Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants In Vitro Cultures
Abstract
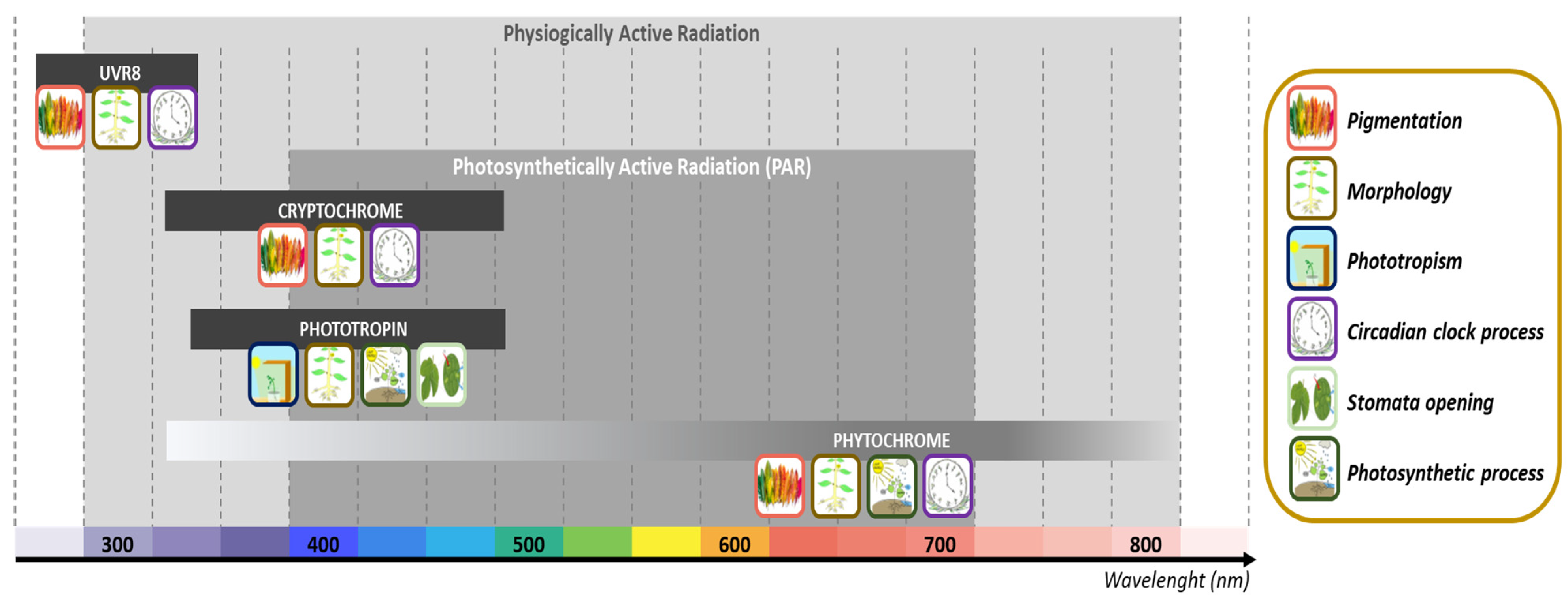
:1. Introduction
2. Light as an Elicitor
2.1. UV Lights
2.1.1. UV-A and UV-B
2.1.2. UV-C
2.2. Fluorescent Lights
2.3. LED Lights
2.3.1. Blue LED
2.3.2. Red LED
2.3.3. Blue and Red LED in Combination
3. Mechanistic Considerations and Future Prospects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wang, J.W.; Wu, J.Y. Effective elicitors and process strategies for enhancement of secondary metabolite production in hairy root cultures. In Biotechnology of Hairy Root Systems; Springer: Berlin, Germany, 2013; pp. 55–89. [Google Scholar]
- Murthy, H.N.; Lee, E.-J.; Paek, K.-Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ. Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Yue, W.; Ming, Q.-l.; Lin, B.; Rahman, K.; Zheng, C.-J.; Han, T.; Qin, L.-P. Medicinal plant cell suspension cultures: Pharmaceutical applications and high-yielding strategies for the desired secondary metabolites. Crit. Rev. Biotechnol. 2016, 36, 215–232. [Google Scholar] [CrossRef]
- Gonçalves, S.; Romano, A. Production of plant secondary metabolites by using biotechnological tools. In Secondary Metabolites, Sources and Applications; IntechOpen: London, UK, 2018; pp. 81–99. [Google Scholar]
- Hussain, M.S.; Fareed, S.; Saba Ansari, M.; Rahman, A.; Ahmad, I.Z.; Saeed, M. Current approaches toward production of secondary plant metabolites. J. Pharm. Bioall. Sci. 2012, 4, 10. [Google Scholar] [CrossRef] [PubMed]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, X.-H.; Chakrabarty, D.; Lee, E.-J.; Paek, K.-Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresour. Technol. 2010, 101, 4708–4716. [Google Scholar] [CrossRef]
- Yang, L.; Stöckigt, J. Trends for diverse production strategies of plant medicinal alkaloids. Nat. Prod. Rep. 2010, 27, 1469–1479. [Google Scholar] [CrossRef]
- Tariq, U.; Ali, M.; Abbasi, B.H. Morphogenic and biochemical variations under different spectral lights in callus cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 130, 264–271. [Google Scholar] [CrossRef]
- Adil, M.; Ren, X.; Jeong, B.R. Light elicited growth, antioxidant enzymes activities and production of medicinal compounds in callus culture of Cnidium officinale Makino. J. Photochem. Photobiol. B Biol. 2019, 196, 111509. [Google Scholar] [CrossRef]
- Abbasi, B.H.; Tian, C.-L.; Murch, S.J.; Saxena, P.K.; Liu, C.-Z. Light-enhanced caffeic acid derivatives biosynthesis in hairy root cultures of Echinacea purpurea. Plant Cell Rep. 2007, 26, 1367–1372. [Google Scholar] [CrossRef] [PubMed]
- Shohael, A.; Ali, M.; Yu, K.; Hahn, E.; Islam, R.; Paek, K. Effect of light on oxidative stress, secondary metabolites and induction of antioxidant enzymes in Eleutherococcus senticosus somatic embryos in bioreactor. Process Biochem. 2006, 41, 1179–1185. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Y.; Wang, Y. Artemisinin: Current state and perspectives for biotechnological production of an antimalarial drug. Appl. Microbiol. Biotechnol. 2006, 72, 11–20. [Google Scholar] [CrossRef]
- Fazal, H.; Abbasi, B.H.; Ahmad, N.; Ali, S.S.; Akbar, F.; Kanwal, F. Correlation of different spectral lights with biomass accumulation and production of antioxidant secondary metabolites in callus cultures of medicinally important Prunella vulgaris L. J. Photochem. Photobiol. B Biol. 2016, 159, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Moran, J.F.; Becana, M.; Iturbe-Ormaetxe, I.; Frechilla, S.; Klucas, R.V.; Aparicio-Tejo, P. Drought induces oxidative stress in pea plants. Planta 1994, 194, 346–352. [Google Scholar] [CrossRef] [Green Version]
- Alexieva, V.; Sergiev, I.; Mapelli, S.; Karanov, E. The effect of drought and ultraviolet radiation on growth and stress markers in pea and wheat. Plant Cell Environ. 2001, 24, 1337–1344. [Google Scholar] [CrossRef]
- Yu, T.-W.; Anderson, D. Reactive oxygen species-induced DNA damage and its modification: A chemical investigation. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 1997, 379, 201–210. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Ashry, N.A.; Mohamed, H.I. Impact of secondary metabolites and related enzymes in flax resistance and or susceptibility to powdery mildew. World J. Agric. Sci 2011, 7, 78–85. [Google Scholar]
- Samuolienė, G.; Brazaitytė, A.; Urbonavičiūtė, A.; Šabajevienė, G.; Duchovskis, P. The effect of red and blue light component on the growth and development of frigo strawberries. Zemdirbyste Agric. 2010, 97, 99–104. [Google Scholar]
