Selenium Biofortification of Soybean Seeds Influences Physiological Responses of Seedlings to Osmotic Stress
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Growth Conditions and Treatments
2.2. Se Content
2.3. Bioactive Compounds (Proline, Total Phenolics, Lipid Peroxidation, and Vitamin C)
2.3.1. Proline
2.3.2. Total Phenolics (TP)
2.3.3. Lipid Peroxidation
2.3.4. Total Vitamin C Content (Ascorbic Acid)
2.4. Analysis of Antioxidant Activity (FRAP)
2.5. Statistical Analysis
3. Results
3.1. Se Content in Soybean Grain and Analysis of Variance (Three-Way ANOVA)
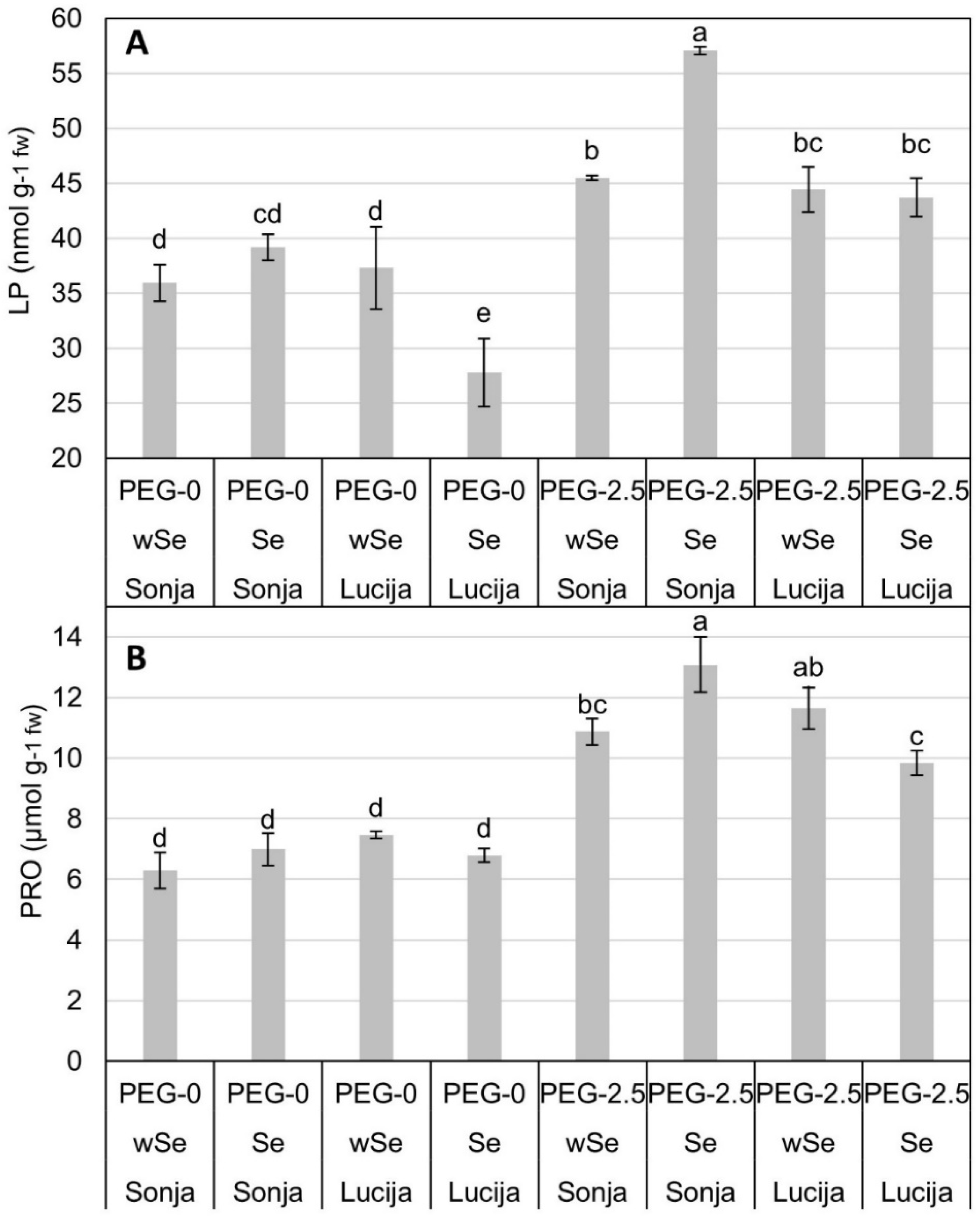
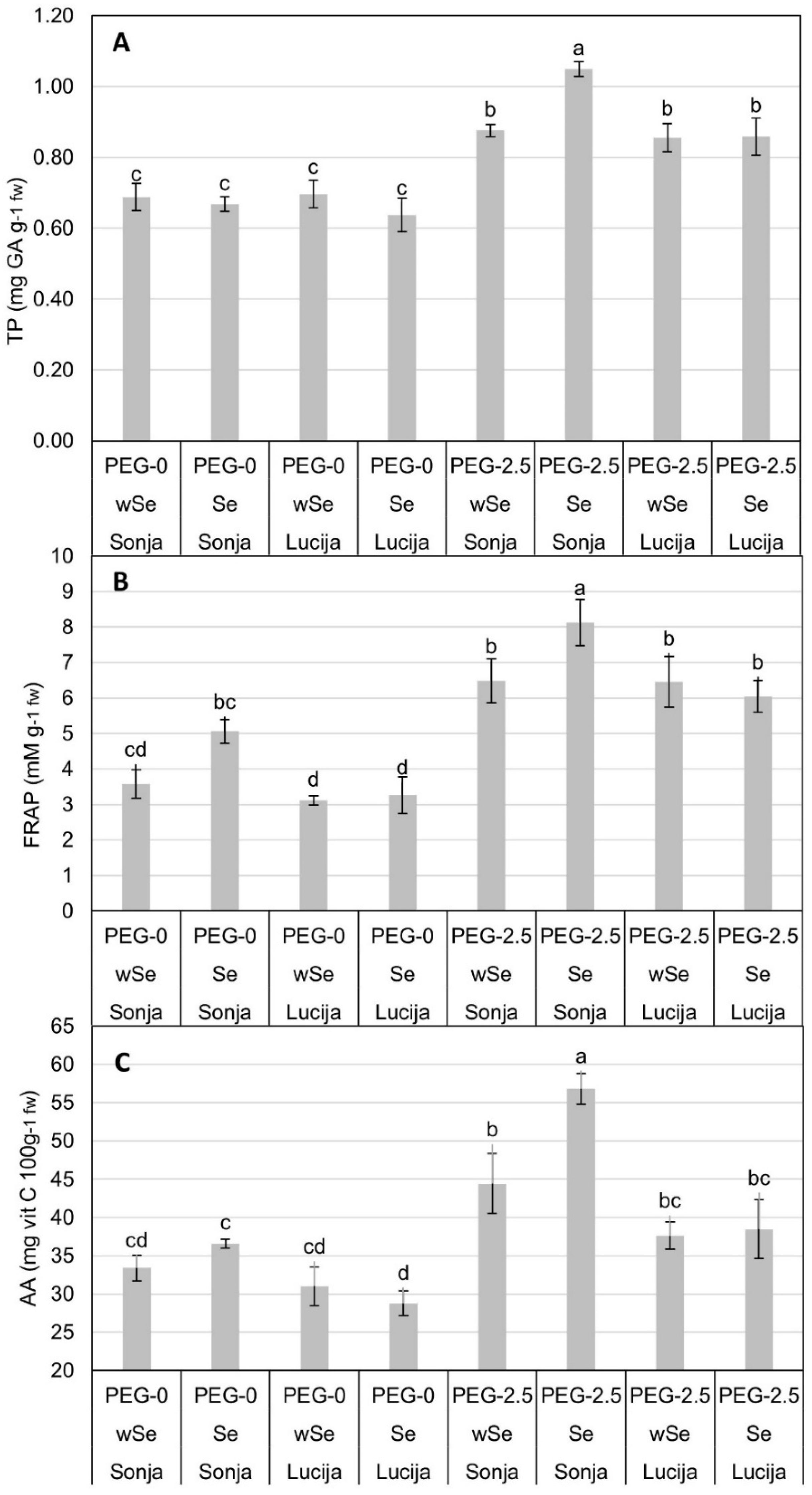
3.2. Concentration of LP, PRO, TP, FRAP, and AA in Soybean Plant Tissue
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hamayun, M.; Khan, S.A.; Shinwari, Z.K.; Khan, A.L.; Ahmad, N.; Lee, I.J. Effect of polyethylene glycol induced drought stress on physio-hormonal attributes of soybean. Pak. J. Bot. 2010, 42, 977–986. [Google Scholar]
- Makbul, S.; Güler, N.S.; Durmuş, N.; Güven, S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Bot. 2011, 35, 369–377. [Google Scholar] [CrossRef]
- Teklić, T.; Parađiković, N.; Špoljarević, M.; Zeljković, S.; Lončarić, Z.; Lisjak, M. Linking abiotic stress, plant metabolites, biostimulants and functional food. Ann. Appl. Biol. 2021, 178, 169–191. [Google Scholar] [CrossRef]
- Hasanuzzaman, M.; Nahar, K.; Fujita, M. Silicon and Selenium: Two Vital Trace Elements that Confer Abiotic Stress Tolerance to Plants. Two Vital Trace Elements that Confer Abiotic Stress Tolerance to Plants.; Elsevier Inc.: Amsterdam, The Netherlands, 2014; Volume 1, ISBN 9780128010884. [Google Scholar]
- Ramasamy, S.; Nandagopal, J.G.T.; Balasubramanian, M.; Girija, S. Effect of abscisic acid and selenium foliar sprays on drought mitigation in tomato (Solanum lycopersicum L.). Mater. Today Proc. 2020. [Google Scholar] [CrossRef]
- Delouche, J.C. Environmental effects on seed development and seed quality. HortScience 1980, 15, 13–18. [Google Scholar]
