotrienols Have a Nephroprotective Action against Lipid-Induced Chronic Renal Dysfunction in Rats. Ren. Fail. 2015, 37, 136–143. [Google Scholar] [CrossRef]
- Kirmizis, D.; Chatzidimitriou, D. Vascular Health and Risk Management Antiatherogenic Effects of Vitamin e: The Search for the Holy Grail. Vasc. Health Risk Manag. 2009, 5, 767–774. [Google Scholar] [CrossRef] [Green Version]
- Minhajuddin, M.; Beg, Z.H.; Iqbal, J. Hypolipidemic and Antioxidant Properties of Tocotrienol Rich Fraction Isolated from Rice Bran Oil in Experimentally Induced Hyperlipidemic Rats. Food Chem. Toxicol. 2005, 43, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Kizhakekuttu, T.J.; Widlansky, M.E. Natural Antioxidants and Hypertension: Promise and Challenges. Cardiovasc. Ther. 2010, 28, 20–32. [Google Scholar] [CrossRef] [PubMed]
- García-Closas, R.; Berenguer, A.; Tormo, M.J.; Sánchez, M.J.; Quirós, J.R.; Navarro, C.; Arnaud, R.; Dorronsoro, M.; Chirlaque, M.D.; Barricarte, A.; et al. Dietary Sources of Vitamin C, Vitamin E and Specific Carotenoids in Spain. Br. J. Nutr. 2004, 91, 1005–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fenech, M.; Amaya, I.; Valpuesta, V.; Botella, M.A. Vitamin C Content in Fruits: Biosynthesis and Regulation. Front. Plant Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Zhang, Z.; Huang, R. Regulation of Ascorbic Acid Synthesis in Plants. Plant Signal. Behav. 2013, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wheeler, G.L.; Jones, M.A.; Smirnoff, N. The Biosynthetic Pathway of Vitamin C in Higher Plants. Nature 1998, 393, 365–369. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. The Water-Water Cycle in Chloroplasts: Scavenging of Active Oxygens and Dissipation of Excess Photons. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 601–639. [Google Scholar] [CrossRef]
- Foyer, C.H.; Halliwell, B. The Presence of Glutathione and Glutathione Reductase in Chloroplasts: A Proposed Role in Ascorbic Acid Metabolism. Planta 1976, 133, 21–25. [Google Scholar] [CrossRef]
- Bilska, K.; Wojciechowska, N.; Alipour, S.; Kalemba, E.M. Ascorbic Acid—the Little-Known Antioxidant in Woody Plants. Antioxidants 2019, 8, 645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Page, M.; Sultana, N.; Paszkiewicz, K.; Florance, H.; Smirnoff, N. The Influence of Ascorbate on Anthocyanin Accumulation during High Light Acclimation in Arabidopsis Thaliana: Further Evidence for Redox Control of Anthocyanin Synthesis. PlantCell Environ. 2012, 35, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Müller-Moulé, P.; Conklin, P.L.; Niyogi, K.K. Ascorbate Deficiency Can Limit Violaxanthin De-Epoxidase Activity in Vivo. Plant Physiol. 2002, 128, 970–977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.-Y.; Chien, C.-T.; Chung, J.-D.; Yang, Y.-S.; Kuo, S.-R. Dormancy-Break and Germination in Seeds of Prunus Campanulata (Rosaceae): Role of Covering Layers and Changes in Concentration of Abscisic Acid and Gibberellins. Seed Sci. Res. 2007, 17, 21–32. [Google Scholar] [CrossRef]
- De Tullio, M.C.; Arrigoni, O. The Ascorbic Acid System in Seeds: To Protect and to Serve. Seed Sci. Res. 2003, 13, 249–260. [Google Scholar] [CrossRef]
- Sauberlich, H.E. A history of scurvy and vitamin C. In Vitamin C in Health and Disease; Marcel Dekker Inc.: New York, NY, USA, 1997; pp. 1–24. ISBN 0824793137. [Google Scholar]
- Ngo, B.; van Riper, J.M.; Cantley, L.C.; Yun, J. Targeting Cancer Vulnerabilities with High-Dose Vitamin C. Nat. Rev. Cancer 2019, 19, 271–282. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary Intake and Blood Concentrations of Antioxidants and the Risk of Cardiovascular Disease, Total Cancer, and All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Bhandari, S.R.; Jo, J.S.; Lee, J.G. Comparison of Glucosinolate Profiles in Different Tissues of Nine Brassica Crops. Molecules 2015, 20, 15827–15841. [Google Scholar] [CrossRef]
