Horseradish Essential Oil as a Promising Anti-Algal Product for Prevention of Phytoplankton Proliferation and Biofouling
Abstract
:1. Introduction
2. Results
2.1. Laboratory Experiments
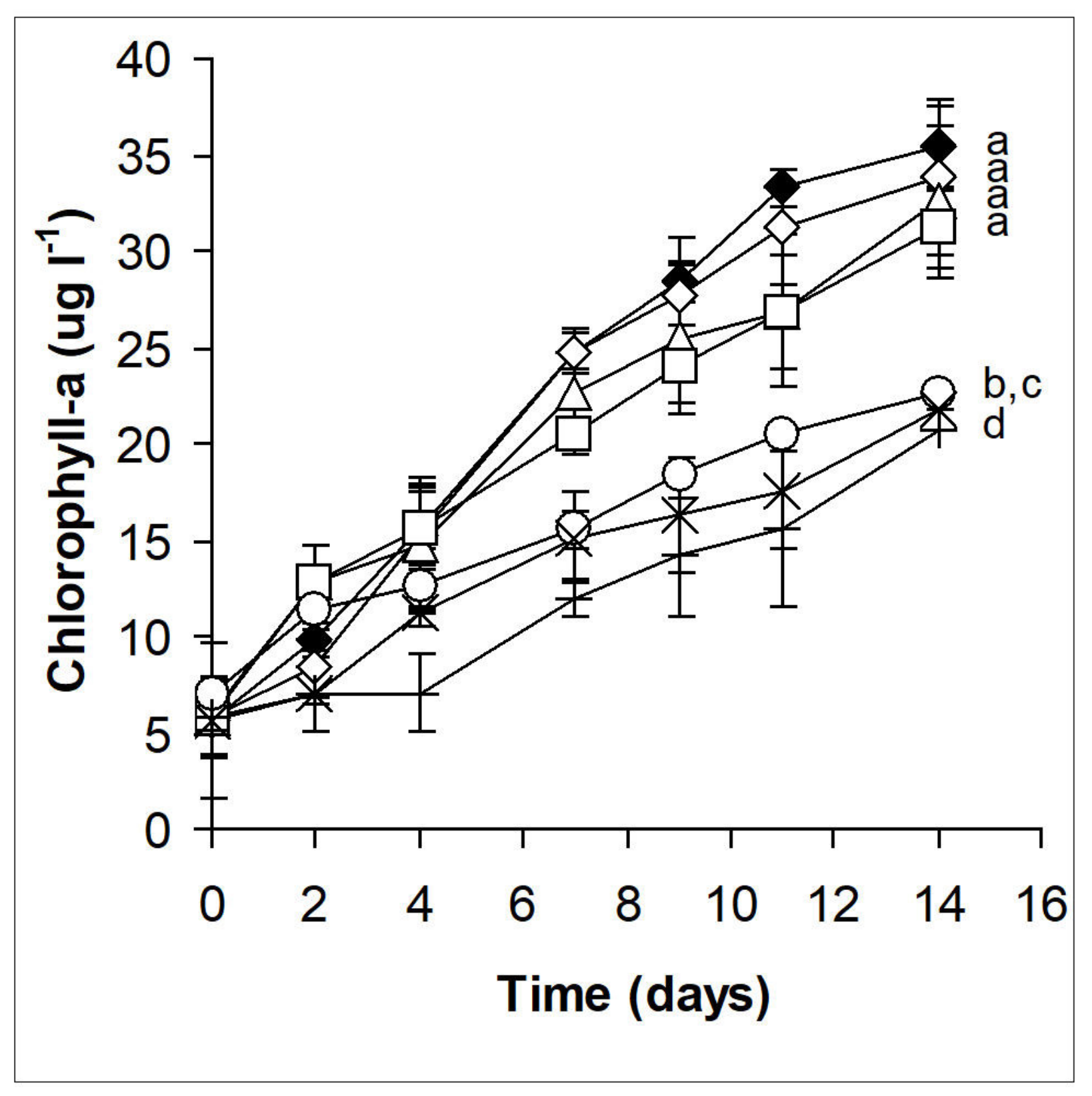
2.2. Phytoplankton Assemblages in Microcosm Experiments
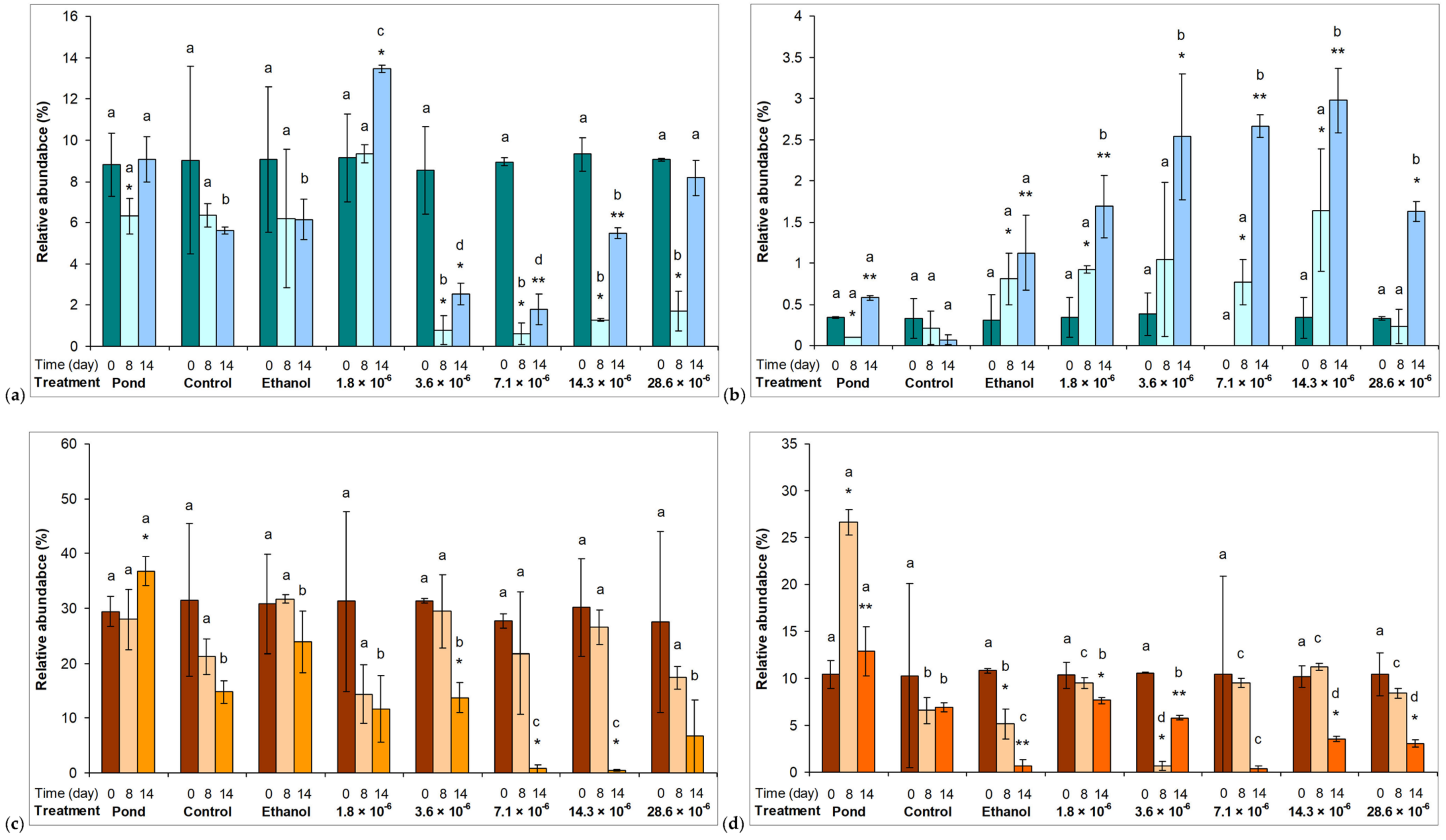
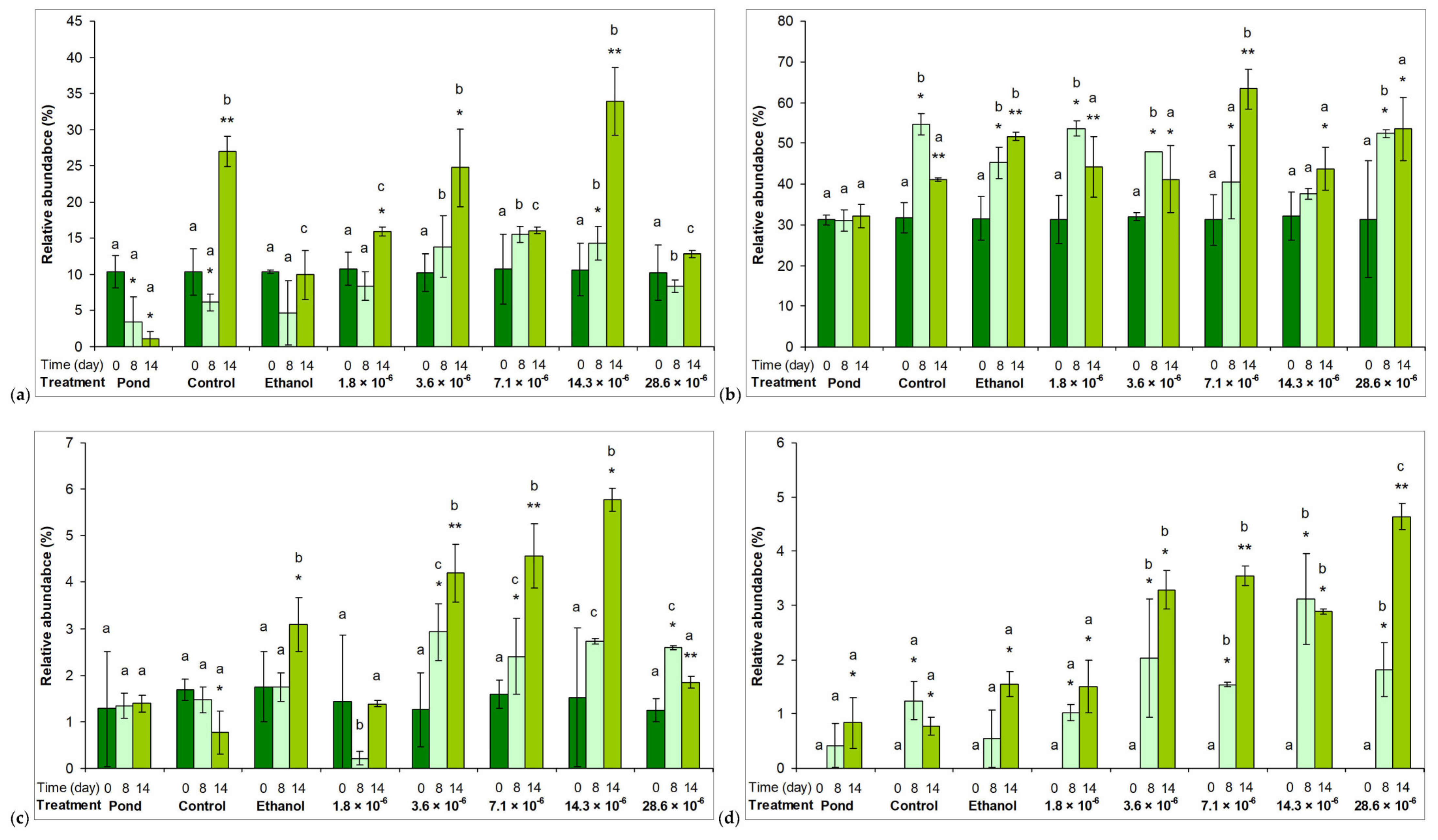
2.3. Benthic Assemblages on Artificial Surfaces
3. Discussion
4. Materials and Methods
4.1. Strains, Culturing Conditions, and Laboratory Experimental Setup
4.2. Microcosm Experiments with Natural Phytoplankton Assemblages
4.3. Algal Biofilm Experiments with Natural Benthic Algal Assemblages
4.4. Data Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Carpenter, S.R.; Caraco, N.F.; Correll, D.L.; Howarth, R.W.; Sharpley, A.N.; Smith, V.H. Nonpoint pollution of surface waters with phosphorus and nitrogen. Ecol. Appl. 1998, 8, 559–568. [Google Scholar] [CrossRef]
- Jančula, D.; Marsalek, B. Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 2011, 85, 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Qi, S.-H.; Ma, X. Antifouling compounds from marine invertebrates. Mar. Drugs 2017, 15, 263. [Google Scholar] [CrossRef] [Green Version]
- Yebra, D.M.; Kiil, S.; Dam-Johansen, K. Antifouling technology—Past, present and future steps towards efficient and environmentally friendly antifouling coatings. Prog. Org. Coatings 2004, 50, 75–104. [Google Scholar] [CrossRef]
- Mohamed, Z.A. Alum and lime–alum removal of toxic and nontoxic phytoplankton from the Nile river water: Laboratory study. Water Resour. Manage. 2001, 15, 213–221. [Google Scholar] [CrossRef]
- Auvray, F.; van Hullebusch, E.D.; Deluchat, V.; Baudu, M. Laboratory investigation of the phosphorus removal (SRP and TP) from eutrophic lake water treated with aluminium. Water Res. 2006, 40, 2713–2719. [Google Scholar] [CrossRef]
- Meers, E.; Rousseau, D.P.L.; Lesage, E.; Demeersseman, E.; Tack, F.M.G. Physico-chemical P removal from the liquid fraction of pig manure as an intermediary step in manure processing. Water Air Soil Pollut. 2006, 169, 317–330. [Google Scholar] [CrossRef]
- Takacs, I.; Murthy, S.; Smith, S.; McGrath, M. Chemical phosphorus removal to extremely low levels: Experience of two plants in the Washington, DC area. Water Sci. Technol. 2006, 53, 21–28. [Google Scholar] [CrossRef]
- Lelkova, E.; Rulik, M.; Hekera, P.; Dobias, P.; Dolejs, P.; Borovickova, M.; Poulickova, A. The influence of the coagulant PAX-18 on Planktothrix agardhii bloom in a shallow eutrophic fishpond. Fottea 2008, 8, 147–154. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.Q.; Graham, N.J.D.; Harward, C. Comparison of polyferric sulfate with other coagulants for the removal of algae and algae-derived organic-matter. Water Sci. Technol. 1993, 27, 221–230. [Google Scholar] [CrossRef]