- Naderi, A.; Naseri, R.; Fathi, A.; Bahamin, S.; Maleki, R. Physiological performance of soybean cultivars under drought stress. Bull. Environ. Pharmacol. Life Sci. 2013, 2, 38–44. [Google Scholar]
- Tyug, T.S.; Prasad, K.N.; Ismail, A. Antioxidant capacity, phenolics and isoflavones in soybean by-products. Food Chem. 2010, 123, 583–589. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Yamaguchi, H.; Yuasa, T.; Iwaya-Inoue, M.; Arima, S.; Zheng, S.H. Hydrogen peroxide spraying alleviates drought stress in soybean plants. J. Plant Physiol. 2011, 168, 1562–1567. [Google Scholar] [CrossRef]
- Khan, M.N.; Komatsu, S. Proteomic analysis of soybean root including hypocotyl during recovery from drought stress. J. Proteomics 2016, 144, 39–50. [Google Scholar] [CrossRef]
- Ahmad, R.; Waraich, E.A.; Nawaz, F.; Ashraf, M.Y.; Khalid, M. Selenium (Se) improves drought tolerance in crop plants—A myth or fact? J. Sci. Food Agric. 2016, 96, 372–380. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.; Larher, F. Osmotic stress induced changes in lipid composition and peroxidation in leaf discs of Brassica napus L. J. Plant Physiol. 1998, 153, 754–762. [Google Scholar] [CrossRef]
- Sadžak, A.; Mravljak, J.; Maltar-Strmečki, N.; Arsov, Z.; Baranović, G.; Erceg, I.; Kriechbaum, M.; Strasser, V.; Přibyl, J.; Šegota, S. The structural integrity of the model lipid membrane during induced lipid peroxidation: The role of flavonols in the inhibition of lipid peroxidation. Antioxidants 2020, 9, 430. [Google Scholar] [CrossRef]
- Halliwell, B.; Chirico, S. Lipid peroxidation: Significance and its mechanism. Am. J. Clin. Nutr. 1993, 57, 715–725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Jiao, H.; Jiang, C.X.; Wang, S.H.; Wei, Z.J.; Luo, J.P.; Jones, R.L. Hydrogen sulfide protects soybean seedlings against drought-induced oxidative stress. Acta Physiol. Plant. 2010, 32, 849–857. