Intra-Individual and Intraspecific Terpenoid Diversity in Erodium cicutarium
Abstract
:1. Introduction
2. Results and Discussion
2.1. Terpenoids in E. cicutarium Plants of Different Geographical Origin
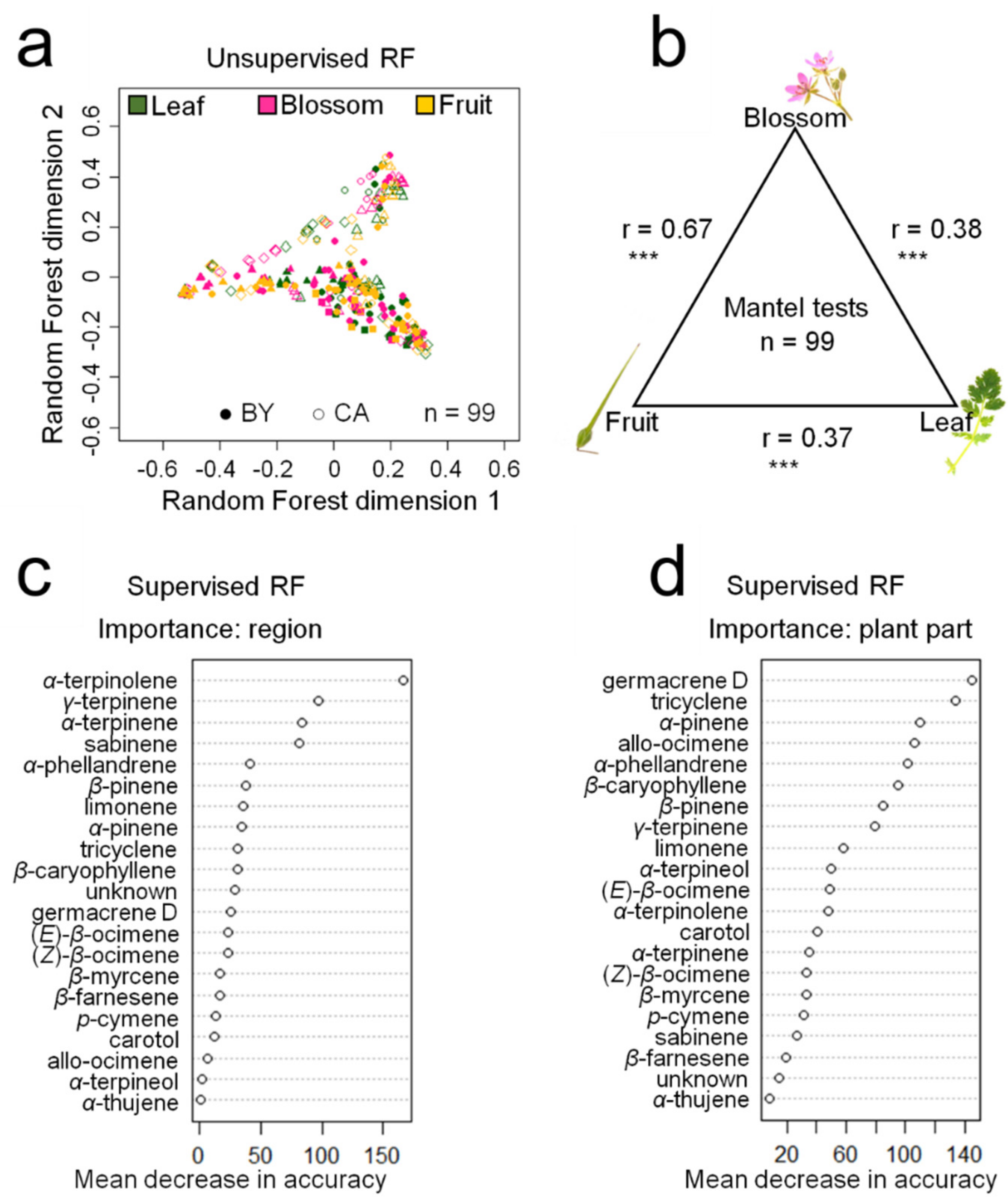
2.2. Ecological Relevance of Terpenoids in E. cicutarium
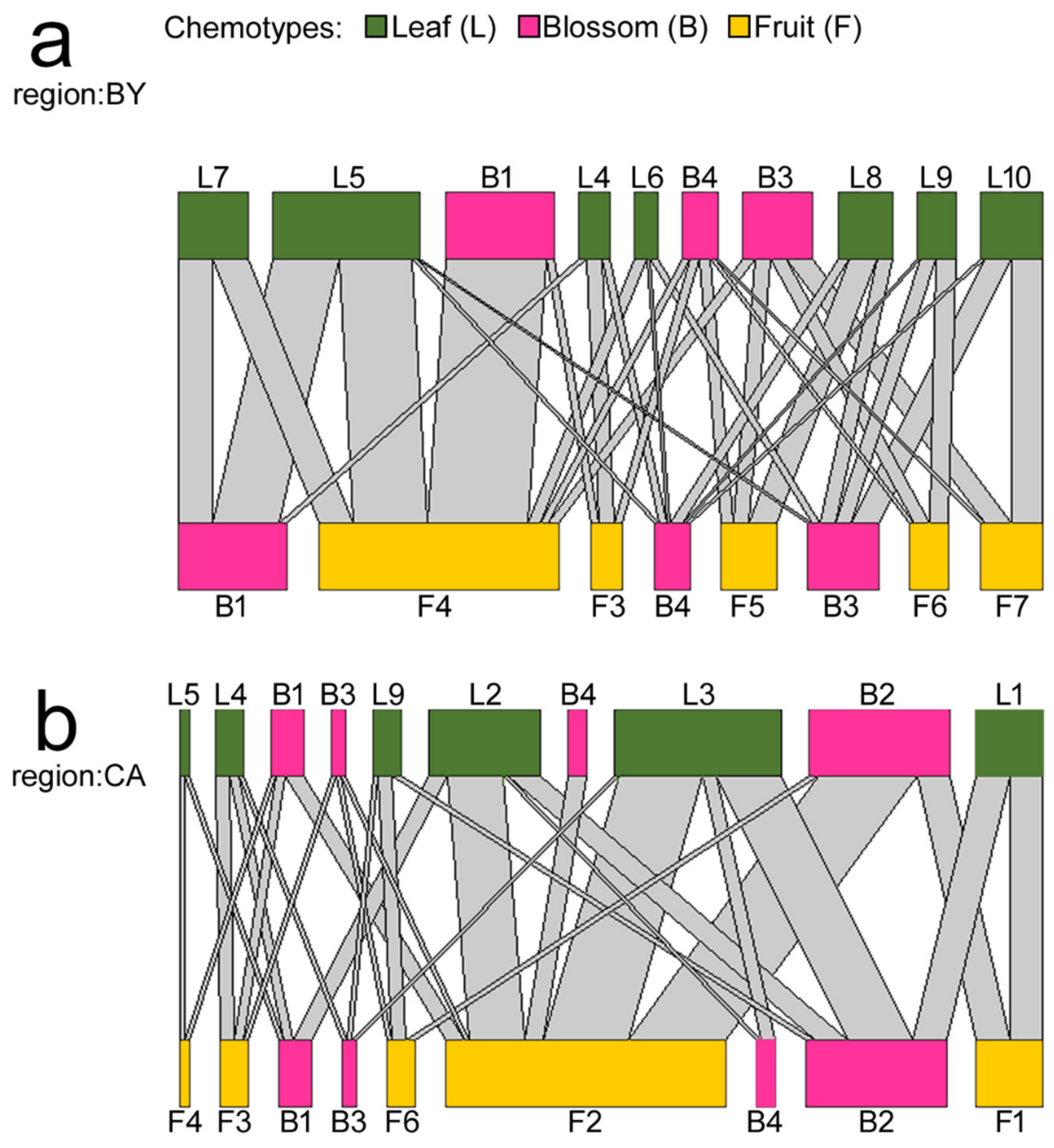
2.3. Terpenoid Chemotypes and Plant Part Specific Combinations Therof in E. cicutarium
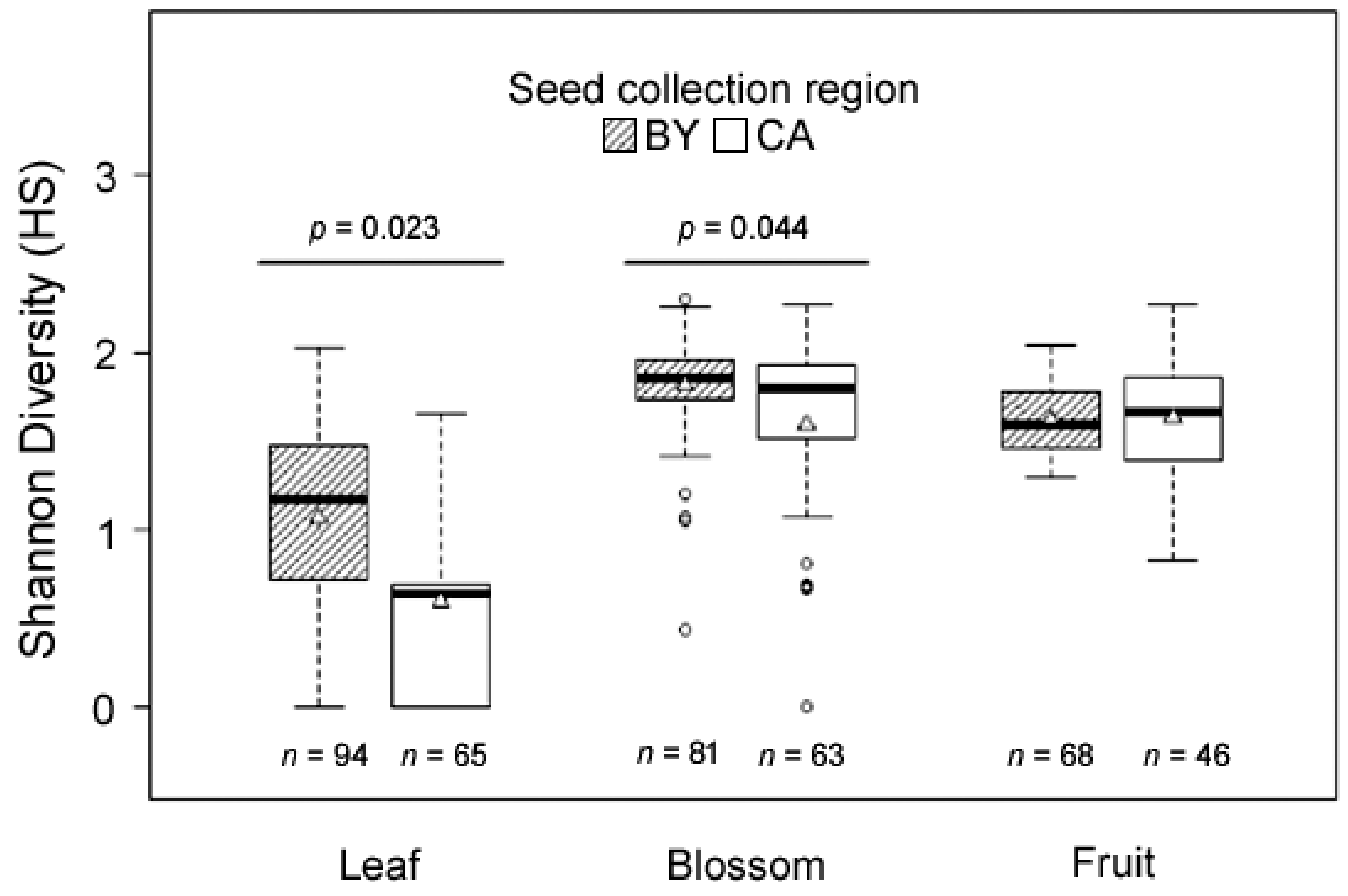
2.4. Intra-Individual and Intraspecific Terpenoid Diversity in E. cicutarium
3. Materials and Methods
3.1. Origin of Plant Seeds
3.2. Cultivation and Harvest of Plants
3.3. Terpenoid Extraction and Analysis of Leaves, Blossoms and Fruits
3.4. Statistical Analyses
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wetzel, W.C.; Whitehead, S.R. The many dimensions of phytochemical diversity: Linking theory to practice. Ecol. Lett. 2020, 23, 16–32. [Google Scholar] [CrossRef]
- Moore, B.D.; Andrew, R.L.; Kulheim, C.; Foley, W.J. Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New. Phytol. 2014, 201, 733–750. [Google Scholar] [CrossRef]
- Jakobs, R.; Müller, C. Effects of intraspecific and intra-individual differences in plant quality on preference and performance of monophagous aphid species. Oecologia 2018, 186, 173–184. [Google Scholar] [CrossRef]
- Müller-Schärer, H.; Schaffner, U. Classical biological control: Exploiting enemy escape to manage plant invasions. Biol. Invasions 2008, 10, 859–874. [Google Scholar] [CrossRef] [Green Version]
- Givnish, T.J. Ecological constraints on the evolution of plasticity in plants. Evol. Ecol. 2002, 16, 213–242. [Google Scholar] [CrossRef]
- Funk, J.L. Differences in plasticity between invasive and native plants from a low resource environment. J. Ecol. 2008, 96, 1162–1173. [Google Scholar] [CrossRef]
- Weidenhamer, J.D.; Hartnett, D.C.; Romeo, J.T. Density-dependent phytotoxicity—Distinguishing resource competition and allelopathic interference in plants. J. Appl. Ecol. 1989, 26, 613–624. [Google Scholar] [CrossRef]