- Chow, C.W.K.; House, J.; Velzeboer, R.M.A.; Drikas, M.; Burch, M.D.; Steffensen, D.A. The effect of ferric chloride flocculation on cyanobacterial cells. Water Res. 1998, 32, 808–814. [Google Scholar] [CrossRef]
- Briley, D.S.; Knappe, D.R.U. Optimizing ferric sulfate coagulation of algae with streaming current measurements. J. Am. Water Works Ass. 2002, 94, 80–90. [Google Scholar] [CrossRef]
- Dafforn, K.A.; Lewis, J.A.; Johnston, E.L. Antifouling strategies: History and regulation, ecological impacts and mitigation. Mar. Pollut. Bull. 2011, 62, 453–465. [Google Scholar] [CrossRef] [PubMed]
- Blomqvist, P. Late summer phytoplankton responses to experimental manipulations of nutrients and grazing in unlimed and limed Lake Njupfatet, central Sweden. Archiv Für Hydrobiol. 1996, 137, 425–455. [Google Scholar] [CrossRef]
- Prepas, E.E.; Pinel-Alloul, B.; Chambers, P.A.; Murphy, T.P.; Reedyk, S.; Sandland, G.; Serediak, M. Lime treatment and its effects on the chemistry and biota of hardwater eutrophic lakes. Freshw. Biol. 2001, 46, 1049–1060. [Google Scholar] [CrossRef]
- Zhang, Y.; Ghadouani, A.; Prepas, E.E.; Pinel-Alloul, B.; Reedyk, S.; Chambers, P.A.; Robarts, R.D.; Methot, G.; Raik, A.; Holst, M. Response of plankton communities to whole-lake Ca(OH)2 and CaCO3 additions in eutrophic hardwater lakes. Freshw. Biol. 2001, 46, 1105–1119. [Google Scholar] [CrossRef]
- Huh, J.-H.; Ahn, J.-W. A perspective of chemical treatment for cyanobacteria control toward sustainable freshwater development. Environ. Eng. Res. 2017, 22, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Omae, I. Organotin antifouling paints and their alternatives. Appl. Organomet. Chem. 2003, 17, 81–105. [Google Scholar] [CrossRef]
- Amara, I.; Miled, W.; Slama, R.B.; Ladhari, N. Antifouling processes and toxicity effects of antifouling paints on marine environment. A review. Environ. Toxicol. Phar. 2018, 57, 115–130. [Google Scholar] [CrossRef]
- Jančula, D.; Drabkova, M.; Cerny, J.; Karaskova, M.; Korinkova, R.; Rakusan, J.; Marsalek, B. Algicidal activity of phthalocyanines—screening of 31 compounds. Environ. Toxicol. 2008, 23, 218–223. [Google Scholar] [CrossRef]
- Jančula, D.; Marsalek, B.; Novotna, Z.; Cerny, J.; Karaskova, M.; Rakusan, J. In search of the main properties of phthalocyanines participating in toxicity against cyanobacteria. Chemosphere 2009, 77, 1520–1525. [Google Scholar] [CrossRef]
- Shen, Q.; Zhu, J.; Cheng, L.; Zhang, J.; Zhang, Z.; Xu, X. Enhanced algae removal by drinking water treatment of chlorination coupled with coagulation. Desalination 2011, 271, 236–240. [Google Scholar] [CrossRef]