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management; Springer: Berlin/Heidelberg, Germany, 2009; Volume 29, ISBN 9789048126668. [Google Scholar]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Hayat, S.; Hayat, Q.; Alyemeni, M.N.; Wani, A.S.; Pichtel, J.; Ahmad, A. Role of proline under changing environments: A review. Plant Signal. Behav. 2012, 7, 37–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamada, M.; Morishita, H.; Urano, K.; Shiozaki, N.; Yamaguchi-Shinozaki, K.; Shinozaki, K.; Yoshiba, Y. Effects of free proline accumulation in petunias under drought stress. J. Exp. Bot. 2005, 56, 1975–1981. [Google Scholar] [CrossRef] [Green Version]
- Hanif, S.; Saleem, M.F.; Sarwar, M.; Irshad, M.; Shakoor, A.; Wahid, M.A.; Khan, H.Z. Biochemically triggered heat and drought stress tolerance in rice by proline application. J. Plant Growth Regul. 2021, 40, 305–312. [Google Scholar] [CrossRef]
- Pérez-Torres, I.; Castrejón-Téllez, V.; Soto, M.E.; Rubio-Ruiz, M.E.; Manzano-Pech, L.; Guarner-Lans, V. Oxidative stress, plant natural antioxidants, and obesity. Int. J. Mol. Sci. 2021, 22, 1786. [Google Scholar] [CrossRef]
- Song, F.L.; Gan, R.Y.; Zhang, Y.; Xiao, Q.; Kuang, L.; Li, H.-B. Total phenolic contents and antioxidant capacities of selected chinese medicinal plants. Int. J. Mol. Sci. 2010, 11, 2362–2372. [Google Scholar] [CrossRef]
- Seminario, A.; Song, L.; Zulet, A.; Nguyen, H.T.; González, E.M.; Larrainzar, E. Drought stress causes a reduction in the biosynthesis of ascorbic acid in soybean plants. Front. Plant Sci. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biosci, I.J.; Razaji, A.; Farzanian, M.; Sayfzadeh, S. The effects of seed priming by ascorbic acid on some morphological and biochemical aspects of rapeseed (Brassica napus L.) under drought stress condition. Int. J. Biosci. 2014, 6655, 432–442. [Google Scholar] [CrossRef]
- Michels, A.J.; Frei, B. Myths, artifacts, and fatal flaws: Identifying limitations and opportunities in vitamin C research. Nutrients 2013, 5, 5161–5192. [Google Scholar] [CrossRef] [Green Version]