- Dou, H.; Niu, G.; Gu, M. Pre-harvest UV-B radiation and photosynthetic photon flux density interactively affect plant photosynthesis, growth, and secondary metabolites accumulation in basil (Ocimum basilicum) plants. Agronomy 2019, 9, 434. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, N.; Rab, A.; Ahmad, N. Light-induced biochemical variations in secondary metabolite production and antioxidant activity in callus cultures of Stevia rebaudiana (Bert). J. Photochem. Photobiol. B Biol. 2016, 154, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-Y.; Park, S.-A.; Park, B.-J.; Lee, Y.; Oh, M.-M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hortic. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Xuan, T.D.; Khanh, T.D.; Khang, D.T.; Quan, N.T.; Elzaawely, A.A. Changes in chemical composition, total phenolics and antioxidant activity of Alpinia (Alpinia zerumbet) leaves exposed to UV. Int. Lett. Nat. Sci. 2016, 55, 25–34. [Google Scholar] [CrossRef] [Green Version]
- Stapleton, A.E. Ultraviolet radiation and plants: Burning questions. Plant Cell 1992, 4, 1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Changing scenario in plant UV-B research: UV-B from a generic stressor to a specific regulator. J. Photochem. Photobiol. B Biol. 2015, 153, 334–343. [Google Scholar] [CrossRef]
- Freitas, A.; Moldão-Martins, M.; Costa, H.S.; Albuquerque, T.G.; Valente, A.; Sanches-Silva, A. Effect of UV-C radiation on bioactive compounds of pineapple (Ananas comosus L. Merr.) by-products. J. Sci. Food Agric. 2015, 95, 44–52. [Google Scholar] [CrossRef]
- Yin, X.; Singer, S.D.; Qiao, H.; Liu, Y.; Jiao, C.; Wang, H.; Li, Z.; Fei, Z.; Wang, Y.; Fan, C. Insights into the mechanisms underlying ultraviolet-C induced resveratrol metabolism in grapevine (V. amurensis Rupr.) cv.“Tonghua-3”. Front. Plant Sci. 2016, 7, 503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abbasi, B.H.; Khan, T.; Khurshid, R.; Nadeem, M.; Drouet, S.; Hano, C. UV-C mediated accumulation of pharmacologically significant phytochemicals under light regimes in in vitro culture of Fagonia indica (L.). Sci. Rep. 2021, 11, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Ellenberger, J.; Siefen, N.; Krefting, P.; Schulze Lutum, J.-B.; Pfarr, D.; Remmel, M.; Schröder, L.; Röhlen-Schmittgen, S. Effect of UV Radiation and Salt Stress on the Accumulation of Economically Relevant Secondary Metabolites in Bell Pepper Plants. Agronomy 2020, 10, 142. [Google Scholar] [CrossRef] [Green Version]
- Nocchi, N.; Duarte, H.M.; Pereira, R.C.; Konno, T.U.P.; Soares, A.R. Effects of UV-B radiation on secondary metabolite production, antioxidant activity, photosynthesis and herbivory interactions in Nymphoides humboldtiana (Menyanthaceae). J. Photochem. Photobiol. B Biol. 2020, 212, 112021. [Google Scholar] [CrossRef] [PubMed]
- Klein, F.R.S.; Reis, A.; Kleinowski, A.M.; Telles, R.T.; Amarante, L.d.; Peters, J.A.; Braga, E.J.B. UV-B radiation as an elicitor of secondary metabolite production in plants of the genus Alternanthera. Acta Bot. Bras. 2018, 32, 615–623. [Google Scholar] [CrossRef]
- Rodríguez-Calzada, T.; Qian, M.; Strid, Å.; Neugart, S.; Schreiner, M.; Torres-Pacheco, I.; Guevara-González, R.G. Effect of UV-B radiation on morphology, phenolic compound production, gene expression, and subsequent drought stress responses in chili pepper (Capsicum annuum L.). Plant Physiol. Biochem. 2019, 134, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wang, L.; Pang, S.; Jia, Z.; Wang, L.; Li, W.; Jin, B. UV-B promotes flavonoid synthesis in Ginkgo biloba leaves. Ind. Crops Prod. 2020, 151, 112483. [Google Scholar] [CrossRef]
- Ramani, S.; Jayabaskaran, C. Enhanced catharanthine and vindoline production in suspension cultures of Catharanthus roseus by ultraviolet-B light. J. Mol. Signal. 2008, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Mosadegh, H.; Trivellini, A.; Ferrante, A.; Lucchesini, M.; Vernieri, P.; Mensuali, A. Applications of UV-B lighting to enhance phenolic accumulation of sweet basil. Sci. Hortic. 2018, 229, 107–116. [Google Scholar] [CrossRef]
- Chen, Z.; Ma, Y.; Weng, Y.; Yang, R.; Gu, Z.; Wang, P. Effects of UV-B radiation on phenolic accumulation, antioxidant activity and physiological changes in wheat (Triticum aestivum L.) seedlings. Food Biosci. 2019, 30, 100409. [Google Scholar] [CrossRef]
- Keskin, N.; Kunter, B. Production of trans-resveratrol in callus tissue of Öküzgözü (Vitis vinifera L.) in response to ultraviolet-C irradiation. J. Anim Plant Sci. 2010, 20, 197–200. [Google Scholar]
