Abiotic Stress Tolerance of Coastal Accessions of a Promising Forage Species, Trifolium fragiferum
Abstract
1. Introduction
2. Results
2.1. Effect of Increased Soil Moisture
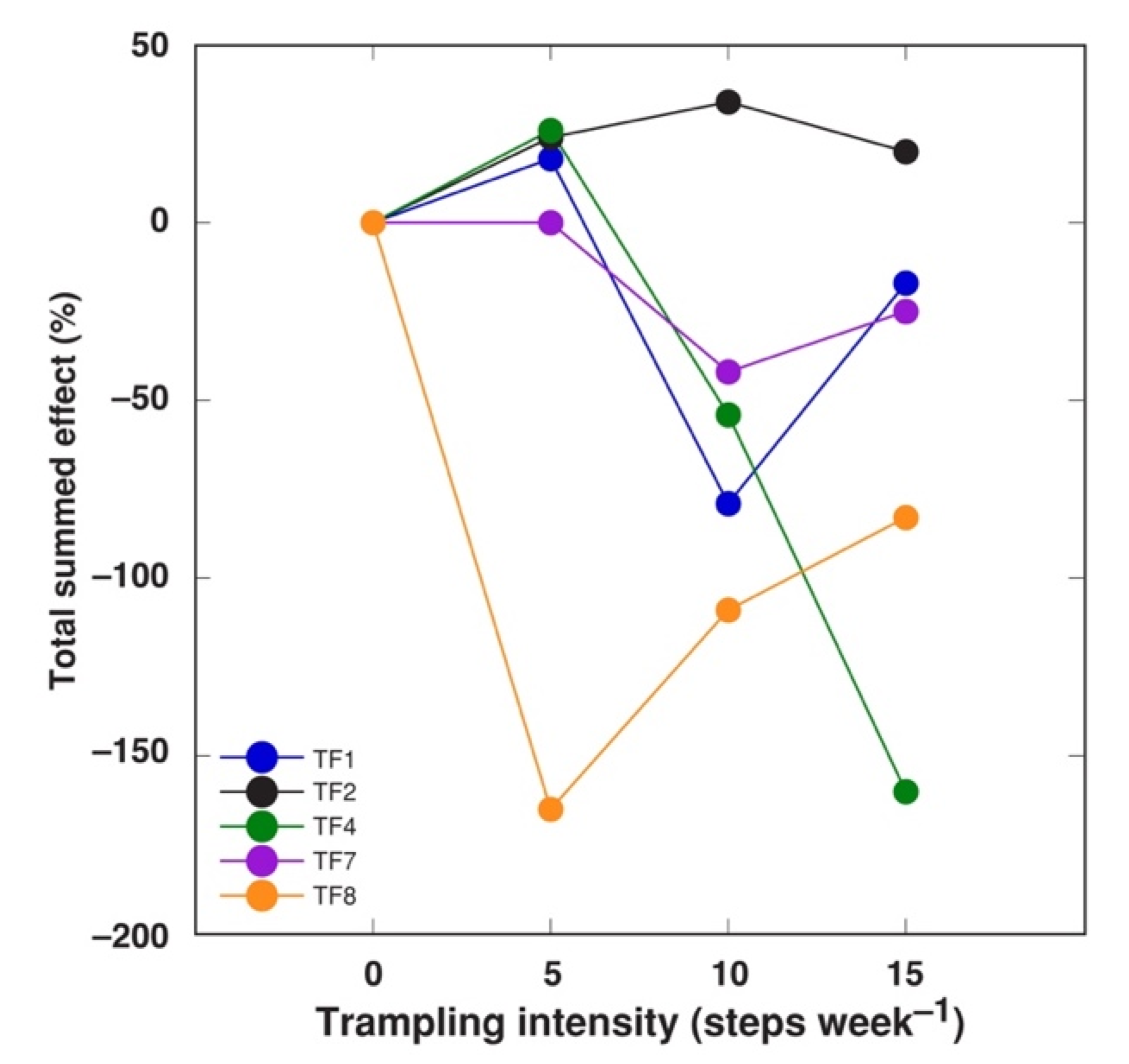
2.2. Effect of Trampling
2.3. Effect of Cutting
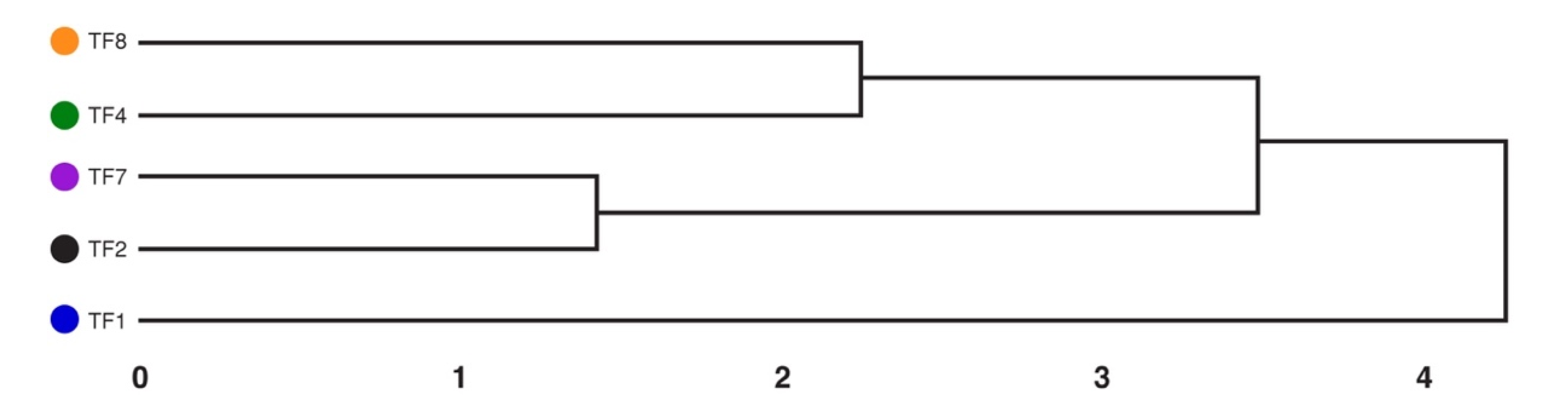
2.4. Average Abiotic Stress Tolerance of T. fragiferum Accessions
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Setup
4.2. Plant Propagation and Establishment of Experimental Material
4.3. Increased Soil Moisture
4.4. Trampling
4.5. Cutting
4.6. Measurements
4.7. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dempewolf, H.; Eastwood, R.J.; Guarino, L.; Khoury, C.K.; Müller, J.V.; Toll, J. Adapting agriculture to climate change: A global initiative to collect, conserve, and use crop wild relatives. Agroecol. Sustain. Food Syst. 2014, 38, 369–377. [Google Scholar] [CrossRef]
- Heywood, V.; Casas, A.; Ford-Lloyd, B.; Kell, S.; Maxted, N. Conservation and sustainable use of crop wild relatives. Agric. Syst. Environ. 2007, 121, 245–255. [Google Scholar] [CrossRef]
- Warschefsky, E.; Penmetsa, R.V.; Cook, D.R.; von Wettberg, E.J.B. Back to the wilds: Tapping evolutionary adaptations for resilient crops through systematic hybridization with crop wild relatives. Am. J. Bot. 2014, 101, 1791–1800. [Google Scholar] [CrossRef]
- Zhang, H.; Mittal, N.; Leamy, L.J.; Barazani, O.; Song, B.-H. Back into the wild—Apply untapped genetic diversity of wild relatives for crop improvement. Evol. Appl. 2016, 10, 5–24. [Google Scholar] [CrossRef]
- Maxted, N.; Scholten, M.; Codd, R.; Ford-Lloyd, B. Creation and use of a national inventory of crop wild relatives. Biol. Conserv. 2007, 140, 142–159. [Google Scholar] [CrossRef]
- Zhang, H.; Yasmin, F.; Song, B.-H. Neglected treasures in the wild—Legume wild relatives in food security and human health. Curr. Opin. Plant Biol. 2019, 49, 17–26. [Google Scholar] [CrossRef]
- Dabkevičiene, G.; Dabkevičius, Z. Evaluation of wild red clover (Trifolium pratense L.) ecotypes and hybrid populations (Trifolium pratense L. × Trifolium diffusum Ehrh.) for clover rot resistance (Sclerotinia trifoliorum Erikss.). Biologija 2005, 3, 54–58. [Google Scholar]