- Jóźwiak, W.; Politycka, B. Effect of selenium on alleviating oxidative stress caused by a water deficit in cucumber roots. Plants 2019, 8, 217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camelina, C.; Brassica, L.; Ahmad, Z.; Anjum, S.; Skalicky, M.; Waraich, E.A.; Muhammad, R.; Tariq, S.; Ayub, M.A.; Hossain, A.; et al. Selenium alleviates the adverse effect of drought in oilseed. Molecules 2021, 26, 1699. [Google Scholar] [CrossRef]
- Nawaz, F.; Ahmad, R.; Ashraf, M.Y.; Waraich, E.A.; Khan, S.Z. Effect of selenium foliar spray on physiological and biochemical processes and chemical constituents of wheat under drought stress. Ecotoxicol. Environ. Saf. 2015, 113, 191–200. [Google Scholar] [CrossRef]
- Yao, X.; Chu, J.; Wang, G. Effects of selenium on wheat seedlings under drought stress. Biol. Trace Elem. Res. 2009, 130, 283–290. [Google Scholar] [CrossRef]
- Iqbal, M.; Hussain, I.; Liaqat, H.; Ashraf, M.A.; Rasheed, R.; Rehman, A.U. Exogenously applied selenium reduces oxidative stress and induces heat tolerance in spring wheat. Plant Physiol. Biochem. 2015, 94, 95–103. [Google Scholar] [CrossRef]
- Kamran, M.; Parveen, A.; Ahmar, S.; Malik, Z.; Hussain, S.; Chattha, M.S.; Saleem, M.H.; Adil, M.; Heidari, P.; Chen, J.T. An overview of hazardous impacts of soil salinity in crops, tolerance mechanisms, and amelioration through selenium supplementation. Int. J. Mol. Sci. 2020, 21, 148. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.; Chu, J.; Liang, L.; Geng, W.; Li, J.; Hou, G. Selenium improves recovery of wheat seedlings at rewatering after drought stress. Russ. J. Plant Physiol. 2012, 59, 701–707. [Google Scholar] [CrossRef]
- Chu, J.; Yao, X.; Yue, Z.; Li, J.; Zhao, J. The effects of selenium on physiological traits, grain selenium content and yield of winter wheat at different development stages. Biol. Trace Elem. Res. 2013, 151, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Gibson, R.S. Content and bioavailability of trace elements in vegetarian diets. Am. J. Clin. Nutr. 1994, 59. [Google Scholar] [CrossRef] [PubMed]