- Cetin, E.S. Induction of secondary metabolite production by UV-C radiation in Vitis vinifera L. Öküzgözü callus cultures. Biol. Res. 2014, 47, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.A.; Tungmunnithum, D.; Garros, L.; Drouet, S.; Hano, C.; Abbasi, B.H. Effect of ultraviolet-C radiation and melatonin stress on biosynthesis of antioxidant and antidiabetic metabolites produced in in vitro callus cultures of Lepidium sativum L. Int. J. Mol. Sci. 2019, 20, 1787. [Google Scholar] [CrossRef] [Green Version]
- Anjum, S.; Abbasi, B.H.; Doussot, J.; Favre-Réguillon, A.; Hano, C. Effects of photoperiod regimes and ultraviolet-C radiations on biosynthesis of industrially important lignans and neolignans in cell cultures of Linum usitatissimum L. (Flax). J. Photochem. Photobiol. B Biol. 2017, 167, 216–227. [Google Scholar] [CrossRef]
- Nazir, M.; Asad Ullah, M.; Mumtaz, S.; Siddiquah, A.; Shah, M.; Drouet, S.; Hano, C.; Abbasi, B.H. Interactive effect of melatonin and UV-C on phenylpropanoid metabolite production and antioxidant potential in callus cultures of purple basil (Ocimum basilicum L. var. purpurascens). Molecules 2020, 25, 1072. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Aal, M.S.; Rabie, K.A.; Manaf, H.H. The effect of UV-C on secondary metabolites production of Echinacea purpurea culture in vitro. J. Biol. Chem. Environ. Sci. 2016, 11, 465–483. [Google Scholar]
- Zuiter, A. Proanthocyanidin: Chemistry and Biology: From Phenolic Compounds to Proanthocyanidins; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Sun, X.; Sun, G.B.; Wang, M.; Xiao, J.; Sun, X.B. Protective effects of cynaroside against H2O2-induced apoptosis in H9c2 cardiomyoblasts. J. Cell. Biochem. 2011, 112, 2019–2029. [Google Scholar] [CrossRef]
- Schreiner, M.; Krumbein, A.; Mewis, I.; Ulrichs, C.; Huyskens-Keil, S. Short-term and moderate UV-B radiation effects on secondary plant metabolism in different organs of nasturtium (Tropaeolum majus L.). Innov. Food Sci. Emerg. Technol. 2009, 10, 93–96. [Google Scholar] [CrossRef]
- Zhang, W.J.; Björn, L.O. The effect of ultraviolet radiation on the accumulation of medicinal compounds in plants. Fitoterapia 2009, 80, 207–218. [Google Scholar] [CrossRef]
- Schreiner, M.; Mewis, I.; Huyskens-Keil, S.; Jansen, M.; Zrenner, R.; Winkler, J.; O’brien, N.; Krumbein, A. UV-B-induced secondary plant metabolites-potential benefits for plant and human health. Crit. Rev. Plant Sci. 2012, 31, 229–240. [Google Scholar] [CrossRef]
- Flint, S.D.; Ryel, R.J.; Caldwell, M.M. Ecosystem UV-B experiments in terrestrial communities: A review of recent findings and methodologies. Agric. For. Meteorol. 2003, 120, 177–189. [Google Scholar] [CrossRef]
- Caldwell, M.M.; Bornman, J.; Ballaré, C.; Flint, S.D.; Kulandaivelu, G. Terrestrial ecosystems, increased solar ultraviolet radiation, and interactions with other climate change factors. Photochem. Photobiol. Sci. 2007, 6, 252–266. [Google Scholar] [CrossRef]
- Burak, M.; Imen, Y. Flavonoids and their antioxidant properties. Turkiye Klin. Tip. Bil. Derg. 1999, 19, 296–304. [Google Scholar]
- Castañeda-Ovando, A.; de Lourdes Pacheco-Hernández, M.; Páez-Hernández, M.E.; Rodríguez, J.A.; Galán-Vidal, C.A. Chemical studies of anthocyanins: A review. Food Chem. 2009, 113, 859–871. [Google Scholar] [CrossRef]
- Lee, Y.K.; Yuk, D.Y.; Lee, J.W.; Lee, S.Y.; Ha, T.Y.; Oh, K.W.; Yun, Y.P.; Hong, J.T. (−)-Epigallocatechin-3-gallate prevents lipopolysaccharide-induced elevation of beta-amyloid generation and memory deficiency. Brain Res. 2009, 1250, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Nisar, A.; Mamat, A.S.; Hatim, M.I.; Aslam, M.S.; Ahmad, M.S. An updated review on Catharanthus roseus: Phytochemical and pharmacological analysis. Indian Res. J. Pharm. Sci. 2016, 3, 631–653. [Google Scholar]
- Bora, K.S.; Arora, S.; Shri, R. Role of Ocimum basilicum L. in prevention of ischemia and reperfusion-induced cerebral damage, and motor dysfunctions in mice brain. J. Ethnopharmacol. 2011, 137, 1360–1365. [Google Scholar] [CrossRef]
- Loughrin, J.H.; Kasperbauer, M.J. Light reflected from colored mulches affects aroma and phenol content of sweet basil (Ocimum basilicum L.) leaves. J. Agric. Food Chem. 2001, 49, 1331–1335. [Google Scholar] [CrossRef]
- Petkovšek, M.M.; Štampar, F.; Veberič, R. Accumulation of phenolic compounds in apple in response to infection by the scab pathogen, Venturia inaequalis. Physiol. Mol. Plant Pathol. 2009, 74, 60–67. [Google Scholar] [CrossRef]
- Bridgen, M. Using ultraviolet-C (UV-C) irradiation on greenhouse ornamental plants for growth regulation. In Proceedings of the VIII International Symposium on Light in Horticulture 1134, East Lansing, MI, USA, 22–26 May 2016; pp. 49–56. [Google Scholar]