- Rancane, S.; Jansone, B.; Sparnina, M. The evaluation of genetic resources of forage legumes collected from natural grassland. In Sustainable Grassland Productivity, Proceedings of the 21st General Meeting of the European Grassland Federation, Badajoz, Spain, 3–6 April 2006; Lloveras, J., González-Rodríguez, A., Vázquez-Yañez, O., Piñeiro, J., Santamaría, O., Olea, L., Poblaciones, M.J., Eds.; European Grassland Federation: Madrid, Spain, 2006; pp. 327–329. [Google Scholar]
- Bērziņa, I.; Zhuk, A.; Veinberga, I.; Rashal, I.; Ruņģis, D. Genetic fingerprinting of Latvian red clover (Trifolium pratense L.) varieties using simple sequence repeat (SSR) markers: Comparison over time and space. Latv. J. Agron. 2008, 11, 28–33. [Google Scholar]
- Paplauskienė, V.; Dabkevičienė, G. A study of genetic diversity in Trifolium hybridum varieties using morphological characters and ISSR markers. Žemdirb.-Agric. 2012, 99, 313–318. [Google Scholar]
- Lemežienė, N.; Stukonis, V.; Kemešytė, V.; Norkevičienė, E. Wild and semi natural ecotypes of perennial grasses and legumes—For breeding purposes. In Breeding Grasses and Protein Crops in the Era of Genomics; Brazauskas, G., Statkeviciute, G., Jonaviciene, K., Eds.; Springer International Publishing AG: Basel, Switzerland, 2018; pp. 88–95. [Google Scholar]
- Heywood, V.H.; Zohary, D. A catalogue of the wild relatives of cultivated plants native to Europe. Flora Mediterr. 1995, 5, 375–415. [Google Scholar]
- Taylor, N.; Gillett, J. Crossing and morphological relationships among Trifolium species closely related to strawberry and Persian clover. Crop Sci. 1988, 28, 636–639. [Google Scholar] [CrossRef]
- Nichols, P.G.H.; Revell, C.K.; Humphries, A.W.; Howie, J.H.; Hall, E.J.; Sandral, G.A.; Ghamkhar, K.; Harris, C.A. Temperate pasture legumes in Australia—Their history, current use, and future prospects. Crop Pasture Sci. 2012, 63, 691–725. [Google Scholar] [CrossRef]
- Townsend, C.E. Miscellaneous perennial clovers. In Clover Science and Technology; Taylor, J.L., Ed.; ASA/CSSA/SSSA: Madison, WI, USA, 1985; pp. 563–578. [Google Scholar]
- Janssen, J.A.M.; Rodwell, J.S. European Red List of Habitats: Part 2. Terrestrial and Freshwater Habitats; European Union: Brussels, Belgium, 2016. [Google Scholar]
- Hilligardt, M.; Weberling, F. Wuchsformen bei Trifolium L. Flora 1989, 182, 13–41. [Google Scholar] [CrossRef]
- Huber, H.; Wiggerman, L. Shade avoidance in the clonal herb Trifolium fragiferum: A field study with experimentally manipulated vegetation height. Plant Ecol. 1997, 130, 53–62. [Google Scholar] [CrossRef]