- Bellostas, N.; Kachlicki, P.; Sørensen, J.C.; Sørensen, H. Glucosinolate Profiling of Seeds and Sprouts of B. Oleracea Varieties Used for Food. Sci. Hortic. 2007, 114, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, S.R.; Kwak, J.H. Chemical Composition and Antioxidant Activity in Different Tissues of Brassica Vegetables. Molecules 2015, 20, 1228–1243. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.Y.; Ibrahim, K.E.; Juvik, J.A.; Kim, D.H.; Kang, W.J. Genetic and Environmental Variation of Glucosinolate Content in Chinese Cabbage. HortScience 2006, 41, 1382–1385. [Google Scholar] [CrossRef] [Green Version]
- Pék, Z.; Daood, H.; Nagyné, M.G.; Berki, M.; Tóthné, M.M.; Neményi, A.; Helyes, L. Yield and Phytochemical Compounds of Broccoli as Affected by Temperature, Irrigation, and Foliar Sulfur Supplementation. HortScience 2012, 47, 1646–1652. [Google Scholar] [CrossRef] [Green Version]
- Song, L.; Thornalley, P.J. Effect of Storage, Processing and Cooking on Glucosinolate Content of Brassica Vegetables. Food Chem. Toxicol. 2007, 45, 216–224. [Google Scholar] [CrossRef]
- Wang, J.; Gu, H.; Yu, H.; Zhao, Z.; Sheng, X.; Zhang, X. Genotypic Variation of Glucosinolates in Broccoli (Brassica Oleracea Var. Italica) Florets from China. Food Chem. 2012, 133, 735–741. [Google Scholar] [CrossRef]
- Mitreiter, S.; Gigolashvili, T. Regulation of Glucosinolate Biosynthesis. J. Exp. Bot. 2021, 72, 70–91. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, H.; Xie, J.; Lv, J.; Zhang, G.; Hu, L.; Luo, S.; Li, L.; Yu, J. The Roles of Cruciferae Glucosinolates in Disease and Pest Resistance. Plants 2021, 10, 1097. [Google Scholar] [CrossRef] [PubMed]
- Rios, J.J.; Agudelo, A.; Moreno, D.A.; Carvajal, M. Growing Broccoli under Salinity: The Influence of Cultivar and Season on Glucosinolates Content. Sci. Agric. 2020, 77. [Google Scholar] [CrossRef] [Green Version]
- Cocetta, G.; Mishra, S.; Raffaelli, A.; Ferrante, A. Effect of Heat Root Stress and High Salinity on Glucosinolates Metabolism in Wild Rocket. J. Plant Physiol. 2018, 231, 261–270. [Google Scholar] [CrossRef]
- Aghajanzadeh, T.A.; Reich, M.; Kopriva, S.; de Kok, L.J. Impact of Chloride (NaCl, KCl) and Sulphate (Na2SO4, K2SO4) Salinity on Glucosinolate Metabolism in Brassica Rapa. J. Agron. Crop Sci. 2018, 204, 137–146. [Google Scholar] [CrossRef]
- Shawon, R.A.; Kang, B.S.; Lee, S.G.; Kim, S.K.; Ju Lee, H.; Katrich, E.; Gorinstein, S.; Ku, Y.G. Influence of Drought Stress on Bioactive Compounds, Antioxidant Enzymes and Glucosinolate Contents of Chinese Cabbage (Brassica Rapa). Food Chem. 2020, 308. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, P.; Kim, J.S. Anti-Carcinogenic Glucosinolates in Cruciferous Vegetables and Their Antagonistic Effects on Prevention of Cancers. Molecules 2018, 23, 2983. [Google Scholar] [CrossRef] [Green Version]
- Dubey, S.; Guignard, F.; Pellaud, S.; Pedrazzetti, M.; van der Schuren, A.; Gaume, A.; Schnee, S.; Gindro, K.; Dubey, O. Isothiocyanate Derivatives of Glucosinolates as Efficient Natural Fungicides. PhytoFrontiersTM 2021, 1, 40–50. [Google Scholar] [CrossRef]
- Muntean, D.; Ştefănuţ, M.N.; Căta, A.; Buda, V.; Danciu, C.; Bănică, R.; Pop, R.; Licker, M.; Ienaşcu, I.M.C. Symmetrical Antioxidant and Antibacterial Properties of Four Romanian Cruciferous Extracts. Symmetry 2021, 13, 893. [Google Scholar] [CrossRef]
- Nam, T.G.; Kim, D.O.; Eom, S.H. Effects of Light Sources on Major Flavonoids and Antioxidant Activity in Common Buckwheat Sprouts. Food Sci. Biotechnol. 2018, 27, 169–176. [Google Scholar] [CrossRef]
- Son, K.-H.; Oh, M.-M. Leaf Shape, Growth, and Antioxidant Phenolic Compounds of Two Lettuce Cultivars Grown under Various Combinations of Blue and Red Light-Emitting Diodes. Am. Soc. Hortic. Sci. 2013, 48, 988–995. [Google Scholar] [CrossRef]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of Different LED Sources on the Growth and Nitrogen Metabolism of Lettuce. Plant Cell Tissue Organ Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Li, Q.; Kubota, C. Effects of Supplemental Light Quality on Growth and Phytochemicals of Baby Leaf Lettuce. Environ. Exp. Bot. 2009, 67, 59–64. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of Light Quality on Growth and Vegetable Quality in Leaf Lettuce, Spinach and Komatsuna. Environ. Control Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Samuoliene, G.; Brazaityte, A.; Sirtautas, R.; Viršile, A.; Sakalauskaite, J.; Sakalauskiene, S.; Duchovskis, P. LED Illumination Affects Bioactive Compounds in Romaine Baby Leaf Lettuce. J. Sci. Food Agric. 2013, 93, 3286–3291. [Google Scholar] [CrossRef]