- Fiod Riccio, B.V.; Fonseca-Santos, B.; Colerato Ferrari, P.; Chorilli, M. Characteristics, biological properties and analytical methods of trans-resveratrol: A review. Crit. Rev. Anal. Chem. 2020, 50, 339–358. [Google Scholar] [CrossRef] [PubMed]
- Hardman, W.E.; Avula, C.R.; Fernandes, G.; Cameron, I.L. Three percent dietary fish oil concentrate increased efficacy of doxorubicin against MDA-MB 231 breast cancer xenografts. Clin. Cancer Res. 2001, 7, 2041–2049. [Google Scholar] [PubMed]
- Kassie, F.; Pool-Zobel, B.; Parzefall, W.; Knasmüller, S. Genotoxic effects of benzyl isothiocyanate, a natural chemopreventive agent. Mutagenesis 1999, 14, 595–604. [Google Scholar] [CrossRef] [Green Version]
- Kassie, F.; Laky, B.; Gminski, R.; Mersch-Sundermann, V.; Scharf, G.; Lhoste, E.; Kansmüller, S. Effects of garden and water cress juices and their constituents, benzyl and phenethyl isothiocyanates, towards benzo (a) pyrene-induced DNA damage: A model study with the single cell gel electrophoresis/Hep G2 assay. Chem. Biol. Interact. 2003, 142, 285–296. [Google Scholar] [CrossRef]
- Schmidt, T.J.; Klaes, M.; Sendker, J. Lignans in seeds of Linum species. Phytochemistry 2012, 82, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, J.; Qiu, C.; Ye, Y.; Guo, X.; Chen, G.; Li, T.; Wang, Y.; Fu, X.; Liu, R.H. Comparison of phytochemical profiles and health benefits in fiber and oil flaxseeds (Linum usitatissimum L.). Food Chem. 2017, 214, 227–233. [Google Scholar] [CrossRef]
- Omar, K.A.; Shan, L.; Zou, X.; Song, Z.; Wang, X. Effects of two emulsifiers on yield and storage of flaxseed oil powder by response surface methodology. Pak. J. Nutr. 2009, 8, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Waszkowiak, K.; Gliszczyńska-Świgło, A. Binary ethanol–water solvents affect phenolic profile and antioxidant capacity of flaxseed extracts. Eur. Food Res. Technol. 2016, 242, 777–786. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Wang, S.Y. Antioxidant activity and phenolic compounds in selected herbs. J. Agric. Food Chem. 2001, 49, 5165–5170. [Google Scholar] [CrossRef] [PubMed]
- Hernández, R.; Eguchi, T.; Deveci, M.; Kubota, C. Tomato seedling physiological responses under different percentages of blue and red photon flux ratios using LEDs and cool white fluorescent lamps. Sci. Hortic. 2016, 213, 270–280. [Google Scholar] [CrossRef] [Green Version]
- Ali, M.; Abbasi, B.H. Light-induced fluctuations in biomass accumulation, secondary metabolites production and antioxidant activity in cell suspension cultures of Artemisia absinthium L. J. Photochem. Photobiol. B Biol. 2014, 140, 223–227. [Google Scholar] [CrossRef]
- Ohashi-Kaneko, K.; Takase, M.; Kon, N.; Fujiwara, K.; Kurata, K. Effect of light quality on growth and vegetable quality in leaf lettuce, spinach and komatsuna. Environ. Control. Biol. 2007, 45, 189–198. [Google Scholar] [CrossRef] [Green Version]
- Jack, A.; Vrenken, L. Fluorescent lamps and low pressure sodium lamps. IEE Proc. A 1980, 127, 149–157. [Google Scholar] [CrossRef]
- Kawka, B.; Kwiecień, I.; Ekiert, H. Influence of culture medium composition and light conditions on the accumulation of bioactive compounds in shoot cultures of Scutellaria lateriflora L. (American Skullcap) grown in vitro. Appl. Biochem. Biotechnol. 2017, 183, 1414–1425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sobhani Najafabadi, A.; Khanahmadi, M.; Ebrahimi, M.; Moradi, K.; Behroozi, P.; Noormohammadi, N. Effect of different quality of light on growth and production of secondary metabolites in adventitious root cultivation of Hypericum perforatum. Plant Signal. Behav. 2019, 14, 1640561. [Google Scholar] [CrossRef]
- Pedroso, R.C.N.; Branquinho, N.A.A.; Hara, A.C.; Costa, A.C.; Silva, F.G.; Pimenta, L.P.; Silva, M.L.A.; Cunha, W.R.; Pauletti, P.M.; Januario, A.H. Impact of light quality on flavonoid production and growth of Hyptis marrubioides seedlings cultivated in vitro. Rev. Bras. Farmacogn. 2017, 27, 466–470. [Google Scholar] [CrossRef]
- Yu, K.-W.; Murthy, H.N.; Hahn, E.-J.; Paek, K.-Y. Ginsenoside production by hairy root cultures of Panax ginseng: Influence of temperature and light quality. Biochem. Eng. J. 2005, 23, 53–56. [Google Scholar] [CrossRef]
- Murthy, H.N.; Kim, Y.-S.; Park, S.-Y.; Paek, K.-Y. Hypericins: Biotechnological production from cell and organ cultures. Appl. Microbiol. Biotechnol. 2014, 98, 9187–9198. [Google Scholar] [CrossRef] [PubMed]