- Junli, H.; Li, W.; Nenqi, R.; Li, L.X.; Fun, S.R.; Guanle, Y. Disinfection effect of chlorine dioxide on viruses, algae and animal planktons in water. Water Res. 1997, 31, 455–460. [Google Scholar] [CrossRef]
- Chen, J.-J.; Yeh, H.-H. The mechanisms of potassium permanganate on algae removal. Water Res. 2005, 39, 4420–4428. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-J.; Yeh, H.-H.; Tseng, I.-C. Potassium permanganate as an alternative preoxidant for enhancing algal coagulation-pilot and bench scale studies. Environ. Technol. 2008, 29, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Xie, P.; Ma, J.; Fang, J.; Guan, Y.; Yue, S.; Li, X.; Chen, L. Comparison of permanganate preoxidation and preozonation on algae containing water: Cell integrity, characteristics, and chlorinated disinfection byproduct formation. Environ. Sci. Technol. 2013, 47, 14051–14061. [Google Scholar] [CrossRef] [PubMed]
- Plummer, J.D.; Edzwald, J.K. Effect of ozone on algae as precursors for trihalomethane and haloacetic acid production. Envion. Sci. Technol. 2001, 35, 3661–3668. [Google Scholar] [CrossRef]
- Schrader, K.K.; Rimando, A.M.; Tucker, C.S.; Glinski, J.; Cutler, S.J.; Cutler, H.G. Evaluation of the natural product SeaKleen for controlling the musty-odorproducing cyanobacterium Oscillatoria perornata in catfish ponds. North Am. J. Aquacul. 2004, 66, 20–28. [Google Scholar] [CrossRef]
- USEPA 2005. Reregistration Eligibility Decision for Endothall; EPA 738-R-05-008; United States Environmental Protection Agency, Prevention, Pesticides and Toxic Substances (7508C): Washington, DC, USA, 2005. [Google Scholar]
- Giacomazzi, S.; Cochet, N. Environmental impact of diuron transformation: A review. Chemosphere 2004, 56, 1021–1032. [Google Scholar] [CrossRef]
- Osano, O.; Admiraal, W.; Klamer, H.J.C.; Pastor, D.; Bleeker, E.A.J. Comparative toxic and genotoxic effects of chloroacetanilides, formamidines and their degradation products on Vibrio fischeri and Chironomus riparius. Environ. Pollut. 2002, 119, 195–202. [Google Scholar] [CrossRef]
- Holdren, C.; Jones, W.; Taggart, J. Managing Lakes and Reservoirs; North American Lake Management Society and Terrene Institute: Madison, WI, USA, 2001. [Google Scholar]
- Dahms, H.U.; Dobretsov, S. Antifouling compounds from marine macroalgae. Marine Drugs 2017, 15, 265. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-L.; Wu, Z.-H.; Wang, C.-Y.; Xu, Y. Mini-review: Antifouling natural products from marine microorganisms and their synthetic analogs. Marine Drugs 2017, 15, 266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, M.; Goecke, F.; Bhadury, P. Minireview: Algal natural compounds and extracts as antifoulants. J. Appl. Phycol. 2018, 30, 1859–1874. [Google Scholar] [CrossRef] [Green Version]