- Jakobs, R.; Schweiger, R.; Müller, C. Aphid infestation leads to plant part-specific changes in phloem sap chemistry, which may indicate niche construction. New Phytol. 2019, 221, 503–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCall, A.C.; Fordyce, J.A. Can optimal defence theory be used to predict the distribution of plant chemical defences? J. Ecol. 2010, 98, 985–992. [Google Scholar] [CrossRef]
- Fiz-Palacios, O.; Vargas, P.; Vila, R.; Papadopulos, A.S.T.; Aldasoro, J.J. The uneven phylogeny and biogeography of Erodium (Geraniaceae): Radiations in the Mediterranean and recent recurrent intercontinental colonization. Ann. Bot. 2010, 106, 871–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Francis, A.; Darbyshire, S.J.; Legere, A.; Simard, M.J. The biology of Canadian weeds. 151. Erodium cicutarium (L.) L’Her. ex Aiton. Can. J. Plant Sci. 2012, 92, 1359–1380. [Google Scholar] [CrossRef]
- Alarcón, M.L.; Roquet, C.; Aldasoro, J.J. Evolution of pollen/ovule ratios and breeding system in Erodium (Geraniaceae). Syst. Bot. 2011, 36, 661–676. [Google Scholar] [CrossRef]
- Mensing, S.; Byrne, R. Pre-mission invasion of Erodium cicutarium in California. J. Biogeogr. 1998, 25, 757–762. [Google Scholar] [CrossRef]
- Forcella, F. Invasive weeds in the northern Rocky Mountains. West. Wildlands 1992, 18, 2–5. [Google Scholar]
- Blackshaw, R.E.; Harker, K.N. Erodium cicutarium density and duration of interference effects on yield of wheat, oilseed rape, pea and dry bean. Weed Res. 1998, 38, 55–62. [Google Scholar] [CrossRef]
- Harker, K.N.; Blackshaw, R.E.; Clayton, G.W. Wild oat (Avena fatua) vs. redstem filaree (Erodium cicutarium) interference in dry pea. Weed Technol. 2007, 21, 235–240. [Google Scholar] [CrossRef]
- Eilers, E.J.; Heger, T. Past competition affects offspring foliar terpenoid concentrations, seed traits, and fitness in the invasive forb Erodium cicutarium (Geraniaceae). Front. Ecol. Evol. 2019, 7, 392. [Google Scholar] [CrossRef] [Green Version]