- Długosz-Grochowska, O.; Wojciechowska, R.; Kruczek, M.; Habela, A. Supplemental Lighting with LEDs Improves the Biochemical Composition of Two Valerianella Locusta (L.) Cultivars. Hortic. Environ. Biotechnol. 2017, 58, 441–449. [Google Scholar] [CrossRef]
- Wu, M.C.; Hou, C.Y.; Jiang, C.M.; Wang, Y.T.; Wang, C.Y.; Chen, H.H.; Chang, H.M. A Novel Approach of LED Light Radiation Improves the Antioxidant Activity of Pea Seedlings. Food Chem. 2007, 101, 1753–1758. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Sirtautas, R.; Sakalauskiene, S.; Jankauskiene, J.; Duchovskis, P.; Novičkovas, A. The Impact of Supplementary Short-Term Red LED Lighting on the Antioxidant Properties of Microgreens. Acta Hortic. 2012, 956, 649–655. [Google Scholar] [CrossRef]
- Samuoliene, G.; Brazaityte, A.; Viršile, A.; Jankauskiene, J.; Sakalauskiene, S.; Duchovskis, P. Red Light-Dose or Wavelength-Dependent Photoresponse of Antioxidants in Herb Microgreens. PLoS ONE 2016, 11. [Google Scholar] [CrossRef]
- Piovene, C.; Orsini, F.; Bosi, S.; Sanoubar, R.; Bregola, V.; Dinelli, G.; Gianquinto, G. Optimal Red: Blue Ratio in Led Lighting for Nutraceutical Indoor Horticulture. Sci. Hortic. 2015, 193, 202–208. [Google Scholar] [CrossRef]
- Frisch, T.; Motawia, M.S.; Olsen, C.E.; Agerbirk, N.; Møller, B.L.; Bjarnholt, N. Diversified Glucosinolate Metabolism: Biosynthesis of Hydrogen Cyanide and of the Hydroxynitrile Glucoside Alliarinoside in Relation to Sinigrin Metabolism in Alliaria Petiolata. Front. Plant Sci. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Deng, M.; Qian, H.; Chen, L.; Sun, B.; Chang, J.; Miao, H.; Cai, C.; Wang, Q. Influence of Pre-Harvest Red Light Irradiation on Main Phytochemicals and Antioxidant Activity of Chinese Kale Sprouts. Food Chem. 2017, 222, 1–5. [Google Scholar] [CrossRef]
- Qian, H.; Liu, T.; Deng, M.; Miao, H.; Cai, C.; Shen, W.; Wang, Q. Effects of Light Quality on Main Health-Promoting Compounds and Antioxidant Capacity of Chinese Kale Sprouts. Food Chem. 2016, 196, 1232–1238. [Google Scholar] [CrossRef]
- Ma, G.; Zhang, L.; Setiawan, C.K.; Yamawaki, K.; Asai, T.; Nishikawa, F.; Maezawa, S.; Sato, H.; Kanemitsu, N.; Kato, M. Effect of Red and Blue LED Light Irradiation on Ascorbate Content and Expression of Genes Related to Ascorbate Metabolism in Postharvest Broccoli. Postharvest Biol. Technol. 2014, 94, 97–103. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Singh, B.R. Red Light Stimulates Flowering and Anthocyanin Biosynthesis in American Cranberry. Plant Growth Regul. 2002, 38, 165–171. [Google Scholar] [CrossRef]
- Gałazka-Czarnecka, I.; Korzeniewska, E.; Czarnecki, A.; Kiełbasa, P.; Drózdz, T. Modelling of Carotenoids Content in Red Clover Sprouts Using Light of Different Wavelength and Pulsed Electric Field. Appl. Sci. 2020, 10, 4143. [Google Scholar] [CrossRef]
- Cuong, D.M.; Ha, T.W.; Park, C.H.; Kim, N.S.; Yeo, H.J.; Chun, S.W.; Kim, C.; Park, S.U. Effects of LED Lights on Expression of Genes Involved in Phenylpropanoid Biosynthesis and Accumulation of Phenylpropanoids in Wheat Sprout. Agronomy 2019, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Tuan, P.A.; Thwe, A.A.; Kim, Y.B.; Kim, J.K.; Kim, S.J.; Lee, S.; Chung, S.O.; Park, S.U. Effects of White, Blue, and Red Light-Emitting Diodes on Carotenoid Biosynthetic Gene Expression Levels and Carotenoid Accumulation in Sprouts of Tartary Buckwheat (Fagopyrum Tataricum Gaertn.). J. Agric. Food Chem. 2013, 61, 12356–12361. [Google Scholar] [CrossRef]
- Yeo, H.J.; Park, C.H.; Lee, K.B.; Kim, J.K.; Park, J.S.; Lee, J.-W.; Park, S.U. Metabolic Analysis of Vigna Unguiculata Sprouts Exposed to Different Light-Emitting Diodes. Nat. Prod. Commun. 2018, 13, 1349–1354. [Google Scholar] [CrossRef] [Green Version]