- Al-Jumaili, A.; Kumar, A.; Bazaka, K.; Jacob, M.V. Plant secondary metabolite-derived polymers: A potential approach to develop antimicrobial films. Polymers 2018, 10, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hasan, J.; Crawford, R.J.; Ivanova, E.P. Antibacterial surfaces: The quest for a new generation of biomaterials. Trends Biotechnol. 2013, 31, 295–304. [Google Scholar] [CrossRef]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef]
- Andrade, B.F.M.T.; Barbosa, L.N.; da Silva Probst, I.; Júnior, A.F. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Nguyen, N.M.; Gonda, S.; Vasas, G. A review on the phytochemical composition and potential medicinal uses of horseradish (Armoracia rusticana) root. Food Rev. Int. 2013, 29, 261–275. [Google Scholar] [CrossRef]
- Fahey, W.; Zalemann, A.T.; Talalay, P. The chemical diversity and distribution of glucosinolates and isothiocyanates among plants. Phytoehemistry 2000, 56, 5–51. [Google Scholar] [CrossRef]
- Li, X.; Kushad, M.M. Purification and characterization of myrosinase from horseradish (Armoracia rusticana) roots. Plant Physiol. Biochem. 2005, 43, 503–511. [Google Scholar] [CrossRef]
- Bones, A.M.; Rossiter, J.T. The enzymatic and chemically induced decomposition of glucosinolates. Phytochemistry 2006, 67, 1053–1067. [Google Scholar] [CrossRef]
- Vig, A.P.; Rampai, G.; Thind, T.S.S.; Arora, S. Bio-protective effects of glucosinolates. LWT Food Sei. Technol. 2009, 42, 1561–1572. [Google Scholar] [CrossRef]
- Kojima, M.; Oawa, K. Studies on the effect of isothiocyanates and their analogues on microorganisms. J. Ferment. Technol. 1971, 49, 740–746. [Google Scholar]
- Zsolnai, T. Antimicrobial effect of thiocyanates and isothiocyanates. Arzneimittelforschung 1971, 21, 121–127. [Google Scholar]
- Shin, I.S.; Masuda, H.; Naohide, K. Bactericidal activity of wasabi (Wasabia japonica) against Helicobacter pylori. Int. J. Food Microbiol. 2004, 94, 255–261. [Google Scholar] [CrossRef]
- Luciano, F.B.; Holley, R.A. Enzymatic inhibition by allyl isothiocyanate and factors affecting its antimicrobial action against Escherichia coli OI57:H7. Int. J. Food Microbiol. 2009, 131, 240–245. [Google Scholar] [CrossRef] [PubMed]
- Plaszkó, T.; Szűcs, Z.; Vasas, G.; Gonda, S. Effects of glucosinolate-derived isothiocyanates on fungi: A comprehensive review on direct effects, mechanisms, structure-activity relationship data and possible agricultural applications. J. Fungi 2021, 7, 539. [Google Scholar] [CrossRef]
- Kassie, F.; Knasmuller, S. Genotoxic effects of allyl isothiocyanate and phenyl isothiocyanate. Chem. Biol. Interact. 2000, 127, 163–180. [Google Scholar] [CrossRef]
- Saladino, F.; Bordin, K.; Luciano, F.B.; Franzón, M.F.; Mañes, J.; Meca, G. Antimicrobial activity of the glucosinolates. In Glucosinolates; Reference Series in Phytochemistry; Mérillon, J.-M., Ramawat, K.G., Eds.; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar]