- Can, E.; Arslan, M.; Sener, O.; Daghan, H. Response of strawberry clover (Trifolium fragiferum L.) to salinity stress. Res. Crop. 2013, 14, 576–584. [Google Scholar]
- Ciocârlan, V.; Georgescu, M.I.; Sǎvulescu, E.; Anastasiu, P. Plopul salt marshes (Tulcea county)—An unique area for halophytes in Romania. Acta Horti Bot. Bucur. 2013, 40, 27–32. [Google Scholar] [CrossRef]
- Hoveland, C.S.; Mikkelsen, E.E. Flooding tolerance of ladino white, intermediate white, persian and strawberry clovers. Agron. J. 1967, 59, 307–308. [Google Scholar] [CrossRef]
- Rogers, M.E.; West, D.W. The effects of rootzone salinity and hypoxia on shoot and root growth in Trifolium species. Ann. Bot. 1993, 72, 503–509. [Google Scholar] [CrossRef]
- Heinrichs, D.H. Flooding tolerance of legumes. Can. J. Plant Sci. 1970, 50, 435–438. [Google Scholar] [CrossRef]
- Rogers, M.E.; Colmer, T.D.; Frost, K.; Henry, D.; Cornwall, D.; Hulm, E.; Hughes, S.; Snowball, R.; Nichols, P.G.H.; Craig, A.D. The influence of NaCl salinity and hypoxia on aspects of growth in Trifolium species. Crop Pasture Sci. 2009, 60, 71–82. [Google Scholar] [CrossRef]
- Pederson, G.A. White clover and other perennial clovers. In Forages. An Introduction to Grassland Agriculture, 5th ed.; Barnes, R.F., Nelson, C.J., Collins, M., Moore, K.J., Eds.; Iowa State University: Ames, IA, USA, 1995; Volume 1, pp. 227–236. [Google Scholar]
- Sun, D. Trampling resistance, recovery and growth rate of eight plant species. Agric. Ecosyst. Environ. 1992, 38, 265–273. [Google Scholar] [CrossRef]
- Melino, V.J.; Drew, E.A.; Ballard, R.A.; Reeve, W.G.; Thomson, G.; White, R.G.; O’hara, G.W. Identifying abnormalities in symbiotic development between Trifolium spp. and Rhizobium leguminosarum bv. trifolii leading to sub-optimal and ineffective nodule phenotypes. Ann. Bot. 2012, 110, 1559–1572. [Google Scholar] [CrossRef][Green Version]
- Kaushal, M.; Wani, S.P. Rhizobacterial-plant interactions: Strategies ensuring plant growth promotion under drought and salinity stress. Agric. Ecosyst. Environ. 2016, 231, 68–78. [Google Scholar] [CrossRef]
- Rumbaugh, M.D.; Pendery, B.M.; James, D.W. Variation in the salinity tolerance of strawberry clover (Trifolium fragiferum L.). Plant Soil 1993, 153, 265–271. [Google Scholar] [CrossRef]