- McNeil, M.; Facey, P.; Porter, R. Essential oils from the Hyptis genus—A review (1909–2009). Nat. Prod. Commun. 2011, 6, 1934578X1100601149. [Google Scholar] [CrossRef] [Green Version]
- Coon, J.T.; Ernst, E. Panax ginseng. Drug Saf. 2002, 25, 323–344. [Google Scholar] [CrossRef] [PubMed]
- Palazón, J.; Cusidó, R.M.; Bonfill, M.; Mallol, A.; Moyano, E.; Morales, C.; Piñol, M.T. Elicitation of different Panax ginseng transformed root phenotypes for an improved ginsenoside production. Plant Physiol. Biochem. 2003, 41, 1019–1025. [Google Scholar] [CrossRef]
- Sharopov, F.S.; Sulaimonova, V.A.; Setzer, W.N. Composition of the Essential oil of Artemisia absinthium from Tajikistan. Rec. Nat. Prod. 2012, 6, 127–134. [Google Scholar]
- Baker, P. The Book of Absinthe: A Cultural History; Grove Press: Greenwich Village, NY, USA, 2001. [Google Scholar]
- Hayat, M.Q.; Khan, M.A.; Ashraf, M.; Jabeen, S. Ethnobotany of the Genus Artemisia L. (Asteraceae) in Pakistan; University of Hawaii at Manoa: Honolulu, HI, USA, 2009. [Google Scholar]
- Anderson, F.J. An Illustrated History of the Herbals; iUniverse: Bloomington, IN, USA, 1997. [Google Scholar]
- Smith, A.; Secoy, D. Plants used for agricultural pest control in western Europe before 1850. Chem. Ind. 1981, 70, 12–17. [Google Scholar]
- Ferreira, J.F.; Peaden, P.; Keiser, J. In vitro trematocidal effects of crude alcoholic extracts of Artemisia annua, A. absinthium, Asimina triloba, and Fumaria officinalis. Parasitol. Res. 2011, 109, 1585–1592. [Google Scholar] [CrossRef]
- Mohamed, A.E.-H.H.; El-Sayed, M.; Hegazy, M.E.; Helaly, S.E.; Esmail, A.M.; Mohamed, N.S. Chemical constituents and biological activities of Artemisia herba-alba. Rec. Nat. Prod. 2010, 4, 1–25. [Google Scholar]
- Gupta, S.D.; Agarwal, A. Light Emitting Diodes for Agriculture; Springer: Berlin, Germany, 2017. [Google Scholar]
- Terashima, I.; Fujita, T.; Inoue, T.; Chow, W.S.; Oguchi, R. Green light drives leaf photosynthesis more efficiently than red light in strong white light: Revisiting the enigmatic question of why leaves are green. Plant Cell Physiol. 2009, 50, 684–697. [Google Scholar] [CrossRef] [Green Version]
- Zhang, T.; Shi, Y.; Piao, F.; Sun, Z. Effects of different LED sources on the growth and nitrogen metabolism of lettuce. Plant Cell Tissue Organ. Cult. 2018, 134, 231–240. [Google Scholar] [CrossRef]
- Gupta, S.D.; Kumar, A.; Agarwal, A. Impact of light-emitting diodes (LEDs) on the growth and morphogenesis of encapsulated shoot buds of Curculigo orchioides Gaertn., an endangered medicinal herb. Acta Physiol. Plant 2019, 41, 50. [Google Scholar] [CrossRef]
- Wang, W.; Su, M.; Li, H.; Zeng, B.; Chang, Q.; Lai, Z. Effects of supplemental lighting with different light qualities on growth and secondary metabolite content of Anoectochilus roxburghii. PeerJ 2018, 6, e5274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taulavuori, K.; Pyysalo, A.; Taulavuori, E.; Julkunen-, R. Responses of phenolic acid and flavonoid synthesis to blue and blue-violet light depends on plant species. Environ. Exp. Bot. 2018, 150, 183–187. [Google Scholar] [CrossRef] [Green Version]
- Cioć, M.; Szewczyk, A.; Żupnik, M.; Kalisz, A.; Pawłowska, B. LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tissue Organ. Cult. 2018, 132, 433–447. [Google Scholar] [CrossRef] [Green Version]
- Younas, M.; Drouet, S.; Nadeem, M.; Giglioli-Guivarc’h, N.; Hano, C.; Abbasi, B.H. Differential accumulation of silymarin induced by exposure of Silybum marianum L. callus cultures to several spectres of monochromatic lights. J. Photochem. Photobiol. B Biol. 2018, 184, 61–70. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Halimah, N.; Ko, C.H.; Jeong, B.R. Blue LED light enhances growth, phytochemical contents, and antioxidant enzyme activities of Rehmannia glutinosa cultured in vitro. Hortic. Environ. Biotechnol. 2015, 56, 105–113. [Google Scholar] [CrossRef]
- Zhang, R.-X.; Li, M.-X.; Jia, Z.-P. Rehmannia glutinosa: Review of botany, chemistry and pharmacology. J. Ethnopharmacol. 2008, 117, 199–214. [Google Scholar] [CrossRef]
- Nadeem, M.; Abbasi, B.H.; Younas, M.; Ahmad, W.; Zahir, A.; Hano, C. LED-enhanced biosynthesis of biologically active ingredients in callus cultures of Ocimum basilicum. J. Photochem. Photobiol. B Biol. 2019, 190, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Cuong, D.M.; Ha, T.W.; Park, C.H.; Kim, N.S.; Yeo, H.J.; Chun, S.W.; Kim, C.; Park, S.U. Effects of LED lights on expression of genes involved in phenylpropanoid biosynthesis and accumulation of phenylpropanoids in wheat sprout. Agronomy 2019, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Seo, J.-M.; Arasu, M.V.; Kim, Y.-B.; Park, S.U.; Kim, S.-J. Phenylalanine and LED lights enhance phenolic compound production in Tartary buckwheat sprouts. Food Chem. 2015, 177, 204–213. [Google Scholar] [CrossRef]