- Zsolnai, T. Antimicrobial effect of thiocyanates and isothiocyanates. Arztl. Forsch. 1966, 16, 870–876. [Google Scholar]
- Lin, C.M.; Kim, J.; Du, W.X.; Wei, C.I. Bactericidal activity of isothiocyanate against pathogens on fresh produce. J. Food Prot. 2000, 63, 25–30. [Google Scholar] [CrossRef] [PubMed]
- Hyldgaard, M.; Mygind, T.; Meyer, R.L. Essential oils in food preservation: Mode of action; synergies; and interactions with food matrix components. Front. Microbiol. 2012, 3, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Calmes, B.; Guyen, G.N.; Durmur, J.; Brisach, C.A.; Campion, C.; Iacomi, B.; Pigné, S.; Dias, E.; Macherel, D.; Guillemette, T.; et al. Glucosinolate-derived isothiocyanates impact mitochondrial function in fungal cells and elicit an oxidative stress response necessary for growth recovery. Front. Plant Sci. 2015, 6, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weenink, E.F.J.; Luimstra, V.M.; Schuurmans, J.M.; Van Herk, M.J.; Visser, P.M.; Matthijs, H.C.P. Combatting cyanobacteria with hydrogenperoxide: A laboratory study on the consequences for phytoplankton community and diversity. Front. Microbiol. 2015, 6, 714. [Google Scholar] [CrossRef] [Green Version]
- Bácsi, I.; Gonda, S.; B-Béres, V.; Novák, Z.; Nagy, S.A.; Vasas, G. Alterations of phytoplankton assemblages treated with chlorinated hydrocarbons: Effects of dominant species sensitivity and initial diversity. Ecotoxicology 2015, 24, 823–834. [Google Scholar] [CrossRef] [PubMed]
- Felföldy, L. Water Management Hydrobiology 16: Biological Water Quality Assessment. (Vízügyi Hidrobiológia 16: A Biológiai Vízminősítés); Institute of Water Management (Vízgazdálkodási Intézet): Budapest, Hungary, 1987; p. 258. [Google Scholar]
- Borics, G. Phytoplankton Based Ecological Status Assesment of Surface Waters. (Felszíni Vizek Fitoplankton Alapú Ökológiai Állapotértékelése); Hungarian Academy of Sciences; Centre for Ecological Research; Danube Research Institute; Department of Tisza Research: Debrecen, Hungary, 2015. [Google Scholar]
- Ghernaout, B.; Ghernaout, D.; Saiba, A. Algae and cyanotoxins removal by coagulation/flocculation: A review. Desalin. Water Treat. 2010, 20, 133–143. [Google Scholar] [CrossRef] [Green Version]
- Molino, P.J.; Wetherbee, R. The biology of biofouling diatoms and their role in the development of microbial slimes. Biofouling 2008, 24, 365–379. [Google Scholar] [CrossRef]
- Van de Poll, W.H.; van Leeuwe, M.A.; Roggeveld, J.; Buma, A.G.J. Nutrient limitation and high irradiance acclimation reduce par and uv-induced viability loss in the antarctic diatom Chaetoceros brevis (Bacillariophyceae). J. Phycol. 2005, 41, 840–850. [Google Scholar] [CrossRef] [Green Version]