- Gibberd, M.R.; Gray, J.D.; Cocks, P.S.; Colmer, T.D. Waterlogging tolerance among a diverse range of Trifolium accessions is related to root porosity, lateral root formation and ‘aerotropic rooting’. Ann. Bot. 2001, 88, 579–589. [Google Scholar] [CrossRef]
- Oram, N.J.; Sun, Y.; Abalos, D.; van Groeningen, J.W.; Hartley, S.; De Deyn, G.B. Plant traits of grass and legume species for flood resilience and N2O mitigation. Funct. Ecol. 2021. [CrossRef]
- Warwick, S.I. The genecology of lawn weeds. VII. The response of different growth forms of Plantago major L. and Poa annua L. to simulated trampling. New Phytol. 1980, 85, 461–469. [Google Scholar] [CrossRef]
- lijima, M.; Kono, Y.; Yamauchi, A.; Pardales, J.R., Jr. Effects of soil compaction on the development of rice and maize root systems. Environ. Exp. Bot. 1991, 31, 333–342. [Google Scholar]
- Engelaar, W.M.H.G.; Blom, C.W.P.M. Effects of flooding and trampling on the performance of river foreland species of Rumex and Plantago. Acta Bot. Neerl. 1995, 44, 225–245. [Google Scholar] [CrossRef]
- Sunohara, Y.; Ikeda, H.; Tsukagoshi, S.; Murata, Y.; Sakurai, N.; Noma, Y. Effects of trampling on morphology and ethylene production in asiatic plantain. Weed Sci. 2002, 50, 479–484. [Google Scholar] [CrossRef]
- Rumball, W.; Claydon, R.B.; Miller, J.E. ‘Grasslands Upward’ strawberry clover (Trifolium fragiferum L.). N. Z. J. Agric. Res. 1991, 34, 135–136. [Google Scholar] [CrossRef]
- Sun, D.; Liddle, M.J. Plant morphological characteristics and resistance to simulated trampling. Environ. Manage. 1993, 17, 511–521. [Google Scholar] [CrossRef]
- Tälle, M.; Deák, B.; Poschlod, P.; Valkó, O.; Westerberg, L.; Milberg, P. Grazing vs. mowing: A meta-analysis of biodiversity benefits for grassland management. Agric. Ecosyst. Environ. 2016, 222, 200–212. [Google Scholar] [CrossRef]
- Brummer, E.C.; Moore, K.J. Persistence of perennial cool-season grass and legume cultivars under continuous grazing by beef cattle. Agron. J. 2000, 92, 466–471. [Google Scholar] [CrossRef]
- Caradus, J.R.; Mackay, A.C. Performance of white clover cultivars and breeding lines in a mixed species sward. 2. Plant characters contributing to differences in clover proportion in swards. N. Z. J. Agric. Sci. 1991, 34, 155–160. [Google Scholar]