- Zoratti, L.; Sarala, M.; Carvalho, E.; Karppinen, K.; Martens, S.; Giongo, L.; Häggman, H.; Jaakola, L. Monochromatic Light Increases Anthocyanin Content during Fruit Development in Bilberry. BMC Plant Biol. 2014, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, X.; Chen, Y.; Mei, X.; Katsuno, T.; Kobayashi, E.; Dong, F.; Watanabe, N.; Yang, Z. Regulation of Formation of Volatile Compounds of Tea (Camellia Sinensis) Leaves by Single Light Wavelength. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Frede, K.; Schreiner, M.; Zrenner, R.; Graefe, J.; Baldermann, S. Carotenoid Biosynthesis of Pak Choi (Brassica Rapa Ssp. Chinensis) Sprouts Grown under Different Light-Emitting Diodes during the Diurnal Course. Photochem. Photobiol. Sci. 2018, 17, 1289–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, J.; Jeong, M.J.; Lee, S.I.; Lee, J.G.; Hwang, H.; Yu, J.; Kim, Y.R.; Park, S.W.; Kim, J.A. Effect of LED Mixed Light Conditions on the Glucosinolate Pathway in Brassica Rapa. J. Plant Biotechnol. 2015, 42, 245–256. [Google Scholar] [CrossRef] [Green Version]
- Ma, G.; Zhang, L.; Kato, M.; Yamawaki, K.; Kiriiwa, Y.; Yahata, M.; Ikoma, Y.; Matsumoto, H. Effect of Blue and Red LED Light Irradiation on β-Cryptoxanthin Accumulation in the Flavedo of Citrus Fruits. J. Agric. Food Chem. 2012, 60, 197–201. [Google Scholar] [CrossRef] [PubMed]
- Kancheva, V.D. Phenolic Antioxidants-Radical-Scavenging and Chain-Breaking Activity: A Comparative Study. Eur. J. Lipid Sci. Technol. 2009, 111, 1072–1089. [Google Scholar] [CrossRef]
- Burri, B.J. Beta-Cryptoxanthin as a Source of Vitamin A. J. Sci. Food Agric. 2015, 95, 1786–1794. [Google Scholar] [CrossRef] [PubMed]
- Grune, T.; Lietz, G.; Palou, A.; Ross, A.C.; Stahl, W.; Tang, G.; Thurnham, D.; Yin, S.A.; Biesalski, H.K. β-Carotene Is an Important Vitamin A Source for Humans. J. Nutr. 2010, 140, 2268–2285. [Google Scholar] [CrossRef] [Green Version]
- Pérez-gálvez, A.; Viera, I.; Roca, M. Carotenoids and Chlorophylls as Antioxidants. Antioxidants 2020, 9, 505. [Google Scholar] [CrossRef]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green Light Drives Leaf Photosynthesis More Efficiently than Red Light in Strong White Light: Revisiting the Enigmatic Question of Why Leaves Are Green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Smith, H.L.; Mcausland, L.; Murchie, E.H. Don’t Ignore the Green Light: Exploring Diverse Roles in Plant Processes. J. Exp. Bot. 2017, 68, 2099–2110. [Google Scholar] [CrossRef]
- Battle, M.W.; Jones, M.A. Cryptochromes Integrate Green Light Signals into the Circadian System. Plant Cell Environ. 2020, 43, 16–27. [Google Scholar] [CrossRef] [Green Version]
- Schenkels, L.; Saeys, W.; Lauwers, A.; de Proft, M.P. Green Light Induces Shade Avoidance to Alter Plant Morphology and Increases Biomass Production in Ocimum Basilicum L. Sci. Hortic. 2020, 261. [Google Scholar] [CrossRef]
- Zheng, C.; Ma, J.Q.; Ma, C.L.; Shen, S.Y.; Liu, Y.F.; Chen, L. Regulation of Growth and Flavonoid Formation of Tea Plants (Camellia Sinensis) by Blue and Green Light. J. Agric. Food Chem. 2019, 67, 2408–2419. [Google Scholar] [CrossRef]
- Liu, H.; Fu, Y.; Wang, M.; Liu, H. Green Light Enhances Growth, Photosynthetic Pigments and CO2 Assimilation Efficiency of Lettuce as Revealed by ‘Knock out’ of the 480–560 Nm Spectral Waveband. Photosynthetica 2017, 55, 144–152. [Google Scholar] [CrossRef]
- Bian, Z.; Yang, Q.; Li, T.; Cheng, R.; Barnett, Y.; Lu, C. Study of the Beneficial Effects of Green Light on Lettuce Grown under Short-Term Continuous Red and Blue Light-Emitting Diodes. Physiol. Plant. 2018, 164, 226–240. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Weerheim, K.; Schipper, R.; Dieleman, J.A. Partial Replacement of Red and Blue by Green Light Increases Biomass and Yield in Tomato. Sci. Hortic. 2019, 249, 271–279. [Google Scholar] [CrossRef]
- Dou, H.; Niu, G.; Gu, M. Photosynthesis, Morphology, Yield, and Phytochemical Accumulation in Basil Plants Influenced by Substituting Green Light for Partial Red and/or Blue Light. HortScience 2019, 54, 1769–1776. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bian, Z.; Li, S.; Chen, X.; Lu, C. Comparative Analysis of Phenolic Compound Profiles, Antioxidant Capacities, and Expressions of Phenolic Biosynthesis-Related Genes in Soybean Microgreens Grown under Different Light Spectra. J. Agric. Food Chem. 2019, 67, 13577–13588. [Google Scholar] [CrossRef]