- Hattab, M.; Genta-Jouve, G.; Bouzidi, N.; Ortalo-Magné, A.; Hellio, C.; Maréchal, J.-P.; Piovetti, L.; Thomas, O.; Culioli, G. Cystophloroketals A–E; unusual phloroglucinol–meroterpenoid hybrids from the brown alga Cystoseira tamariscifolia. J. Nat. Prod. 2015, 78, 1663–1670. [Google Scholar] [CrossRef]
- Cho, J. Antifouling chromanols isolated from brown alga Sargassum horneri. J. Appl. Phycol. 2013, 25, 299–309. [Google Scholar] [CrossRef]
- Sun, Y.-Y.; Wang, H.; Guo, G.-L.; Pu, Y.-F.; Yan, B.-L.; Wang, C.-H. Green alga Ulva pertusa—A new source of bioactive compounds with antialgal activity. Environ. Sci. Pollut. Res. 2015, 22, 10351–10359. [Google Scholar]
- Van Alstyne, K.L.; Harvey, E.L.; Cataldo, M. Effects of dopamine; a compound released by the green-tide macroalga Ulvaria obscura (Chlorophyta); on marine algae and invertebrate larvae and juveniles. Phycologia 2014, 53, 195–202. [Google Scholar] [CrossRef]
- Silkina, A.; Bazes, A.; Mouget, L.; Bourgaugnon, N. Comparative efficiency of macroalgal extracts and booster biocides as antifouling agents to control growth of the diatom species. Mar. Pollut. Bull. 2012, 64, 2039–2046. [Google Scholar] [CrossRef]
- Chambers, L.D.; Hellio, C.; Stokes, K.R.; Dennington, S.P.; Goodes, L.R.; Wood, R.J.K.; Walsh, F.C. Investigation of Chondrus crispus as a potential source of new antifouling agents. Int. Biodeter. Biodegr. 2011, 65, 939–946. [Google Scholar] [CrossRef]
- Ktari, L.; Ismail-Ben Ali, A.; Ben Redjem, Y.; Langar, H.; Bour, M.E. Antifouling activity and chemical investigation of the brown alga Dictyota fasciola (Dictyotales) from Tunisian coast. Cah. Biol. Mar. 2010, 51, 109–115. [Google Scholar]
- Águila-Ramírez, R.N.; Arenas-González, A.; Hernández-Guerrero, C.J.; González-Acosta, B.; Borges-Souza, J.M.; Verón, B.; Pope, J.; Hellio, C. Antimicrobial and antifouling activities achieved by extracts of seaweeds from gulf of California; Mexico. Hidrobiológica 2012, 22, 8–15. [Google Scholar]
- Plouguerné, E.; Hellio, C.; Cesconetto, C.; Thabard, M.; Mason, K.; Véron, B.; Pereira, R.C.; Gama, B.A. Antifouling activity as a function of population variation in Sargassum vulgare from the littoral of Rio de Janeiro. J. Appl. Phycol. 2010, 22, 717–724. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Krock, B.; Tillmann, U.; Bickmeyer, U.; Graeve, M.; Cembella, A. Mode of action of membrane-disruptive lytic compounds from the marine dinoflagellate Alexandrium tamarense. Toxicon 2011, 58, 247–258. [Google Scholar] [CrossRef] [Green Version]
- Poulson, K.L.; Sieg, R.D.; Prince, E.K.; Kubanek, J. Allelopathic compounds of a red tide dinoflagellate have species-specific and contextdependent impacts on phytoplankton. Mar. Ecol. Prog. Ser. 2010, 416, 69–78. [Google Scholar] [CrossRef]