- Lobiuc, A.; Vasilache, V.; Oroian, M.; Stoleru, T.; Burducea, M.; Pintilie, O.; Zamfirache, M.-M. Blue and red LED illumination improves growth and bioactive compounds contents in acyanic and cyanic Ocimum basilicum L. microgreens. Molecules 2017, 22, 2111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeow, L.C.; Chew, B.L.; Sreeramanan, S. Elevation of secondary metabolites production through light-emitting diodes (LEDs) illumination in protocorm-like bodies (PLBs) of Dendrobium hybrid orchid rich in phytochemicals with therapeutic effects. Biotechnol. Rep. 2020, 27, e00497. [Google Scholar] [CrossRef]
- Khurshid, R.; Ullah, M.A.; Tungmunnithum, D.; Drouet, S.; Shah, M.; Zaeem, A.; Hameed, S.; Hano, C.; Abbasi, B.H. Lights triggered differential accumulation of antioxidant and antidiabetic secondary metabolites in callus culture of Eclipta alba L. PLoS ONE 2020, 15, e0233963. [Google Scholar] [CrossRef] [PubMed]
- Davis, P.A.; Burns, C. Photobiology in protected horticulture. Food Energy Secur. 2016, 5, 223–238. [Google Scholar] [CrossRef]
- García-Closas, R.; Berenguer, A.; Tormo, M.J.; Sánchez, M.J.; Quiros, J.R.; Navarro, C.; Arnaud, R.; Dorronsoro, M.; Chirlaque, M.D.; Barricarte, A. Dietary sources of vitamin C, vitamin E and specific carotenoids in Spain. Br. J. Nutr. 2004, 91, 1005–1011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Snafi, A.E. Phenolics and flavonoids contents of medicinal plants, as natural ingredients for many therapeutic purposes-A review. IOSR J. Pharm. 2020, 10, 42–81. [Google Scholar]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Ieperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, F.; Şan, B.; Yildirim, A.; Polat, M.; Ercişli, S. Mineral composition of leaves and fruit in some myrtle (Myrtus communis L.) genotypes. Erwerbs Obstbau 2015, 57, 149–152. [Google Scholar] [CrossRef]
- Bouaziz, A.; Abdalla, S.; Baghiani, A.; Charef, N. Phytochemical analysis, hypotensive effect and antioxidant properties of Myrtus communis L. growing in Algeria. Asian Pac. J. Trop. Biomed. 2015, 5, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Kazi, T.; Baig, J.; Afridi, H.; Kandhro, G.; Khan, S.; Kolachi, N.; Wadhwa, S. Determination of total mercury in chicken feed, its translocation to different tissues of chicken and their manure using cold vapour atomic absorption spectrometer. Food Chem. Toxicol. 2010, 48, 1550–1554. [Google Scholar] [CrossRef] [PubMed]
- Chung, I.M.; Kim, J.J.; Lim, J.D.; Yu, C.Y.; Kim, S.H.; Hahn, S.J. Comparison of resveratrol, SOD activity, phenolic compounds and free amino acids in Rehmannia glutinosa under temperature and water stress. Environ. Exp. Bot. 2006, 56, 44–53. [Google Scholar] [CrossRef]
- Machlin, L.J.; Bendich, A. Free radical tissue damage: Protective role of antioxidant nutrients 1. FASEB J. 1987, 1, 441–445. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.S.; Landau, J.M.; Huang, M.-T.; Newmark, H.L. Inhibition of carcinogenesis by dietary polyphenolic compounds. Annu. Rev. Nutr. 2001, 21, 381–406. [Google Scholar] [CrossRef] [Green Version]
- Yao, L.H.; Jiang, Y.-M.; Shi, J.; Tomas-Barberan, F.; Datta, N.; Singanusong, R.; Chen, S. Flavonoids in food and their health benefits. Plant Foods Hum. Nutr. 2004, 59, 113–122. [Google Scholar] [CrossRef]
- Naoumkina, M.A.; Zhao, Q.; Gallego-Giraldo, L.; Dai, X.; Zhao, P.X.; Dixon, R.A. Genome-wide analysis of phenylpropanoid defence pathways. Mol. Plant Pathol. 2010, 11, 829–846. [Google Scholar] [CrossRef]
- Thoma, F.; Somborn-Schulz, A.; Schlehuber, D.; Keuter, V.; Deerberg, G. Effects of light on secondary metabolites in selected leafy greens: A review. Front. Plant Sci. 2020, 11, 497. [Google Scholar] [CrossRef]
- Folta, K.M.; Carvalho, S.D. Photoreceptors and control of horticultural plant traits. HortScience 2015, 50, 1274–1280. [Google Scholar] [CrossRef] [Green Version]
- Brennicke, A.; Schopfer, P. Pflanzenphysiologie; Spektrum Akademischer Verlag: Berlin, Germany, 2010. [Google Scholar]
- Brunetti, C.; Fini, A.; Sebastiani, F.; Gori, A.; Tattini, M. Modulation of phytohormone signaling: A primary function of flavonoids in plant–environment interactions. Front. Plant Sci. 2018, 9, 1042. [Google Scholar] [CrossRef] [Green Version]