- Radulovic, N.; Dekic, M.; Stojanovic-Radic, Z.; Palic, R. Volatile constituents of Erodium cicutarium (L.) L’ Hérit. (Geraniaceae). Cent. Eur. J. Biol. 2009, 4, 404–410. [Google Scholar] [CrossRef]
- Stojanovic-Radic, Z.; Comic, L.; Radulovic, N.; Dekic, M.; Randelovic, V.; Stefanovic, O. Chemical composition and antimicrobial activity of Erodium species: E. ciconium L., E. cicutarium L., and E. absinthoides Willd. (Geraniaceae). Chem. Pap. 2010, 64, 368–377. [Google Scholar] [CrossRef]
- Lis-Balchin, M. The essential oils of Pelargonium grossularioides and Erodium cicutarium (Geraniaceae). J. Essent. Oil Res. 1993, 5, 317–318. [Google Scholar] [CrossRef]
- Saleh, N.A.M.; Elkaremy, Z.A.R.; Mansour, R.M.A.; Fayed, A.A.A. A chemosystematic study of some Geraniaceae. Phytochemistry 1983, 22, 2501–2505. [Google Scholar] [CrossRef]
- Fecka, I.; Kowalczyk, A.; Cisowski, W. Phenolic acids and depsides from some species of the Erodium genera. Z. Naturforsch. C 2001, 56, 943–950. [Google Scholar] [CrossRef]
- Tholl, D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr. Opin. Plant Biol. 2006, 9, 297–304. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Johnson, R.H.; Hull-Sanders, H.M.; Meyer, G.A. Comparison of foliar terpenes between native and invasive Solidago gigantea. Biochem. Syst. Ecol. 2007, 35, 821–830. [Google Scholar] [CrossRef]
- Kleine, S.; Müller, C. Intraspecific plant chemical diversity and its relation to herbivory. Oecologia 2011, 166, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Wolf, V.C.; Gassmann, A.; Clasen, B.M.; Smith, A.G.; Müller, C. Genetic and chemical variation of Tanacetum vulgare in plants of native and invasive origin. Biol. Control. 2012, 61, 240–245. [Google Scholar] [CrossRef]