- Hunt, J.R. Bioavailability of iron, zinc, and other trace minerals from vegetarian diets. Am. J. Clin. Nutr. 2003, 78. [Google Scholar] [CrossRef] [PubMed]
- Newman, R.; Waterland, N.; Moon, Y.; Tou, J.C. Selenium biofortification of agricultural crops and effects on plant nutrients and bioactive compounds important for human health and disease prevention—A review. Plant Foods Hum. Nutr. 2019, 74, 449–460. [Google Scholar] [CrossRef]
- Radawiec, A.; Szulc, W.; Rutkowska, B. Agrotechnical biofortification as a method to increase selenium content in spring wheat. Agronomy 2021, 11, 541. [Google Scholar] [CrossRef]
- Galić, L.; Vinković, T.; Ravnjak, B.; Lončarić, Z. Agronomic biofortification of significant cereal crops with selenium—A review. Agronomy 2021, 11, 1015. [Google Scholar] [CrossRef]
- Wang, C.Q. Water-stress mitigation by selenium in Trifolium repens L. J. Plant Nutr. Soil Sci. 2011, 174, 276–282. [Google Scholar] [CrossRef]
- Rady, M.M.; Belal, H.E.E.; Gadallah, F.M.; Semida, W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020, 266, 109290. [Google Scholar] [CrossRef]
- Gouveia, G.C.C.; Galindo, F.S.; Lanza, M.G.D.B.; Silva, A.C.D.R.; Mateus, M.P.D.B.; da Silva, M.S.; Tavanti, R.F.R.; Tavanti, T.R.; Lavres, J.; dos Reis, A.R. Selenium toxicity stress-induced phenotypical, biochemical and physiological responses in rice plants: Characterization of symptoms and plant metabolic adjustment. Ecotoxicol. Environ. Saf. 2020, 202. [Google Scholar] [CrossRef]
- Lyons, G.; Lewis, J.; Lorimer, M. High-selenium wheat: Agronomic biofortification strategies to improve human nutrition. Food Agric. Environ. 2004, 2, 171–178. [Google Scholar]
- Matusiewicz, H.; Sturgeon, R.E.; Berman, S.S. Trace element analysis of biological material following pressure digestion with nitric acid—Hydrogen peroxide and microwave heating. J. Anal. At. Spectrom. 1989, 4, 323–327. [Google Scholar] [CrossRef]
- NIST—National Institute of Standards & Technology. Certificate Certificate of Analysis Standard Reference Material® 2709 San Joaquin Soil Baseline; NIST: Gaithersburg, MD, USA, 2011.