- Williams, T.A.; Abberton, M.T.; Thornley, W.; Rhodes, I. No relationship between leaf size and yield in medium leaf size white clover varieties under rotational sheep grazing and cutting. Grass Forage Sci. 2002, 56, 412–417. [Google Scholar] [CrossRef]
- Ludvíková, V.; Pavlú, V.; Gaisler, J.; Hecjman, M.; Pavlú, L. Long term defoliation by cattle grazing with and without trampling differently affects soil penetration resistance and plant species composition in Agrostis capillaris grassland. Agric. Ecosyst. Environ. 2014, 197, 204–211. [Google Scholar] [CrossRef]
- Karlsons, A.; Druva-Lusite, I.; Osvalde, A.; Necajeva, J.; Andersone-Ozola, U.; Samsone, I.; Ievinsh, G. Adaptation strategies of rare plant species to heterogeneous soil conditions on a coast of a lagoon lake as revealed by analysis of mycorrhizal symbiosis and mineral constituent dynamics. Environ. Exp. Biol. 2017, 15, 113–126. [Google Scholar]
- Ievinsh, G.; Landorfa-Svalbe, Z.; Andersone-Ozola, U.; Bule, A. Wild Rumex species as models in ecophysiological studies: Effect of Na/K salts and nitrogen compounds on growth and electrolyte accumulation. Environ. Exp. Biol. 2020, 18, 43–44. [Google Scholar]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2020, 58, 275–292. [Google Scholar] [CrossRef]
- McDonald, J.H. Handbook of Biological Statistics, 3rd ed.; Sparky House Publishing: Baltimore, MD, USA, 2014; p. 299. [Google Scholar]
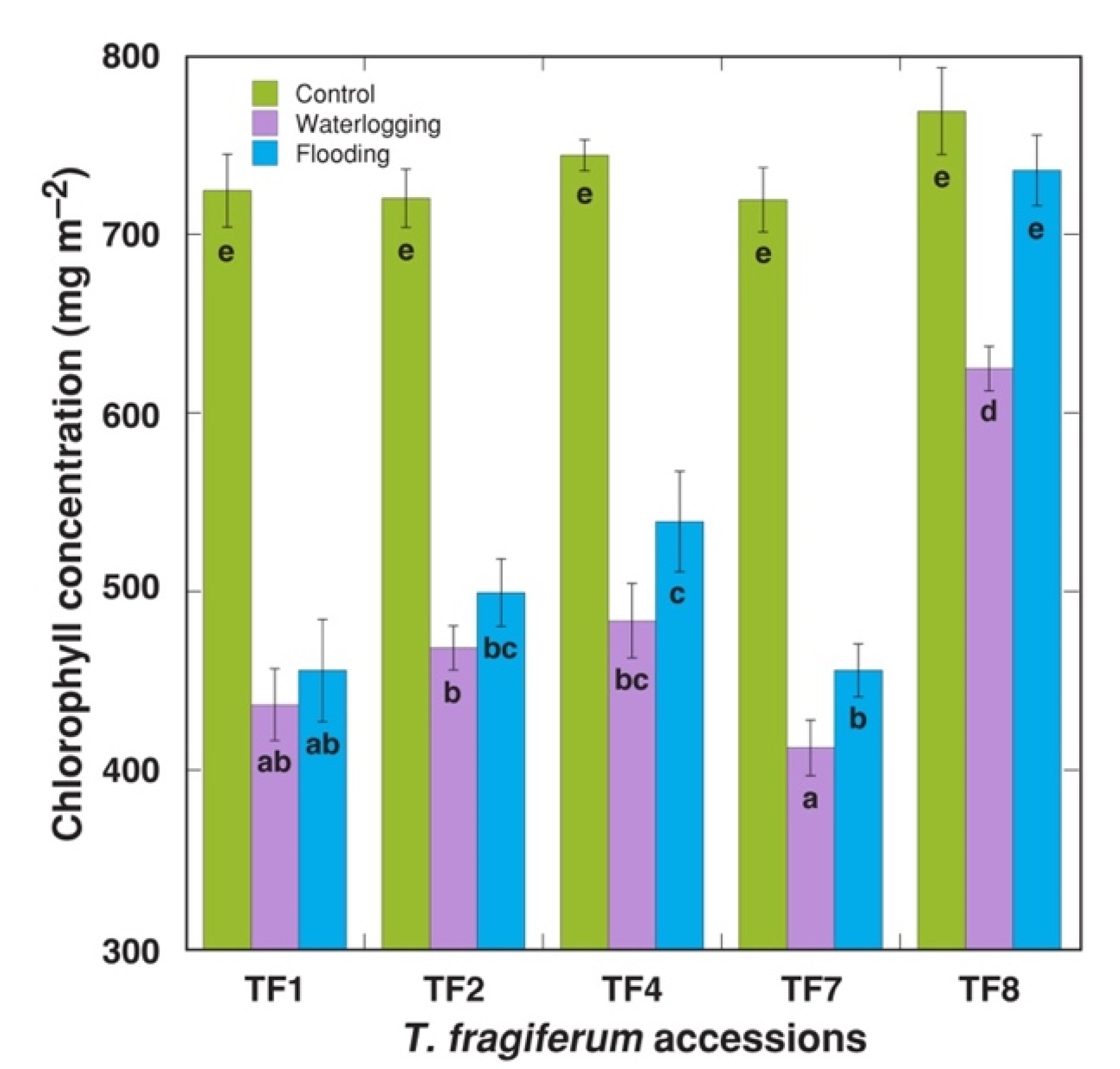
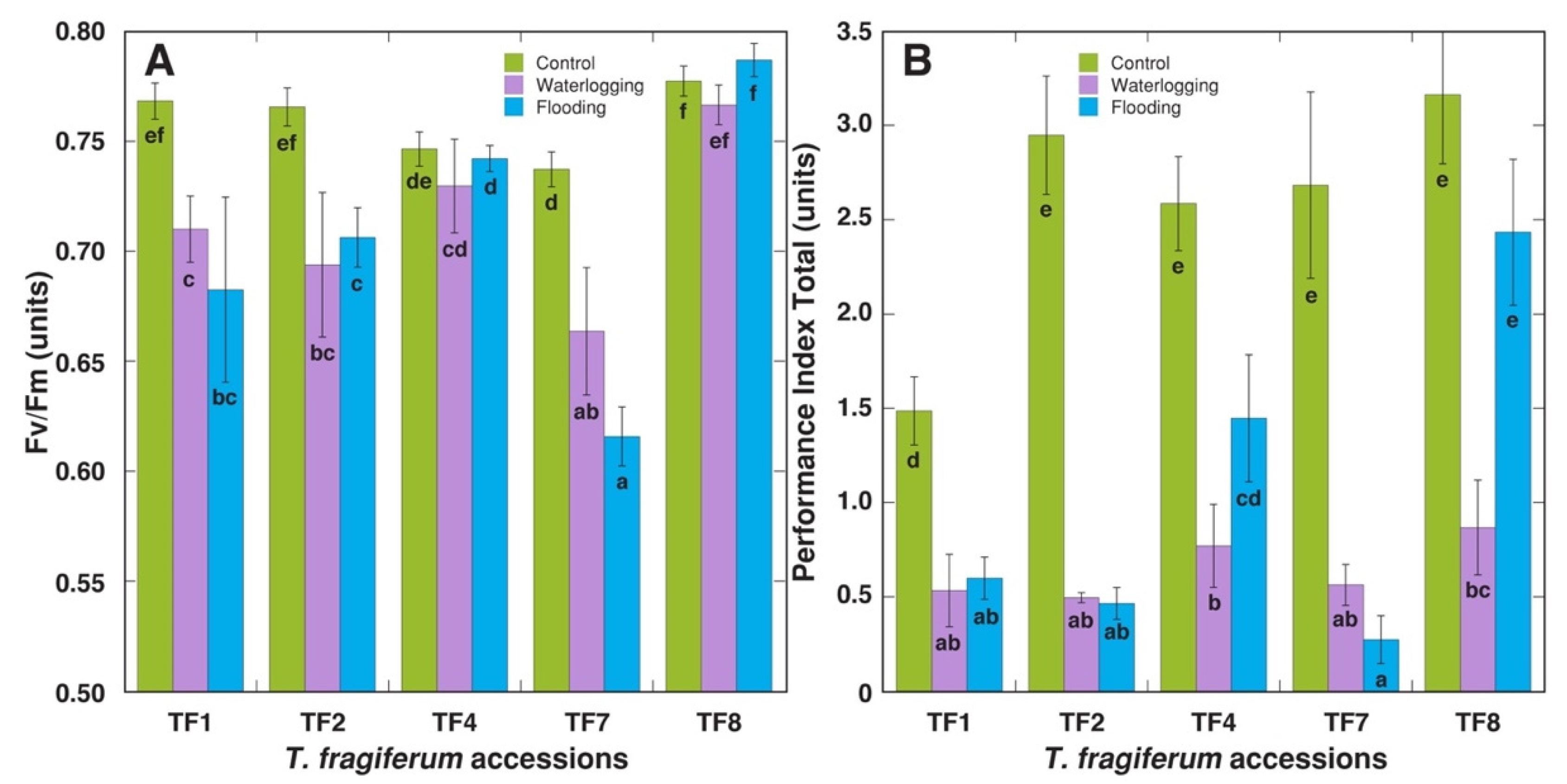
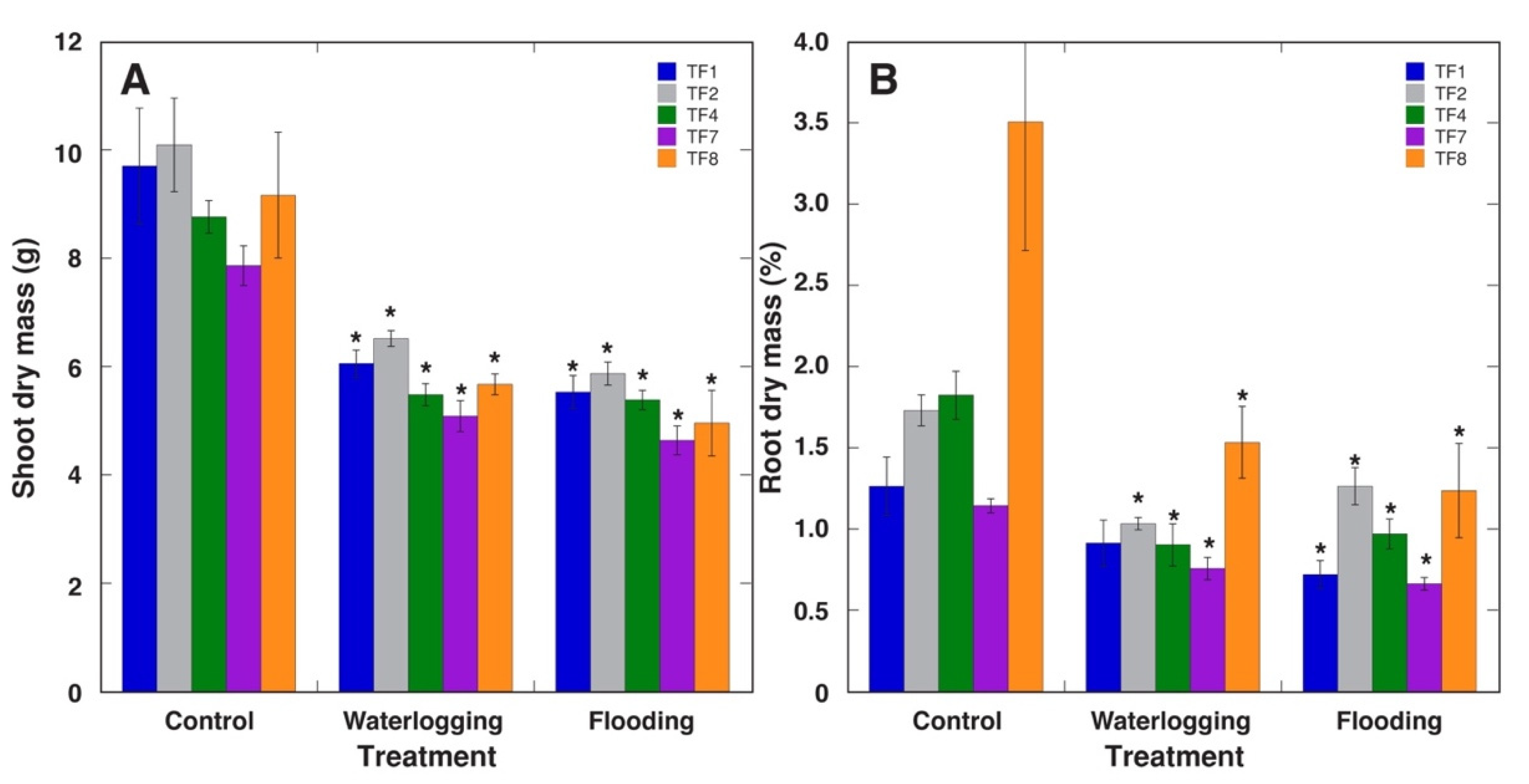

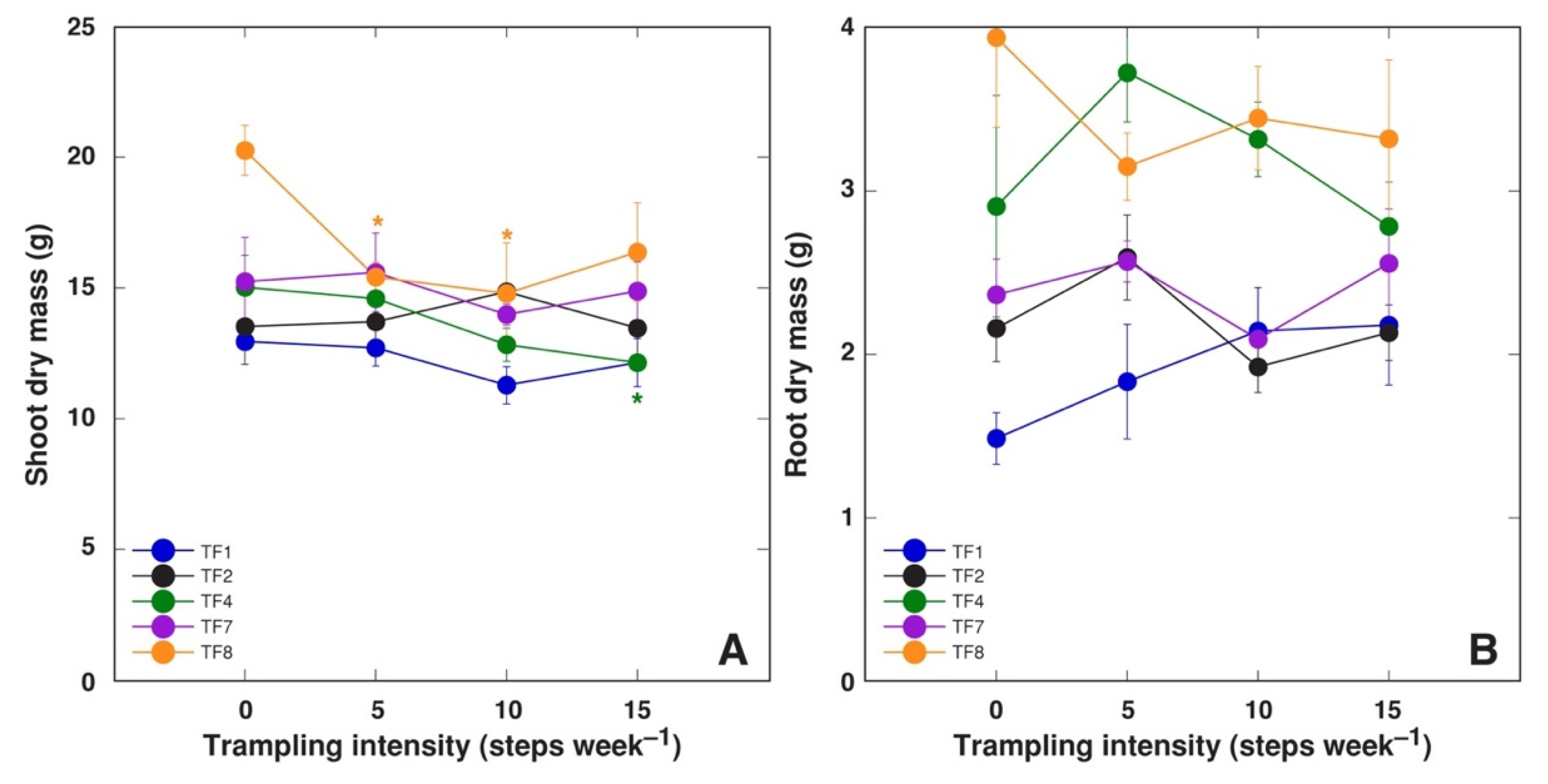


| Table 1 | No. of Stolons per Plant | Average Length of Stolons (mm) | Total Length of Stolons (cm) | DM of Stolons (g) | No. of Leaves per Plant | DM of Leaf Petioles (g) | DM of Leaf Blades (g) |
|---|---|---|---|---|---|---|---|
| TF1 | |||||||
| control | 17.6 ± 1.6 | 322 ± 19 | 560 ± 41 | 3.23 ± 0.31 | 290 ± 30 | 1.46 ± 0.33 | 2.81 ± 0.55 |
| WLG | 15.8 ± 1.0 | 216 ± 9 | 337 ± 14 | 2.02 ± 0.09 | 254 ± 27 | 1.13 ± 0.14 | 2.06 ± 0.18 |
| FLOOD | 14.0 ± 1.1 | 236 ± 13 | 322 ± 13 | 1.78 ± 0.03 | 204 ± 32 | 1.05 ± 0.08 | 1.89 ± 0.05 |
| TF2 | |||||||
| control | 19.4 ± 3.3 | 337 ± 36 | 1193 ± 53 | 3.77 ± 0.32 | 264 ± 33 | 1.86 ± 0.27 | 3.30 ± 0.22 |
| WLG | 19.2 ± 0.7 | 196 ± 6 | 721 ± 33 | 2.15 ± 0.08 | 236 ± 6 | 1.30 ± 0.06 | 2.50 ± 0.04 |
| FLOOD | 15.0 ± 1.0 | 227 ± 19 | 625 ± 73 | 1.77 ± 0.09 | 171 ± 7 | 1.41 ± 0.13 | 2.39 ± 0.11 |
| TF4 | |||||||