- Lukas, B.; Schmiderer, C.; Novak, J. Essential oil diversity of European Origanum vulgare L. (Lamiaceae). Phytochemistry 2015, 119, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Loreto, F.; Bagnoli, F.; Fineschi, S. One species, many terpenes: Matching chemical and biological diversity. Trends Plant Sci. 2009, 14, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Bustos-Segura, C.; Kulheim, C.; Foley, W. Effects of terpene chemotypes of Melaleuca alternifolia on two specialist leaf beetles and susceptibility to myrtle rust. J. Chem. Ecol. 2015, 41, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Duan, Q.X.; Bonn, B.; Kreuzwieser, J. Terpenoids are transported in the xylem sap of Norway spruce. Plant Cell Environ. 2020, 43, 1766–1778. [Google Scholar] [CrossRef] [Green Version]
- Boachon, B.; Lynch, J.H.; Ray, S.; Yuan, J.; Caldo, K.M.P.; Junker, R.R.; Kessler, S.A.; Morgan, J.A.; Dudareva, N. Natural fumigation as a mechanism for volatile transport between flower organs. Nat. Chem. Biol. 2019, 15, 583–588. [Google Scholar] [CrossRef] [PubMed]
- Judzentiene, A.; Mockute, D. The inflorescence and leaf essential oils of Tanacetum vulgare L. var. vulgare growing wild in Lithuania. Biochem. Syst. Ecol. 2005, 33, 487–498. [Google Scholar] [CrossRef]
- Eilers, E.J.; Kleine, S.; Eckert, S.; Waldherr, S.; Müller, C. Flower production, headspace volatiles, pollen nutrients, and florivory in Tanacetum vulgare chemotypes. Front. Plant Sci. 2021, 11, 611877. [Google Scholar] [CrossRef] [PubMed]
- Clark, E.L.; Carroll, A.L.; Huber, D.P.W. Differences in the constitutive terpene profile of lodgepole pine across a geographical range in British Columbia, and correlation with historical attack by mountain pine beetle. Can. Entomol. 2010, 142, 557–573. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Eriksson, R.; Gershenzon, J.; Stahl, B. Diversity and distribution of floral scent. Bot. Rev. 2006, 72, 1. [Google Scholar] [CrossRef]
- Nason, J.D.; Herre, E.A.; Hamrick, J.L. Paternity analysis of the breeding structure of strangler fig populations: Evidence for substantial long-distance wasp dispersal. J. Biogeogr. 1996, 23, 501–512. [Google Scholar] [CrossRef]