- Zha, L.; Liu, W.; Yang, Q.; Zhang, Y.; Zhou, C.; Shao, M. Regulation of Ascorbate Accumulation and Metabolism in Lettuce by the Red:Blue Ratio of Continuous Light Using LEDs. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Amoozgar, A.; Mohammadi, A.; Sabzalian, M.R. Impact of Light-Emitting Diode Irradiation on Photosynthesis, Phytochemical Composition and Mineral Element Content of Lettuce Cv. Grizzly. Photosynthetica 2017, 55, 85–95. [Google Scholar] [CrossRef]
- Li, H.; Tang, C.; Xu, Z.; Liu, X.; Han, X. Effects of Different Light Sources on the Growth of Non-Heading Chinese Cabbage (Brassica Campestris L.). J. Agric. Sci. 2012, 4. [Google Scholar] [CrossRef] [Green Version]
- Samuolienė, G.; Viršilė, A.; Brazaitytė, A.; Jankauskienė, J.; Sakalauskienė, S.; Vaštakaitė, V.; Novičkovas, A.; Viškelienė, A.; Sasnauskas, A.; Duchovskis, P. Blue Light Dosage Affects Carotenoids and Tocopherols in Microgreens. Food Chem. 2017, 228, 50–56. [Google Scholar] [CrossRef]
- Arakawa, O.; Kikuya, S.; Pungpomin, P.; Zhang, S.; Tanaka, N. Accumulation of Anthocyanin in Apples in Response to Blue Light at 450 Nm: Recommendations for Producing Quality Fruit Color under Global Warming. Eur. J. Hortic. Sci. 2016, 81, 297–302. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, L.; Li, Y.; Chen, Q.; Ye, Y.; Zhang, Y.; Luo, Y.; Sun, B.; Wang, X.; Tang, H. Effect of Red and Blue Light on Anthocyanin Accumulation and Differential Gene Expression in Strawberry (Fragaria × Ananassa). Molecules 2018, 23, 820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, F.; Cao, S.; Shi, L.; Chen, W.; Su, X.; Yang, Z. Blue Light Irradiation Affects Anthocyanin Content and Enzyme Activities Involved in Postharvest Strawberry Fruit. J. Agric. Food Chem. 2014, 62, 4778–4783. [Google Scholar] [CrossRef]
- Xu, F.; Shi, L.; Chen, W.; Cao, S.; Su, X.; Yang, Z. Effect of Blue Light Treatment on Fruit Quality, Antioxidant Enzymes and Radical-Scavenging Activity in Strawberry Fruit. Sci. Hortic. 2014, 175, 181–186. [Google Scholar] [CrossRef]
- Xie, B.X.; Wei, J.J.; Zhang, Y.T.; Song, S.W.; Su, W.; Sun, G.W.; Hao, Y.W.; Liu, H.C. Supplemental Blue and Red Light Promote Lycopene Synthesis in Tomato Fruits. J. Integr. Agric. 2019, 18, 590–598. [Google Scholar] [CrossRef] [Green Version]
- Hammock, H.A.; Kopsell, D.A.; Sams, C.E. Narrowband Blue and Red LED Supplements Impact Key Flavor Volatiles in Hydroponically Grown Basil Across Growing Seasons. Front. Plant Sci. 2021, 12. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, G.; Yamawaki, K.; Ikoma, Y.; Matsumoto, H.; Yoshioka, T.; Ohta, S.; Kato, M. Regulation of Ascorbic Acid Metabolism by Blue LED Light Irradiation in Citrus Juice Sacs. Plant Sci. 2015, 233, 134–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Kim, N.S.; Park, J.S.; Lee, S.Y.; Lee, J.W.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Glucosinolates and Phenolic Compounds in Sprouting Canola (Brassica Napus L.). Foods 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Park, Y.E.; Yeo, H.J.; Kim, J.K.; Park, S.U. Effects of Light-Emitting Diodes on the Accumulation of Phenolic Compounds and Glucosinolates in Brassica Juncea Sprouts. Horticulturae 2020, 6, 77. [Google Scholar] [CrossRef]
- Li, Y.Y.; Mao, K.; Zhao, C.; Zhao, X.Y.; Zhang, R.F.; Zhang, H.L.; Shu, H.R.; Hao, Y.J. Molecular Cloning and Functional Analysis of a Blue Light Receptor Gene MdCRY2 from Apple (Malus Domestica). Plant Cell Rep. 2013, 32, 555–566. [Google Scholar] [CrossRef]
- Kadomura-Ishikawa, Y.; Miyawaki, K.; Noji, S.; Takahashi, A. Phototropin 2 Is Involved in Blue Light-Induced Anthocyanin Accumulation in Fragaria x Ananassa Fruits. J. Plant Res. 2013, 126, 847–857. [Google Scholar] [CrossRef]
- Fraser, P.D.; Romer, S.; Shipton, C.A.; Mills, P.B.; Kiano, J.W.; Misawa, N.; Drake, R.G.; Schuch, W.; Bramley, P.M. Evaluation of Transgenic Tomato Plants Expressing an Additional Phytoene Synthase in a Fruit-Specific Manner. Proc. Natl. Acad. Sci. USA 2002, 99, 1092–1097. [Google Scholar] [CrossRef] [Green Version]