- Prince, E.K.; Poulson, K.L.; Myers, T.L.; Sieg, R.D.; Kubanek, J. Characterization of allelopathic compounds from the red tide dinoflagellate Karenia brevis. Harmful Algae 2010, 10, 39–48. [Google Scholar] [CrossRef]
- Poulson-Ellestad, K.; McMillan, E.; Montoya, J.P.; Kubanek, J. Are offshore phytoplankton susceptible to Karenia brevis allelopathy? J. Plankton Res. 2014, 36, 1344–1356. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.; Zhu, H.; Duan, S. Allelopathic interactions between the redtide causative dinoflagellate Prorocentrum donghaiense and the diatom Phaeodactylum tricornutum. Oceanologia 2014, 56, 639–650. [Google Scholar] [CrossRef]
- Sala-Pérez, M.; Alpermann, T.J.; Krock, B.; Tillmann, U. Growth and bioactive secondary metabolites of arctic Protoceratium reticulatum (Dinophyceae). Harmful Algae 2016, 55, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Szűcs, Z.; Plaszkó, T.; Cziáky, Z.; Kiss-Szikszai, A.; Emri, T.; Bertóti, R.; Sinka, L.T.; Vasas, G.; Gonda, S. Endophytic fungi from the roots of horseradish (Armoracia rusticana) and their interactions with the defensive metabolites of the glucosinolate-myrosinase-isothiocyanate system. BMC Plant Biol. 2018, 18, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Bendall, D.S.; Bowes, J.M.; Stewart, A.C.; Taylor, M.E. Oxygen evolving photosystem II particles from Phormidium laminosum. Method. Enzymol. 1988, 167, 272–280. [Google Scholar]
- Allen, M.M. Simple conditions for the growth of unicellular blue-green algae on plates. J. Phycol. 1968, 4, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.K.; Johnson, E.J.; MacElroy, R.D.; Speer, H.L.; Bruff, B.S. Effects of salts on the halophilic alga Dunaliella viridis. J. Bacteriol. 1968, 95, 1461–1468. [Google Scholar] [CrossRef] [Green Version]
- CCAP Media Recipes. Available online: https://www.ccap.ac.uk/wp-content/uploads/MR_JM.pdf (accessed on 29 June 2021).
- CCAP Media Recipes. Available online: https://www.ccap.ac.uk/wp-content/uploads/MR_BB.pdf (accessed on 29 June 2021).
- Bácsi, I.; B-Béres, V.; Kókai, Z.; Gonda, S.; Novák, Z.; Nagy, S.A.; Vasas, G. Effects of non-steroidal anti-inflammatory drugs on cyanobacteria and algae in laboratory strains and in natural algal assemblages. Environ. Poll. 2016, 212, 508–518. [Google Scholar] [CrossRef] [PubMed]
- European Commitee for Standardizationrue. Water Quality e Guidance Standard on the Enumeration of Phytoplankton Using Inverted Microscopy (Utermöhl Technique); European Standard EN 15204: 2006; European Commitee for Standardizationrue: Brussels, Belgium, 2006. [Google Scholar]
- Guiry, M.D.; Guiry, G.M. AlgaeBase. In World-Wide Electronic Publication; National University of Ireland: Galway, Ireland, 1996; Available online: http://www.algaebase.org (accessed on 16 January 2021).