- Baythavong, B.S.; Stanton, M.L.; Rice, K.J. Understanding the consequences of seed dispersal in a heterogeneous environment. Ecology 2009, 90, 2118–2128. [Google Scholar] [CrossRef]
- Kimball, S.; Gremer, J.R.; Barron-Gafford, G.A.; Angert, A.L.; Huxman, T.E.; Venable, D.L. High water-use efficiency and growth contribute to success of non-native Erodium cicutarium in a Sonoran Desert winter annual community. Conserv. Physiol. 2014, 2, cou006. [Google Scholar] [CrossRef] [Green Version]
- Strauss, S.Y.; Stanton, M.L.; Emery, N.C.; Bradley, C.A.; Carleton, A.; Dittrich-Reed, D.R.; Ervin, O.A.; Gray, L.N.; Hamilton, A.M.; Rogge, J.H.; et al. Cryptic seedling herbivory by nocturnal introduced generalists impacts survival, performance of native and exotic plants. Ecology 2009, 90, 419–429. [Google Scholar] [CrossRef]
- Martin, G.R.; Twigg, L.E.; Zampichelli, L. Seasonal changes in the diet of the European rabbit (Oryctolagus cuniculus) from three different Mediterranean habitats in south-western Australia. Wildlife Res. 2007, 34, 25–42. [Google Scholar] [CrossRef]
- Bhadresa, R. Food preferences of rabbits Oryctolagus cuniculus L. at Holkham sand dunes, Norfolk. J. Appl. Ecol. 1977, 14, 287–291. [Google Scholar] [CrossRef]
- Fischer, N.H.; Williamson, G.B.; Weidenhamer, J.D.; Richardson, D.R. In search of allelopathy in the florida scrub—The role of terpenoids. J. Chem. Ecol. 1994, 20, 1355–1380. [Google Scholar] [CrossRef]
- Ens, E.J.; Bremner, J.B.; French, K.; Korth, J. Identification of volatile compounds released by roots of an invasive plant, bitou bush (Chrysanthemoides monilifera spp. rotundata), and their inhibition of native seedling growth. Biol. Invasions 2009, 11, 275–287. [Google Scholar]
- Viljoen, A.M.; Vanderwalt, J.J.A.; Demarne, F.E.; Swart, J.P.J. A study of the variation in the essential oil and morphology of Pelargonium capitatum (L.) L’Herit (Geraniaceae). III. Geographical variation in essential oil composition and floral structure. S. Afr. J. Bot. 1995, 61, 105–113. [Google Scholar] [CrossRef] [Green Version]