| control | 13.0 ± 0.7 | 260 ± 17 | 333 ± 11 | 2.48 ± 0.11 | 157 ± 4 | 1.34 ± 0.08 | 2.37 ± 0.08 |
| WLG | 9.0 ± 0.6 | 230 ± 11 | 205 ± 7 | 1.61 ± 0.09 | 106 ± 5 | 0.92 ± 0.02 | 1.57 ± 0.08 |
| FLOOD | 10.2 ± 1.3 | 237 ± 18 | 234 ± 15 | 1.88 ± 0.08 | 136 ± 9 | 1.21 ± 0.08 | 1.99 ± 0.04 |
| TF7 | |||||||
| control | 14.6 ± 1.0 | 493 ± 38 | 713 ± 56 | 3.76 ± 0.19 | 231 ± 9 | 1.26 ± 0.08 | 2.38 ± 0.05 |
| WLG | 8.0 ± 0.3 | 377 ± 13 | 302 ± 17 | 2.02 ± 0.11 | 170 ± 10 | 1.04 ± 0.09 | 1.80 ± 0.10 |
| FLOOD | 9.2 ± 1.0 | 354 ± 29 | 317 ± 21 | 1.78 ± 0.10 | 170 ± 7 | 0.89 ± 0.05 | 1.54 ± 0.08 |
| TF8 | |||||||
| control | 12.8 ± 1.9 | 235 ± 33 | 294 ± 42 | 2.97 ± 0.52 | 188 ± 30 | 1.99 ± 0.42 | 4.08 ± 0.72 |
| WLG | 10.0 ± 0.6 | 203 ± 38 | 196 ± 31 | 1.77 ± 0.14 | 152 ± 13 | 1.31 ± 0.08 | 2.46 ± 0.23 |
| FLOOD | 9.8 ± 1.0 | 159 ± 18 | 153 ± 18 | 1.50 ± 0.22 | 132 ± 18 | 1.29 ± 0.20 | 2.01 ± 0.31 |
| Trampling Intensity (Steps Week–1) | No. of Stolons per Plant | Average Length of Stolons (mm) | Total Length of Stolons (cm) | DM of Stolons (g) | No. of Leaves per Plant | DM of Leaf Petioles (g) | DM of Leaf Blades (g) |
|---|---|---|---|---|---|---|---|
| TF1 | |||||||
| 0 | 24.6 ± 0.9 | 275 ± 17 | 675 ± 36 | 4.47 ± 0.25 | 276 ± 11 | 1.78 ± 0.06 | 3.47 ± 0.28 |
| 5 | 28.2 ± 1.9 | 234 ± 11 | 660 ± 55 | 4.12 ± 0.31 | 326 ± 11 | 1.82 ± 0.10 | 3.67 ± 0.29 |
| 10 | 29.8 ± 2.7 | 198 ± 15 | 576 ± 26 | 3.32 ± 0.09 | 285 ±10 | 1.60 ± 0.21 | 3.66 ± 0.30 |
| 15 | 40.0 ± 3.9 | 157 ± 10 | 648 ±90 | 3.67 ± 0.49 | 385 ± 55 | 1.55 ± 0.15 | 3.45 ± 0.30 |
| TF2 | |||||||
| 0 | 34.6 ± 2.7 | 266 ± 20 | 943 ± 144 | 5.20 ± 0.97 | 483 ± 53 | 2.08 ± 0.04 | 4.34 ± 0.35 |
| 5 | 37.6 ± 1.3 | 229 ± 18 | 864 ± 88 | 5.02 ± 0.54 | 469 ± 38 | 2.33 ± 0.06 | 4.90 ± 0.23 |
| 10 | 36.8 ± 1.0 | 275 ± 16 | 1015 ± 74 | 6.03 ± 0.45 | 478 ± 15 | 2.46 ± 0.07 | 4.95 ± 0.08 |
| 15 | 32.6 ± 1.3 | 242 ± 10 | 788 ± 64 | 5.19 ± 0.48 | 443 ± 15 | 2.22 ± 0.18 | 4.41 ± 0.10 |
| TF4 | |||||||
| 0 | 32.2 ± 2.7 | 166 ± 9 | 535 ± 49 | 3.75 ± 0.38 | 319 ± 20 | 1.68 ± 0.12 | 3.27 ± 0.13 |
| 5 | 37.2 ± 3.8 | 137 ± 12 | 480 ± 30 | 3.49 ± 0.40 | 401 ± 35 | 1.76 ± 0.20 | 3.62 ± 0.31 |
| 10 | 36.2 ± 3.8 | 123 ± 8 | 410 ± 37 | 3.08 ± 0.51 | 331 ± 18 | 1.52 ± 0.09 | 3.06 ± 0.18 |
| 15 | 34.4 ± 0.8 | 109 ± 4 | 374 ± 14 | 2.37 ± 0.15 | 300 ± 8 | 1.34 ± 0.08 | 2.47 ± 0.17 |
| TF7 | |||||||