- Mao, P.; Duan, F.; Zheng, Y.; Yang, Q. Blue and UV-A Light Wavelengths Positively Affected Accumulation Profiles of Healthy Compounds in Pak-Choi. J. Sci. Food Agric. 2021, 101, 1676–1684. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, B.; Sun, M.; Li, Y.; Kawabata, S. UV-A Light Induces Anthocyanin Biosynthesis in a Manner Distinct from Synergistic Blue + UV-B Light and UV-A/Blue Light Responses in Different Parts of the Hypocotyls in Turnip Seedlings. Plant Cell Physiol. 2012, 53, 1470–1480. [Google Scholar] [CrossRef] [Green Version]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light Doses and Harvesting Time Differentially Tailor Glucosinolate and Phenolic Profiles in Broccoli Sprouts. Molecules 2017, 22, 1065. [Google Scholar] [CrossRef]
- Rechner, O.; Neugart, S.; Schreiner, M.; Wu, S.; Poehling, H.M. Different Narrow-Band Light Ranges Alter Plant Secondary Metabolism and Plant Defense Response to Aphids. J. Chem. Ecol. 2016, 42, 989–1003. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Rechner, O.; Neugart, S.; Schreiner, M.; Poehling, H.M. Effects of Light-Emitting Diode Treatments on Brevicoryne Brassicae Performance Mediated by Secondary Metabolites in Brussels Sprouts. J. Plant Dis. Prot. 2016, 123, 321–330. [Google Scholar] [CrossRef]
- Brazaityte, A.; Viršile, A.; Jankauskiene, J.; Sakalauskiene, S.; Samuoliene, G.; Sirtautas, R.; Novičkovas, A.; Dabašinskas, L.; Miliauskiene, J.; Vaštakaite, V.; et al. Effect of Supplemental UV-A Irradiation in Solid-State Lighting on the Growth and Phytochemical Content of Microgreens. Int. Agrophysics 2015, 29, 13–22. [Google Scholar] [CrossRef]
- He, R.; Zhang, Y.; Song, S.; Su, W.; Hao, Y.; Liu, H. UV-A and FR Irradiation Improves Growth and Nutritional Properties of Lettuce Grown in an Artificial Light Plant Factory. Food Chem. 2021, 345. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, T.; Yang, Q.; Zhang, Y.; Zou, J.; Bian, Z.; Wen, X. UVA Radiation Is Beneficial for Yield and Quality of Indoor Cultivated Lettuce. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldwell, C.R.; Britz, S.J. Effect of Supplemental Ultraviolet Radiation on the Carotenoid and Chlorophyll Composition of Green House-Grown Leaf Lettuce (Lactuca Sativa L.) Cultivars. J. Food Compos. Anal. 2006, 19, 637–644. [Google Scholar] [CrossRef]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and Phenolic Compounds of Lactuca Sativa L. Grown in a Closed-Type Plant Production System with UV-A, -B, or -C Lamp. J. Sci. Food Agric. 2014, 94, 197–204. [Google Scholar] [CrossRef]
- Mariz-Ponte, N.; Mendes, R.J.; Sario, S.; Melo, P.; Santos, C. Moderate UV-A Supplementation Benefits Tomato Seed and Seedling Invigoration: A Contribution to the Use of UV in Seed Technology. Sci. Hortic. 2018, 235, 357–366. [Google Scholar] [CrossRef]
- Guo, J.; Wang, M.H. Ultraviolet A-Specific Induction of Anthocyanin Biosynthesis and PAL Expression in Tomato (Solanum Lycopersicum L.). Plant Growth Regul. 2010, 62, 1–8. [Google Scholar] [CrossRef]
- Dyshlyuk, L.; Babich, O.; Prosekov, A.; Ivanova, S.; Pavsky, V.; Chaplygina, T. The Effect of Postharvest Ultraviolet Irradiation on the Content of Antioxidant Compounds and the Activity of Antioxidant Enzymes in Tomato. Heliyon 2020, 6. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.J.; Son, J.E.; Oh, M.M. Growth and Phenolic Content of Sowthistle Grown in a Closed-Type Plant Production System with a UV-A or UV-B Lamp. Hortic. Environ. Biotechnol. 2013, 54, 492–500. [Google Scholar] [CrossRef]
- Zhang, Z.Z.; Che, X.N.; Pan, Q.H.; Li, X.X.; Duan, C.Q. Transcriptional Activation of Flavan-3-Ols Biosynthesis in Grape Berries by UV Irradiation Depending on Developmental Stage. Plant Sci. 2013, 208, 64–74. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, C.T.T.; Kim, J.; Yoo, K.S.; Lim, S.; Lee, E.J. Effect of Prestorage UV-A, -B, and -C Radiation on Fruit Quality and Anthocyanin of “Duke” Blueberries during Cold Storage. J. Agric. Food Chem. 2014, 62, 12144–12151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Dong, W.; Wang, K.; Zhang, B.; Allan, A.C.; Lin-Wang, K.; Chen, K.; Xu, C. Differential Sensitivity of Fruit Pigmentation to Ultraviolet Light between Two Peach Cultivars. Front. Plant Sci. 2017, 8. [Google Scholar] [CrossRef] [PubMed]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B Lighting to Enhance Phenolic Accumulation of Sweet Basil. Sci. Hortic. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Nascimento, L.B.D.S.; Brunetti, C.; Agati, G.; Iacono, C.L.; Detti, C.; Giordani, E.; Ferrini, F.; Gori, A. Short-Term Pre-Harvest Uv-b Supplement Enhances the Polyphenol Content and Antioxidant Capacity of Ocimum Basilicum Leaves during Storage. Plants 2020, 9, 797. [Google Scholar] [CrossRef]