- Renouard, S.; Corbin, C.; Lopez, T.; Montguillon, J.; Gutierrez, L.; Lamblin, F.; Lainé, E.; Hano, C. Abscisic acid regulates pinoresinol–lariciresinol reductase gene expression and secoisolariciresinol accumulation in developing flax (Linum usitatissimum L.) seeds. Planta 2012, 235, 85–98. [Google Scholar] [CrossRef]
- Markulin, L.; Corbin, C.; Renouard, S.; Drouet, S.; Durpoix, C.; Mathieu, C.; Lopez, T.; Auguin, D.; Hano, C.; Lainé, É. Characterization of LuWRKY36, a flax transcription factor promoting secoisolariciresinol biosynthesis in response to Fusarium oxysporum elicitors in Linum usitatissimum L. hairy roots. Planta 2019, 250, 347–366. [Google Scholar] [CrossRef] [PubMed]
- Casal, J.J. Phytochromes, cryptochromes, phototropin: Photoreceptor interactions in plants. Photochem. Photobiol. 2000, 71, 1–11. [Google Scholar] [CrossRef]
- Fox, A.R.; Soto, G.C.; Jones, A.M.; Casal, J.J.; Muschietti, J.P.; Mazzella, M.A. cry1 and GPA1 signaling genetically interact in hook opening and anthocyanin synthesis in Arabidopsis. Plant Mol. Biol. 2012, 80, 315–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Folta, K.M. Green light stimulates early stem elongation, antagonizing light-mediated growth inhibition. Plant Physiol. 2004, 135, 1407–1416. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodyoung, A.; Masuda, Y.; Tomiyama, H.; Saito, T.; Okawa, K.; Ohara, H.; Kondo, S. Effects of light emitting diode irradiation at night on abscisic acid metabolism and anthocyanin synthesis in grapes in different growing seasons. Plant Growth Regul. 2016, 79, 39–46. [Google Scholar] [CrossRef]
- Hano, C.; Addi, M.; Fliniaux, O.; Bensaddek, L.; Duverger, E.; Mesnard, F.; Lamblin, F.; Lainé, E. Molecular characterization of cell death induced by a compatible interaction between Fusarium oxysporum f. sp. linii and flax (Linum usitatissimum) cells. Plant Physiol. Biochem. 2008, 46, 590–600. [Google Scholar] [CrossRef]

| Light Sources | Intensity | Exposure Time | Plant Species | Culture System | Secondary Metabolite | Yield Increase | References |
|---|---|---|---|---|---|---|---|
| UV-A/B | 4–5 Wm−2/10–14 Wm−2 | 3 h per day for 16 days | Capsicum annum | CGC (leaf) | Cynaroside | - | [30] |
| UV-B | 73.08 kJ/m2/day | 7 h per day for 13 days | Nymphoides humboldtiana | CGC (leaf) | Flavonoids | - | [31] |
| UV-B | 40 J/cm2 | 8 h | Alternanthera Sessilis | CGC (shoot) | Flavonoids | 51% | [32] |
| Alternanthera Brasiliana | 62% | ||||||
| UV-B | 1.14 kJ/m2/day | 4 h per day for 14 days | Capsicum annuum | CGC (leaf) | Flavonoids | - | [33] |
| UV-B | 313 nm | 7–14 days | Ginkgo biloba | CGC (leaf) | Quercetin, kaempferol, and isorhamnetin | 2.05- to 2.4-fold and 16.67- to −42-fold, respectively | [34] |
| UV-B | 1.26 μW/cm2 | 5 min | Catharanthus roseus | Cell suspension culture | Catharanthine and vindoline | 3-fold and 12-fold | [35] |
| UV-B | 224 μmol m−2 s −1 | 1 h for 2 days and 2 h for 2 days | Ocimum basilicum Green basil | CGC (leaf) | Anthocyanin, phenolics, and flavonoids | 9–23%, 28–126% and 80–169%, respectively | [21] |
| 2 h for 2 days and 2 h for five days | Ocimum basilicum Purple Basil | Phenolics and flavonoids | 29–63% and 37–79% | ||||
| UV-B | 102 kJ/m2/day | 3 days | Ocimum basilicum | CGC (plantlet) | Phenolics | [36] | |
| UV-B | 20 µW/cm2 | 4 days | Triticum aestivium | CGC (seedling) | Phenolics | 26.3% | [37] |
| UV-C | 254 nm | 15 min | Vitis vinifera | Callus culture | trans-resveratrol | 26-fold | [38] |
| UV-C | 254 nm | 5 min after 24 h | Vitis vinifera | Callus culture | trans-resveratrol | 8-fold | [39] |
| 10 min after 48 h | Catechin | - | |||||
| UV-C | 3 W/m2 | 60 min | Lepidium sativum | Callus culture | Chlorogenic acid, kaemferol, and quercetin | 2.5-fold | [40] |
| UV-C + Photoperiod UV-C + Dark | 3.6 kJ/m2 + 16/8 h 1.8 kJ/m2 + 24 h dark | 10–60 min | Linum usitatissimum | Callus culture | Secoisolariciresinol diglucoside (SDG) | 1.86-fold | [41] |
| Lariciresinol diglucoside (LDG) | 2.25-fold | ||||||
| Guaiacylglycerol-β-coniferyl alcohol ether glucoside (GGCG) | 1.33-fold | ||||||
| Total phenolic production | 2.82-fold | ||||||
| Total flavonoid production | 2.94-fold | ||||||
| Dehydrodiconiferyl alcohol glucoside (DCG) | 1.36-fold | ||||||
| UV-C | 3 W/m2 | 10 min | Ocimum basilicum | Callus culture | Rosmarinic acid | 2.3-fold | [42] |
| Chichoric acid and cyanide | 4.1-fold | ||||||
| Peonidin | 2.7-fold | ||||||
| UV-C | 254 nm | 60 min | Echinacea purpurea | Callus culture | Phenolics | - | [43] |
| Cell suspension culture |
| Light Type | Light Characteristic | Exposure Time | Plant Species | Culture System | Secondary Metabolite | Yield Increase | References |