| 0 | 37.6 ± 3.0 | 432 ± 26 | 1611 ± 131 | 8.09 ± 0.55 | 360 ± 56 | 1.43 ± 0.20 | 4.03 ± 0.52 |
| 5 | 33.2 ± 3.6 | 406 ± 14 | 1335 ± 112 | 7.27 ± 0.56 | 411 ± 74 | 1.51 ± 0.19 | 4.18 ± 0.22 |
| 10 | 35.4 ± 4.7 | 362 ± 27 | 1233 ± 79 | 6.75 ± 0.20 | 387 ± 53 | 1.42 ± 0.08 | 4.01 ± 0.06 |
| 15 | 31.4 ± 2.7 | 383 ± 15 | 1208 ± 133 | 7.10 ± 0.56 | 341 ± 19 | 1.42 ± 0.18 | 4.33 ± 0.16 |
| TF8 | |||||||
| 0 | 25.8 ± 0.4 | 344 ± 34 | 892 ± 97 | 9.58 ± 0.70 | 380 ± 31 | 3.16 ± 0.26 | 6.35 ± 0.38 |
| 5 | 24.2 ± 1.2 | 234 ± 32 | 634 ± 98 | 6.73 ± 0.61 | 421 ± 45 | 2.54 ± 0.24 | 5.55 ± 0.38 |
| 10 | 29.4 ± 3.9 | 198 ± 33 | 534 ± 88 | 7.00 ± 1.28 | 466 ± 61 | 2.57 ± 0.32 | 5.19 ± 0.48 |
| 15 | 24.2 ± 1.8 | 248 ± 27 | 610 ± 94 | 8.41 ± 1.31 | 456 ± 50 | 2.77 ± 0.31 | 5.14 ± 0.78 |
| Cutting Intensity (times) | No. of Stolons per Plant | Average Length of Stolons (mm) | Total Length of Stolons (cm) | DM of Stolons (g) | No. of Leaves per Plant | DM of Leaf Petioles (g) | DM of Leaf Blades (g) |
|---|---|---|---|---|---|---|---|
| TF1 | |||||||
| 0 | 40.4 ± 4.4 | 299 ± 22 | 1056 ± 108 | 4.48 ± 0.24 | 488 ± 49 | 2.71 ± 0.16 | 4.39 ± 0.48 |
| 1 | 39.2 ± 3.8 | 289 ± 20 | 1109 ± 64 | 4.58 ± 0.86 | 511 ± 55 | 2.29 ± 0.24 | 4.02 ± 0.17 |
| 2 | 34.0 ± 2.8 | 267 ± 25 | 891 ± 58 | 3.25 ± 0.23 | 355 ± 54 | 2.06 ± 0.06 | 3.10 ± 0.13 |
| 3 | 20.2 ± 2.4 | 235 ± 28 | 474 ± 66 | 1.74 ± 0.20 | 177 ± 11 | 1.32 ± 0.13 | 1.79 ± 0.13 |
| TF2 | |||||||
| 0 | 36.8 ± 2.6 | 360 ± 6 | 1321 ± 87 | 5.19 ± 0.25 | 554 ± 42 | 3.37 ± 0.10 | 4.72 ± 0.09 |
| 1 | 32.0 ± 0.9 | 320 ± 9 | 1023 ± 37 | 3.95 ± 0.09 | 418 ± 14 | 2.89 ± 0.08 | 3.85 ± 0.09 |
| 2 | 31.2 ± 2.6 | 240 ± 7 | 749 ± 67 | 2.74 ± 0.31 | 358 ± 38 | 2.14 ± 0.11 | 2.89 ± 0.13 |
| 3 | 25.6 ± 2.3 | 165 ± 12 | 421 ± 51 | 1.42 ± 0.18 | 238 ± 16 | 1.47 ± 0.09 | 1.70 ± 0.13 |
| TF4 | |||||||
| 0 | 29.8 ± 1.4 | 297 ± 12 | 880 ± 34 | 3.73 ± 0.21 | 338 ± 19 | 2.42 ± 0.13 | 3.19 ± 0.18 |
| 1 | 35.8 ± 2.6 | 250 ± 22 | 873 ± 38 | 3.39 ± 0.22 | 327 ± 8 | 2.41 ± 0.06 | 3.08 ± 0.15 |
| 2 | 28.4 ± 3.5 | 233 ± 24 | 635 ± 53 | 2.43 ± 0.21 | 248 ± 23 | 1.86 ± 0.10 | 2.11 ± 0.17 |
| 3 | 20.0 ± 0.8 | 112 ± 6 | 226 ± 16 | 0.91 ± 0.06 | 112 ± 11 | 0.56 ± 0.05 | 0.75 ± 0.06 |
| TF7 | |||||||
| 0 | 28.6 ± 1.3 | 478 ± 11 | 1370 ± 75 | 6.18 ± 0.45 | 345 ± 20 | 2.26 ± 0.10 | 3.75 ± 0.16 |
| 1 | 23.4 ± 0.4 | 439 ± 5 | 1028 ± 19 | 4.49 ± 0.26 | 272 ± 10 | 2.16 ± 0.14 | 3.28 ± 0.17 |