- Ioannidis, D.; Bonner, L.; Johnson, C.B. UV-B Is Required for Normal Development of Oil Glands in Ocimum Basilicum L. (Sweet Basil). Ann. Bot. 2002, 90, 453–460. [Google Scholar] [CrossRef] [Green Version]
- Chang, X.; Alderson, P.G.; Wright, C.J. Enhanced UV-B Radiation Alters Basil (Ocimum Basilicum L.) Growth and Stimulates the Synthesis of Volatile Oils. J. Hortic. For. 2009, 1, 27–31. [Google Scholar]
- Faseela, P.; Puthur, J.T. The Imprints of the High Light and UV-B Stresses in Oryza Sativa L. ‘Kanchana’ Seedlings Are Differentially Modulated. J. Photochem. Photobiol. B Biol. 2018, 178, 551–559. [Google Scholar] [CrossRef]
- Moreira-Rodríguez, M.; Nair, V.; Benavides, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. UVA, UVB Light, and Methyl Jasmonate, Alone or Combined, Redirect the Biosynthesis of Glucosinolates, Phenolics, Carotenoids, and Chlorophylls in Broccoli Sprouts. Int. J. Mol. Sci. 2017, 18, 2330. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B Radiation on Phenolic Accumulation, Antioxidant Activity and Physiological Changes in Wheat (Triticum Aestivum L.)Seedlings. Food Biosci. 2019, 30. [Google Scholar] [CrossRef]
- Wang, H.; Gui, M.; Tian, X.; Xin, X.; Wang, T.; Li, J. Effects of UV-B on Vitamin C, Phenolics, Flavonoids and Their Related Enzyme Activities in Mung Bean Sprouts (Vigna Radiata). Int. J. Food Sci. Technol. 2017, 52, 827–833. [Google Scholar] [CrossRef]
- Dolzhenko, Y.; Bertea, C.M.; Occhipinti, A.; Bossi, S.; Maffei, M.E. UV-B Modulates the Interplay between Terpenoids and Flavonoids in Peppermint (Mentha × piperita L.). J. Photochem. Photobiol. B Biol. 2010, 100, 67–75. [Google Scholar] [CrossRef]
- Assumpção, C.F.; Assis, R.Q.; Hermes Poletto, V.S.; Castagna, A.; Ranieri, A.; Neugart, S.; Flôres, S.H.; de Oliveira Rios, A. Application of Supplemental UV-B Radiation in Pre-Harvest to Enhance Health-Promoting Compounds Accumulation in Green and Red Lettuce. J. Food Process. Preserv. 2019, 43. [Google Scholar] [CrossRef]
- Rodríguez-Calzada, T.; Qian, M.; Strid, Å.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B Radiation on Morphology, Phenolic Compound Production, Gene Expression, and Subsequent Drought Stress Responses in Chili Pepper (Capsicum Annuum L.). Plant Physiol. Biochem. 2019, 134, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Sakalauskaite, J.; Viskelis, P.; Dambrauskiene, E.; Sakalauskiene, S.; Samuoliene, G.; Brazaityte, A.; Duchovskis, P.; Urbonavičiene, D. The Effects of Different UV-B Radiation Intensities on Morphological and Biochemical Characteristics in Ocimum Basilicum L. J. Sci. Food Agric. 2013, 93, 1266–1271. [Google Scholar] [CrossRef]
- Mewis, I.; Schreiner, M.; Nguyen, C.N.; Krumbein, A.; Ulrichs, C.; Lohse, M.; Zrenner, R. UV-B Irradiation Changes Specifically the Secondary Metabolite Profile in Broccoli Sprouts: Induced Signaling Overlaps with Defense Response to Biotic Stressors. Plant Cell Physiol. 2012, 53, 1546–1560. [Google Scholar] [CrossRef] [Green Version]
- Scattino, C.; Castagna, A.; Neugart, S.; Chan, H.M.; Schreiner, M.; Crisosto, C.H.; Tonutti, P.; Ranieri, A. Post-Harvest UV-B Irradiation Induces Changes of Phenol Contents and Corresponding Biosynthetic Gene Expression in Peaches and Nectarines. Food Chem. 2014, 163, 51–60. [Google Scholar] [CrossRef] [PubMed]
- Sgherri, C.; Scattino, C.; Pinzino, C.; Tonutti, P.; Ranieri, A.M. Ultraviolet-B Radiation Applied to Detached Peach Fruit: A Study of Free Radical Generation by EPR Spin Trapping. Plant Physiol. Biochem. 2015, 96, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Lucini, L.; Castagna, A.; Rocchetti, G.; Hauser, M.T.; Ranieri, A. Comparative “Phenol-Omics” and Gene Expression Analyses in Peach (Prunus Persica) Skin in Response to Different Postharvest UV-B Treatments. Plant Physiol. Biochem. 2019, 135, 511–519. [Google Scholar] [CrossRef]