- Junker, R.R.; Kuppler, J.; Amo, L.; Blande, J.D.; Borges, R.M.; van Dam, N.M.; Dicke, M.; Dötterl, S.; Ehlers, B.K.; Etl, F.; et al. Covariation and phenotypic integration in chemical communication displays: Biosynthetic constraints and eco-evolutionary implications. New Phytol. 2018, 220, 739–749. [Google Scholar] [CrossRef]
- Bohlmann, J.; Steele, C.L.; Croteau, R. Monoterpene synthases from Grand fir (Abies grandis)—cDNA isolation, characterization, and functional expression of myrcene synthase, (-)(4S)-limonene synthase, and (-)-(1S,5S)-pinene synthase. J. Biol. Chem. 1997, 272, 21784–21792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, V.C.; Berger, U.; Gassmann, A.; Müller, C. High chemical diversity of a plant species is accompanied by increased chemical defence in invasive populations. Biol. Invasions 2011, 13, 2091–2102. [Google Scholar] [CrossRef]
- Keane, R.M.; Crawley, M.J. Exotic plant invasions and the enemy release hypothesis. Trends Ecol. Evol. 2002, 17, 164–170. [Google Scholar] [CrossRef]
- Heger, T.; Jacobs, B.S.; Latimer, A.M.; Kollmann, J.; Rice, K.J. Does experience with competition matter? Effects of source competitive environment on mean and plastic trait expression in Erodium cicutarium. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 236–246. [Google Scholar] [CrossRef]
- Kleine, S.; Müller, C. Differences in shoot and root terpenoid profiles and plant responses to fertilisation in Tanacetum vulgare. Phytochemistry 2013, 96, 123–131. [Google Scholar] [CrossRef]
- Kováts, V.E. Gas-chromatographische charakterisierung organischer verbindungen. Teil 1: Retentionsindices aliphatischer halogenide, alkohole, aldehyde und ketone. Helv. Chim. Acta 1958, 41, 1915–1932. [Google Scholar] [CrossRef]
- El-Sayed, A.M. The Pherobase: Database of Pheromones and Semiochemicals. 2012. Available online: http://www.pherobase.com (accessed on 28 June 2021).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; ISBN 3-900051-07-0. Available online: http://www.R-project.org (accessed on 28 June 2021).