|---|---|---|---|---|---|---|---|
| Blue light | 380–560 nm | 30 days | Stevia rebaudiana | Callus culture | Phenolics and Flavonoids | - | [22] |
| Blue light | 40–50 µmol m−2 s−1 | 3 weeks | Prunella vulgaris | Callus culture | Phenolics and Flavonoids | - | [14] |
| Blue light | 60 µmol m−2 s−1 | 6 weeks | Scutellaria lateriflora L. | Shoot culture | Glucuronides, Baicalin, Wogonoside, Verbascoside | 1.54-, 1.49-, 2.05- and 1.86-fold, respectively | [72] |
| Red light | 660 nm | 5 weeks | Hypericum perforatum | Root culture | Hypericins, Flavonoids | - | [73] |
| Blue light | 470 nm | 1 week | Hypericins, Phenolics | 52% and 26% | |||
| Blue light | 50 µmol m−2 s−1 | 30 days | Hyptis marrubioides | Micro propagation | Rutin | - | [74] |
| White light | |||||||
| Fluores cent light | 50 µmol m−2 s−1 | 4 weeks | Panax ginseng | Hairy root culture | Ginsenoside | - | [75] |
| Green light | 40–50 µmol m−2 s−1 | 3 weeks | Artemisia absinthium | Callus culture | Phenolics and Flavonoids | - | [9] |
| Light Types | Light Characteristic | Exposure Time | Plant Species | Culture System | Secondary Metabolite | Yield Increase | References |
|---|---|---|---|---|---|---|---|
| Monochromatic Blue LED | 456 nm | 27 days | Solanum lycopersicum | Closed-type plant production system (seedling) | Phenolics and flavonoids | - | [23] |
| Blue LED | 200 µmol m−2 s−1 | 14/10 h | Lettuce | CGC (leaf) | Flavonoids | 2.07-fold | [89] |
| Blue LED | 50 µmol m−2 s−1 | 28 days | Curculigo Orchioides | CGC (shoot bud) | Phenolics and flavonoids | - | [90] |
| Blue LED | 30 ±1 µmol m−2 s−1 | 8 h per day 40 days | Anoectochilus roxburghii | CGC (leaf) | Flavonoids and polyphenols | 24.2% | [91] |
| Blue (+B) and Blue-violet (+BV) LED | 450 nm and 420–440 nm | 10 days | Ocimum basilicum | CGC (leaf) | Phenolics | [92] | |
| Eruca sativa | Flavonoids | ||||||
| Red LED | 35 µmol m−2 s−1 | 6 weeks | Myrtus communis | In vitro shoot culture | Gallic acid and Myricetin | - | [93] |
| Red LED | 40–50 μmol m−2 s −1 | 24 h | Silybum marianum | Callus culture | Silymarin | 2-fold | [94] |
| Blue LED | 50 µmol m−2 s−1 | 4 weeks | Rehmannia glutinosa | In vitro shoot culture | Phenolics and flavonoids | 39.3%, 33.6% | [95] |
| Red LED | 450 nm | CGC (root and leaf) | Phenolics, Flavonoids | 33.6%, 61.7% | [96] | ||
| Blue LED | 40–50 µmol m−2 s−1 | 4 weeks | Ocimum basilicum | Callus culture | Rosmarinic acid and eugenol | 2.46- and 2.25-fold | [97] |
| Red LED | Peonidin and cyanidin | 3.5- and 4.53-fold | |||||
| White LED | Chicoric acid | 4.52-fold | |||||
| White LED | 380 nm | 4 days | Triticum aestivium | CGC (seedling) | Epicatechin | 1.3- to 1.46-fold | [98] |
| Blue LED | 470 nm | 8 days | Gallic acid and quercetin | 1.2–1.5 fold | |||
| Red LED | 660 nm | 8 days | Ferulic acid | - | |||
| 12 days | p-coumaric acid | 1.27- to 1.77-fold | |||||
| Red Blue light | 137 µmol m−2 s−1 | 96 h | Fagopyrum sp. | CGC (seedling) | Flavonoids | - | [99] |
| Blue LED | 177 µmol m−2 s−1 | Anthocyanins | - | ||||
| Red and Blue 2R:1B | 120 µmol m−2 s−1 | 12 h each day for 17 days | Ocimum basilicum | CGC (seedling) | Phenolics and flavonoids | 1.63–1.87-fold and 2.06-fold | [100] |
| Blue–red (1:1) LED | 44.80 µmol/s | 30 days for 16 h | Dendrobium | In vitro protocorn-like body culture | Flavonoids | - | [101] |
| Red: Blue LED | 33 µmol m−2 s−1 | 4 weeks | Cnidium officinale | Callus culture | Phenolics and flavonoids | - | [10] |
| Red LED | 660 nm | 24 h | Eclipta alba | Callus culture | Phenolics and flavonoids | 2-fold | [102] |
| Blue LED | 460 nm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hashim, M.; Ahmad, B.; Drouet, S.; Hano, C.; Abbasi, B.H.; Anjum, S. Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants In Vitro Cultures. Plants 2021, 10, 1521. https://doi.org/10.3390/plants10081521
Hashim M, Ahmad B, Drouet S, Hano C, Abbasi BH, Anjum S. Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants In Vitro Cultures. Plants. 2021; 10(8):1521. https://doi.org/10.3390/plants10081521
Chicago/Turabian StyleHashim, Mariam, Bushra Ahmad, Samantha Drouet, Christophe Hano, Bilal Haider Abbasi, and Sumaira Anjum. 2021. "Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants In Vitro Cultures" Plants 10, no. 8: 1521. https://doi.org/10.3390/plants10081521
APA StyleHashim, M., Ahmad, B., Drouet, S., Hano, C., Abbasi, B. H., & Anjum, S. (2021). Comparative Effects of Different Light Sources on the Production of Key Secondary Metabolites in Plants In Vitro Cultures. Plants, 10(8), 1521. https://doi.org/10.3390/plants10081521