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 207, 205–207. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Viticul. 1965, 16, 144–158. [Google Scholar]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplasts. Arch. Biochem. Biophys. 1968, 125, 189–198. [Google Scholar] [CrossRef]
- Roe, J.H.; Kuether, C.A. the determination of ascorbic acid in whole blood and urine through the 2,4-dinitrophenylhydrazine derivative of dehydroascorbic acid. J. Biol. Chem. 1943, 147, 399–407. [Google Scholar] [CrossRef]
- Benzie, I.; Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of ‘“antioxidant power”’: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Basal, O.; Szabó, A.; Veres, S. Physiology of soybean as affected by PEG-induced drought stress. Curr. Plant Biol. 2020, 22, 100135. [Google Scholar] [CrossRef]
- Jiang, Y.; Huang, B. Drought and heat stress injury to two cool-season turfgrasses in relation to antioxidant metabolism and lipid peroxidation. Crop. Sci. 2001, 41, 436–442. [Google Scholar] [CrossRef]
- Xue, T.; Hartikainen, H.; Piironen, V. Antioxidative and growth-promoting effect of selenium on senescing lettuce. Plant Soil 2001, 237, 55–61. [Google Scholar] [CrossRef]
- Hartikainen, H.; Xue, T.; Piironen, V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 2000, 225, 193–200. [Google Scholar] [CrossRef]
- Ardebili, N.O.; Saadatmand, S.; Niknam, V.; Khavari-Nejad, R.A. The alleviating effects of selenium and salicylic acid in salinity exposed soybean. Acta Physiol. Plant. 2014, 36, 3199–3205. [Google Scholar] [CrossRef]
- Anjum, S.A.; Farooq, M.; Xie, X.-Y.; Liu, X.-J.; Ijaz, M.F. Antioxidant defense system and proline accumulation enables hot pepper to perform better under drought. Sci. Hortic. 2012, 140, 66–73. [Google Scholar] [CrossRef]
- van Heerden, P.D.R.; de Villiers, O.T. Evaluation of proline accumulation as an indicator of drought tolerance in spring wheat cultivars. South Afr. J. Plant Soil 1996, 13, 17–21. [Google Scholar] [CrossRef]
- Zahedi, S.M.; Moharrami, F.; Sarikhani, S.; Padervand, M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef]
- Kähkönen, M.P.; Hopia, A.I.; Vuorela, H.J.; Rauha, J.P.; Pihlaja, K.; Kujala, T.S.; Heinonen, M. Antioxidant activity of plant extracts containing phenolic compounds. J. Agric. Food Chem. 1999, 47, 3954–3962. [Google Scholar] [CrossRef]
- Pannico, A.; El-Nakhel, C.; Graziani, G.; Kyriacou, M.C.; Giordano, M.; Soteriou, G.A.; Zarrelli, A.; Ritieni, A.; De Pascale, S.; Rouphael, Y.; et al. Selenium biofortification impacts the nutritive value, polyphenolic content, and bioactive constitution of variable microgreens genotypes. Antioxidants 2020, 9, 272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puccinelli, M.; Pezzarossa, B.; Rosellini, I.; Malorgio, F. Selenium enrichment enhances the quality and shelf life of basil leaves. Plants 2020, 9, 801. [Google Scholar] [CrossRef] [PubMed]