- Santin, M.; Lucini, L.; Castagna, A.; Chiodelli, G.; Hauser, M.-T.; Ranieri, A. Post-Harvest UV-B Radiation Modulates Metabolite Profile in Peach Fruit. Postharvest Biol. Technol. 2018, 139, 127–134. [Google Scholar] [CrossRef]
- Castagna, A.; Dall’Asta, C.; Chiavaro, E.; Galaverna, G.; Ranieri, A. Effect of Post-Harvest UV-B Irradiation on Polyphenol Profile and Antioxidant Activity in Flesh and Peel of Tomato Fruits. Food Bioprocess Technol. 2014, 7, 2241–2250. [Google Scholar] [CrossRef] [Green Version]
- Castagna, A.; Chiavaro, E.; Dall’Asta, C.; Rinaldi, M.; Galaverna, G.; Ranieri, A. Effect of Postharvest UV-B Irradiation on Nutraceutical Quality and Physical Properties of Tomato Fruits. Food Chem. 2013, 137, 151–158. [Google Scholar] [CrossRef]
- Liu, C.; Han, X.; Cai, L.; Lu, X.; Ying, T.; Jiang, Z. Postharvest UV-B Irradiation Maintains Sensory Qualities and Enhances Antioxidant Capacity in Tomato Fruit during Storage. Postharvest Biol. Technol. 2011, 59, 232–237. [Google Scholar] [CrossRef]
- Martínez-Zamora, L.; Castillejo, N.; Artés-Hernández, F. Postharvest UV-B and Photoperiod with Blue + Red LEDs as Strategies to Stimulate Carotenogenesis in Bell Peppers. Appl. Sci. 2021, 11, 3736. [Google Scholar] [CrossRef]
- Srilaong, V.; Aiamla-or, S.; Soontornwat, A.; Shigyo, M.; Yamauchi, N. UV-B Irradiation Retards Chlorophyll Degradation in Lime (Citrus Latifolia Tan.) Fruit. Postharvest Biol. Technol. 2011, 59, 110–112. [Google Scholar] [CrossRef]
- DeLong, J.M.; Steffen, K.L. Photosynthetic Function, Lipid Peroxidation, and-Tocopherol Content in Spinach Leaves during Exposure to UV-B Radiation. Can. J. Plant Sci. 1997, 77, 453–459. [Google Scholar] [CrossRef]
- Carletti, P.; Masi, A.; Wonisch, A.; Grill, D.; Tausz, M.; Ferretti, M. Changes in Antioxidant and Pigment Pool Dimensions in UV-B Irradiated Maize Seedlings. Environ. Exp. Bot. 2003, 50, 149–157. [Google Scholar] [CrossRef]
- Jain, K.; Kataria, S.; Guruprasad, K.N. Changes in Antioxidant Defenses of Cucumber Cotyledons in Response to UV-B and to the Free Radical Generating Compound AAPH. Plant Sci. 2003, 165, 551–557. [Google Scholar] [CrossRef]
- Hagen, S.F.; Borge, G.I.A.; Bengtsson, G.B.; Bilger, W.; Berge, A.; Haffner, K.; Solhaug, K.A. Phenolic Contents and Other Health and Sensory Related Properties of Apple Fruit (Malus Domestica Borkh., Cv. Aroma): Effect of Postharvest UV-B Irradiation. Postharvest Biol. Technol. 2007, 45, 1–10. [Google Scholar] [CrossRef]
- Santin, M.; Ranieri, A.; Hauser, M.-T.; Miras-Moreno, B.; Rocchetti, G.; Lucini, L.; Strid, Å.; Castagna, A. The Outer Influences the Inner: Postharvest UV-B Irradiation Modulates Peach Flesh Metabolome Although Shielded by the Skin. Food Chem. 2021, 338, 127782. [Google Scholar] [CrossRef] [PubMed]
- Santin, M.; Castagna, A.; Miras-Moreno, B.; Rocchetti, G.; Lucini, L.; Hauser, M.-T.; Ranieri, A. Beyond the Visible and Below the Peel: How UV-B Radiation Influences the Phenolic Profile in the Pulp of Peach Fruit. A Biochemical and Molecular Study. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.B.; Kirby, J.; Naxakis, G.; Pearson, S. Substantial UV-B-Mediated Induction of Essential Oils in Sweet Basil (Ocimum Basilicum L.). Phytochemistry 1999, 51, 507–510. [Google Scholar] [CrossRef]
- Duarte-Sierra, A.; Munzoor Hasan, S.M.; Angers, P.; Arul, J. UV-B Radiation Hormesis in Broccoli Florets: Glucosinolates and Hydroxy-Cinnamates Are Enhanced by UV-B in Florets during Storage. Postharvest Biol. Technol. 2020, 168. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santin, M.; Ranieri, A.; Castagna, A. Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants. Plants 2021, 10, 1485. https://doi.org/10.3390/plants10071485
Santin M, Ranieri A, Castagna A. Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants. Plants. 2021; 10(7):1485. https://doi.org/10.3390/plants10071485
Chicago/Turabian StyleSantin, Marco, Annamaria Ranieri, and Antonella Castagna. 2021. "Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants" Plants 10, no. 7: 1485. https://doi.org/10.3390/plants10071485
APA StyleSantin, M., Ranieri, A., & Castagna, A. (2021). Anything New under the Sun? An Update on Modulation of Bioactive Compounds by Different Wavelengths in Agricultural Plants. Plants, 10(7), 1485. https://doi.org/10.3390/plants10071485