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Oksanen, J.; Blanchet, F.G.; Kindt, R.; Legendre, P.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; Stevens, M.H.H.; Szoecs, E.; et al. Vegan: Community Ecology Package. R Package Version 2.5-7. 2020. Available online: https://cran.r-project.org/web/packages/vegan/vegan.pdf (accessed on 30 July 2021).
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Fitting linear mixed-effects models using lme4. J. Stat. Softw. 2015, 67, 1–48. [Google Scholar] [CrossRef]
- Ward, J.H., Jr. Hierarchical grouping to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Maechler, M.; Rousseeuw, P.; Struyf, A.; Hubert, M.; Hornik, K. Cluster: Cluster Analysis Basics and Extensions. R Package Version 2.1-2. 2012. Available online: https://CRAN.R-project.org/package=cluster (accessed on 30 July 2021).
- Dormann, C.; Gruber, B.; Fründ, J. Introducing the bipartite package: Analysing ecological networks. Interaction 2008, 8, 8–11. [Google Scholar]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]

| Mean Content (%) ± s.d. | Detected in % of Samples | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| # | Rt | Name | Leaf | Blossom | Fruit | Leaf | Blossom | Fruit | BY | CA | |
| 1 | 930 | tricyclene | M | 0.10 ± 0.56 | 4.99 ± 4.50 | 4.26 ± 1.95 | 3 | 74 | 97 | 58 | 47 |
| 2 | 932 | α-thujene | M | 0 | 0.03 ± 0.21 | 0 | 0 | 2 | 0 | 1 | 0 |
| 3 | 941 | α-pinene * | M | 0.33 ± 1.14 | 5.27 ± 3.62 | 3.75 ± 1.30 | 11 | 71 | 97 | 65 | 52 |
| 4 | 976 | Sabinene * | M | 1.89 ± 4.27 | 2.36 ± 3.39 | 2.35 ± 2.55 | 23 | 56 | 89 | 68 | 31 |
| 5 | 986 | β-pinene * | M | 8.47 ± 11.63 | 22.34 ± 17.57 | 12.22 ± 9.11 | 42 | 76 | 87 | 66 | 65 |
| 6 | 990 | β-myrcene | M | 0.65 ± 1.96 | 1.10 ± 1.59 | 1.60 ± 0.91 | 11 | 40 | 85 | 47 | 33 |
| 7 | 1012 | α-phellandrene | M | 0.22 ± 1.23 | 5.48 ± 10.87 | 3.14 ± 5.54 | 4 | 41 | 50 | 28 | 32 |
| 8 | 1024 | α-terpinene | M | 0.41 ± 1.46 | 1.51 ± 2.94 | 0.60 ± 0.75 | 8 | 44 | 61 | 23 | 51 |
| 9 | 1031 | p-cymene | M | 0 | 0 | 1.11 ± 3.87 | 0 | 0 | 23 | 9 | 3 |
| 10 | 1037 | Limonene * | M | 4.26 ± 9.52 | 6.09 ± 9.37 | 1.77 ± 1.14 | 36 | 66 | 77 | 65 | 48 |
| 11 | 1040 | (Z)-β-ocimene | M | 0.92 ± 4.14 | 5.26 ± 10.18 | 4.13 ± 6.32 | 6 | 39 | 75 | 42 | 29 |