- Hammer, O.; Harper, D.A.T.; Ryan, P.D. PAST: Paleontological statistics software package for education and data analysis. Palaeontol. Electron. 2001, 4, 9. [Google Scholar]
- Papp, N.; Gonda, S.; Kiss-Szikszai, A.; Plaszkó, T.; Lőrincz, P.; Vasas, G. Ethnobotanical and ethnopharmacological data of Armoracia rusticana P. Gaertner, B. Meyer et Scherb. in Hungary and Romania: A case study. Genet. Resour. Crop. Evol. 2018, 65, 1893–1905. [Google Scholar] [CrossRef]
- Norway Grant HU09-0009-A2-2013 Development and Production of Natural Bioactive Compound Based Products from Vegetable Waste of Horseradish Using Environmentally Friendly Technology. Available online: http://tormaolaj.hu/en/index.html (accessed on 16 January 2021).


| Strains | EC50 (× 10−6 v/v % Horseradish Essential Oil) | ||
|---|---|---|---|
| 4th Day | 7th Day | 14th Day | |
| Synechococcus elongatus | 7.66 ± 2.26 | 7.95 ± 0.48 | - |
| Microcystis aeruginosa | 4.97 ± 0.25 | 5.07 ± 0.38 | - |
| Cylindrospermopsis raciborskii | 4.77 ± 2.87 | 4.13 ± 3.07 | - |
| Dunaliella salina | 1.33 ± 0.07 | 1.47 ± 0.08 | - |
| Cryptomonas ovata | 1.81 ± 0.41 | 1.63 ± 0.47 | 2.07 ± 0.39 |
| Chlorella sorokiniana | 1.57 ± 0.05 | 1.67 ± 0.02 | 1.77 ± 0.05 |
| Chlorococcum sp. | 2.37 ± 0.49 | 2.69 ± 0.12 | 2.81 ± 0.05 |
| Desmodesmus communis | 5.67 ± 0.24 | 4.17 ± 1.21 | 4.76 ± 0.27 |
| Prokaryotes | Culturing Medium | Culture Parameters |
|---|---|---|
| Synechococcus elongatus | Allen Medium [80] | 100 mL Erlenmeyer flask on shaker (90 rpm), 35 mL final volume, 28 °C, continuous irradiation (80 µmol photons m−2 s−1). |
| Microcystis aeruginosa | ||
| Cylindrospermopsis raciborskii | Allen Medium (without nitrate) | |
| Eukaryotes | ||
| Dunaliella salina | Johnson’s Medium [81] | 100 mL Erlenmeyer flask on shaker (90 rpm), 35 mL final volume, 28 °C, continuous irradiation (80 µmol photons m−2 s−1). |
| Cryptomonas ovata | Jaworski’s Medium [82] | 100 mL Erlenmeyer flask on shaker (90 rpm), 50 mL final volume, 24 °C, continuous irradiation (40 µmol photons m−2 s−1). |
| Chorella sorokiniana | Bold’s Basal Medium [83] | |
| Chlorococcum sp. | ||
| Desmodesmus communis |
| Treatments (v/v % Horseradish Oil) | µM AITC | µM PEITC |
|---|---|---|
| 1.8 × 10−6 | 0.78 ± 0.012 | 0.016 ± 0.008 |
| 3.6 × 10−6 | 1.57 ± 0.025 | 0.032 ± 0.016 |
| 7.1 × 10−6 | 3.13 ± 0.05 | 0.064 ± 0.032 |
| 14.3 × 10−6 | 6.25 ± 0.10 | 0.128 ± 0.064 |
| 28.6 × 10−6 | 12.51 ± 0.20 | 0.257 ± 0.128 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bácsi, I.; Gonda, S.; Nemes-Kókai, Z.; B-Béres, V.; Vasas, G. Horseradish Essential Oil as a Promising Anti-Algal Product for Prevention of Phytoplankton Proliferation and Biofouling. Plants 2021, 10, 1550. https://doi.org/10.3390/plants10081550
Bácsi I, Gonda S, Nemes-Kókai Z, B-Béres V, Vasas G. Horseradish Essential Oil as a Promising Anti-Algal Product for Prevention of Phytoplankton Proliferation and Biofouling. Plants. 2021; 10(8):1550. https://doi.org/10.3390/plants10081550
Chicago/Turabian StyleBácsi, István, Sándor Gonda, Zsuzsanna Nemes-Kókai, Viktória B-Béres, and Gábor Vasas. 2021. "Horseradish Essential Oil as a Promising Anti-Algal Product for Prevention of Phytoplankton Proliferation and Biofouling" Plants 10, no. 8: 1550. https://doi.org/10.3390/plants10081550