| 2 | 23.4 ± 1.1 | 350 ± 9 | 818 ± 44 | 2.80 ± 0.25 | 221 ± 15 | 1.62 ± 0.11 | 2.25 ± 0.17 |
| 3 | 13.6 ± 0.7 | 210 ± 10 | 287 ± 26 | 1.10 ± 0.13 | 110 ± 4 | 0.86 ± 0.04 | 1.20 ± 0.07 |
| TF8 | |||||||
| 0 | 20.0 ± 1.5 | 273 ± 26 | 545 ± 64 | 4.95 ±0.49 | 338 ± 44 | 2.53 ± 0.16 | 4.61 ± 0.42 |
| 1 | 23.6 ± 2.4 | 234 ± 27 | 540 ± 50 | 4.17 ± 0.46 | 351 ± 29 | 2.44 ± 0.29 | 4.05 ± 0.52 |
| 2 | 23.0 ± 1.7 | 184 ± 14 | 425 ± 48 | 2.57 ± 0.24 | 223 ± 18 | 1.74 ± 0.16 | 2.66 ± 0.14 |
| 3 | 13.4 ± 1.8 | 115 ± 18 | 159 ± 36 | 1.02 ± 0.22 | 141 ± 25 | 0.95 ± 0.17 | 1.30 ± 0.17 |
| Code | Waterlogging | Flooding | Trampling | Cutting | Average Tolerance |
|---|---|---|---|---|---|
| TF1 | 4 | 1 | 3 | 4 | 3.00 |
| TF2 | 2 | 2 | 4 | 1 | 2.25 |
| TF4 | 1 | 3 | 2 | 2 | 2.00 |
| TF7 | 2 | 1 | 3 | 1 | 1.75 |
| TF8 | 1 | 1 | 1 | 2 | 1.25 |
| Code | Location | Habitat | Coordinates | Year of Seed Collection |
|---|---|---|---|---|
| TF1 | Liepāja | wet saline meadow | 56°29′29″ N, 21°1′38″ E | 2016 |
| TF2 | Jūrmala, Lielupe | saline river bank near estuary | 57°0′11″ N, 23°55′56″ E | 2016 |
| TF4 | Rīga, Skanste | degraded land in urban industrial area | 56°57′46″ N, 24°7′2″ E | 2020 |
| TF7 | Ainaži | dry coastal meadow | 57°52′8″ N, 24°21′10″ E | 2020 |
| TF8 | cv. ‘Palestine’ | NA | NA | 2020 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersone-Ozola, U.; Jēkabsone, A.; Purmale, L.; Romanovs, M.; Ievinsh, G. Abiotic Stress Tolerance of Coastal Accessions of a Promising Forage Species, Trifolium fragiferum. Plants 2021, 10, 1552. https://doi.org/10.3390/plants10081552
Andersone-Ozola U, Jēkabsone A, Purmale L, Romanovs M, Ievinsh G. Abiotic Stress Tolerance of Coastal Accessions of a Promising Forage Species, Trifolium fragiferum. Plants. 2021; 10(8):1552. https://doi.org/10.3390/plants10081552
Chicago/Turabian StyleAndersone-Ozola, Una, Astra Jēkabsone, Līva Purmale, Māris Romanovs, and Gederts Ievinsh. 2021. "Abiotic Stress Tolerance of Coastal Accessions of a Promising Forage Species, Trifolium fragiferum" Plants 10, no. 8: 1552. https://doi.org/10.3390/plants10081552
APA StyleAndersone-Ozola, U., Jēkabsone, A., Purmale, L., Romanovs, M., & Ievinsh, G. (2021). Abiotic Stress Tolerance of Coastal Accessions of a Promising Forage Species, Trifolium fragiferum. Plants, 10(8), 1552. https://doi.org/10.3390/plants10081552