| 12 | 1050 | (E)-β-ocimene | M | 0.08 ± 0.34 | 1.42 ± 1.83 | 1.28 ± 0.81 | 5 | 48 | 84 | 48 | 33 |
| 13 | 1065 | γ-terpinene | M | 10.25 ± 11.56 | 7.83 ± 6.21 | 3.97 ± 2.53 | 54 | 84 | 88 | 92 | 49 |
| 14 | 1090 | α-terpinolene | M | 25.24 ± 34.98 | 13.32 ± 21.52 | 5.94 ± 8.34 | 36 | 32 | 44 | 4 | 82 |
| 15 | 1131 | allo-ocimene | M | 0 | 0.14 ± 0.51 | 1.44 ± 1.49 | 0 | 8 | 92 | 29 | 26 |
| 16 | 1206 | α-terpineol | M | 0 | 0.02 ± 0.16 | 0.29 ± 0.38 | 0 | 3 | 49 | 14 | 14 |
| 17 | 1447 | β-caryophyllene * | S | 0.54 ± 1.63 | 4.76 ± 5.22 | 0.65 ± 0.59 | 11 | 69 | 64 | 46 | 45 |
| 18 | 1456 | β-farnesene | S | 0.78 ± 2.54 | 0.93 ± 2.08 | 0.44 ± 1.30 | 11 | 33 | 42 | 26 | 29 |
| 19 | 1506 | germacrene D * | S | 35.00 ± 25.63 | 14.76 ± 9.35 | 50.39 ± 13.11 | 84 | 90 | 100 | 94 | 86 |
| 20 | 1523 | unknown | S? | 0.33 ± 1.58 | 0.21 ± 1.02 | 0.58 ± 1.49 | 4 | 7 | 16 | 5 | 14 |
| 21 | 1638 | carotol | S | 4.24 ± 17.32 | 0.81 ± 1.36 | 0.10 ± 0.15 | 7 | 33 | 30 | 18 | 29 |
| Geographical Region | |||
|---|---|---|---|
| Plant Part | Chemotype No. | Bavaria | California |
| Leaf | L1 | - | 7 |
| Leaf | L2 | - | 12 |
| Leaf | L3 | - | 18 |
| Leaf | L4 | 4 | 3 |
| Leaf | L5 | 19 | 1 |
| Leaf | L6 | 3 | - |
| Leaf | L7 | 9 | - |
| Leaf | L8 | 7 | - |
| Leaf | L9 | 5 | 3 |
| Leaf | L10 | 8 | - |
| Blossom | B1 | 28 | 7 |
| Blossom | B2 | - | 30 |
| Blossom | B3 | 18 | 3 |
| Blossom | B4 | 9 | 4 |
| Fruit | F1 | - | 7 |
| Fruit | F2 | - | 30 |
| Fruit | F3 | 4 | 3 |
| Fruit | F4 | 31 | 1 |
| Fruit | F5 | 7 | - |
| Fruit | F6 | 5 | 3 |
| Fruit | F7 | 8 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eilers, E.J. Intra-Individual and Intraspecific Terpenoid Diversity in Erodium cicutarium. Plants 2021, 10, 1574. https://doi.org/10.3390/plants10081574
Eilers EJ. Intra-Individual and Intraspecific Terpenoid Diversity in Erodium cicutarium. Plants. 2021; 10(8):1574. https://doi.org/10.3390/plants10081574
Chicago/Turabian StyleEilers, Elisabeth Johanna. 2021. "Intra-Individual and Intraspecific Terpenoid Diversity in Erodium cicutarium" Plants 10, no. 8: 1574. https://doi.org/10.3390/plants10081574
APA StyleEilers, E. J. (2021). Intra-Individual and Intraspecific Terpenoid Diversity in Erodium cicutarium. Plants, 10(8), 1574. https://doi.org/10.3390/plants10081574