- Paciolla, C.; Fortunato, S.; Dipierro, N.; Paradiso, A.; De Leonardis, S.; Mastropasqua, L.; de Pinto, M.C. Vitamin C in plants: From functions to biofortification. Antioxidants 2019, 8. [Google Scholar] [CrossRef] [Green Version]
- Yuan, M.; Zhao, L.; Lin, A.; Wang, L.; Li, Q.; She, D.; Qu, S. Impacts of preseason drought on vegetation spring phenology across the Northeast China Transect. Sci. Total Environ. 2020, 738, 140297. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A. Selenium (Se) seed priming induced growth and biochemical changes in wheat under water deficit conditions. Biol. Trace Elem. Res. 2013, 151, 284–293. [Google Scholar] [CrossRef]
- Nawaz, F.; Ashraf, M.Y.; Ahmad, R.; Waraich, E.A.; Shabbir, R.N. Selenium (Se) regulates seedling growth in wheat under drought stress. Adv. Chem. 2014, 2014, 1–7. [Google Scholar] [CrossRef] [Green Version]
| Treatment | Cultivar | µg Se g−1 |
|---|---|---|
| wSe | Lucija | 64.02 ± 36.04 b |
| Sonja | 101.42 ± 65.87 b | |
| Se | Lucija | 2091.67 ± 97.29 a |
| Sonja | 2315.33 ± 331.8 a | |
| LSD 0.05 | 333.09 | |
| LP | PRO | TP | FRAP | AA | |
|---|---|---|---|---|---|
| Cultivar | ** | 0.345 | * | ** | *** |
| Se | 0.485 | 0.783 | 0.356 | 0.064 | 0.061 |
| PEG-2.5 | *** | *** | *** | *** | *** |
| Cultivar * Se | ** | ** | 0.061 | * | * |
| Cultivar * PEG-2.5 | 0.492 | * | 0.086 | 0.923 | * |
| Se * PEG-2.5 | * | 0.813 | * | 0.788 | 0.103 |
| Cultivar * Se * PEG-2.5 | 0.937 | 0.103 | 0.225 | 0.625 | 0.399 |
| PEG | Se | LP | PRO | TP | FRAP | AA |
|---|---|---|---|---|---|---|
| PEG-0 | wSe | 36.64 ± 1.86 b | 6.88 ± 0.38 b | 0.69 ± 0.02 b | 3.35 ± 0.22 b | 32.19 ± 1.45 c |
| Se | 33.47 ± 2.95 b | 6.89 ± 0.26 b | 0.65 ± 0.02 b | 4.16 ± 0.49 b | 32.69 ± 1.91 bc | |
| PEG-2.5 | wSe | 44.97 ± 0.94 a | 11.26 ± 0.4 a | 0.87 ± 0.02 a | 6.47 ± 0.42 a | 41.04 ± 2.46 ab |
| Se | 50.39 ± 3.09 a | 11.46 ± 0.85 a | 0.95 ± 0.05 a | 7.08 ± 0.59 a | 47.62 ± 4.54 a | |
| LSD 0.05 | - | 7.01 | 1.54 | 0.094 | 1.32 | 8.39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galić, L.; Špoljarević, M.; Jakovac, E.; Ravnjak, B.; Teklić, T.; Lisjak, M.; Perić, K.; Nemet, F.; Lončarić, Z. Selenium Biofortification of Soybean Seeds Influences Physiological Responses of Seedlings to Osmotic Stress. Plants 2021, 10, 1498. https://doi.org/10.3390/plants10081498
Galić L, Špoljarević M, Jakovac E, Ravnjak B, Teklić T, Lisjak M, Perić K, Nemet F, Lončarić Z. Selenium Biofortification of Soybean Seeds Influences Physiological Responses of Seedlings to Osmotic Stress. Plants. 2021; 10(8):1498. https://doi.org/10.3390/plants10081498
Chicago/Turabian StyleGalić, Lucija, Marija Špoljarević, Elizabeta Jakovac, Boris Ravnjak, Tihana Teklić, Miroslav Lisjak, Katarina Perić, Franjo Nemet, and Zdenko Lončarić. 2021. "Selenium Biofortification of Soybean Seeds Influences Physiological Responses of Seedlings to Osmotic Stress" Plants 10, no. 8: 1498. https://doi.org/10.3390/plants10081498







