Do Bryophyte Elemental Concentrations Explain Their Morphological Traits?
Abstract
:1. Introduction
2. Methods
2.1. Data Collection and Analyses of Elemental Composition and Morphological Traits
2.2. Statistical Analyses
3. Results
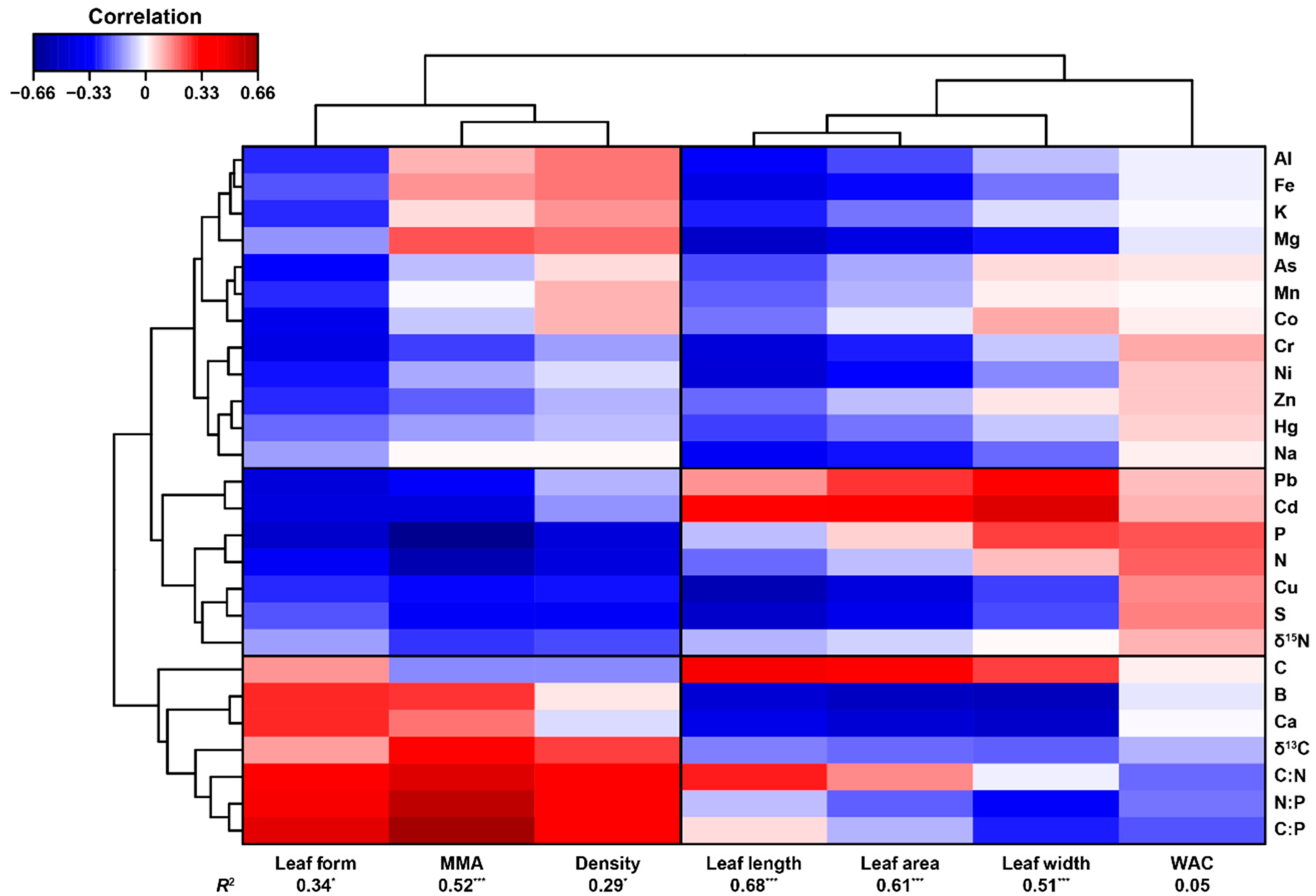
3.1. Elementome Similarity amongst Moss Species
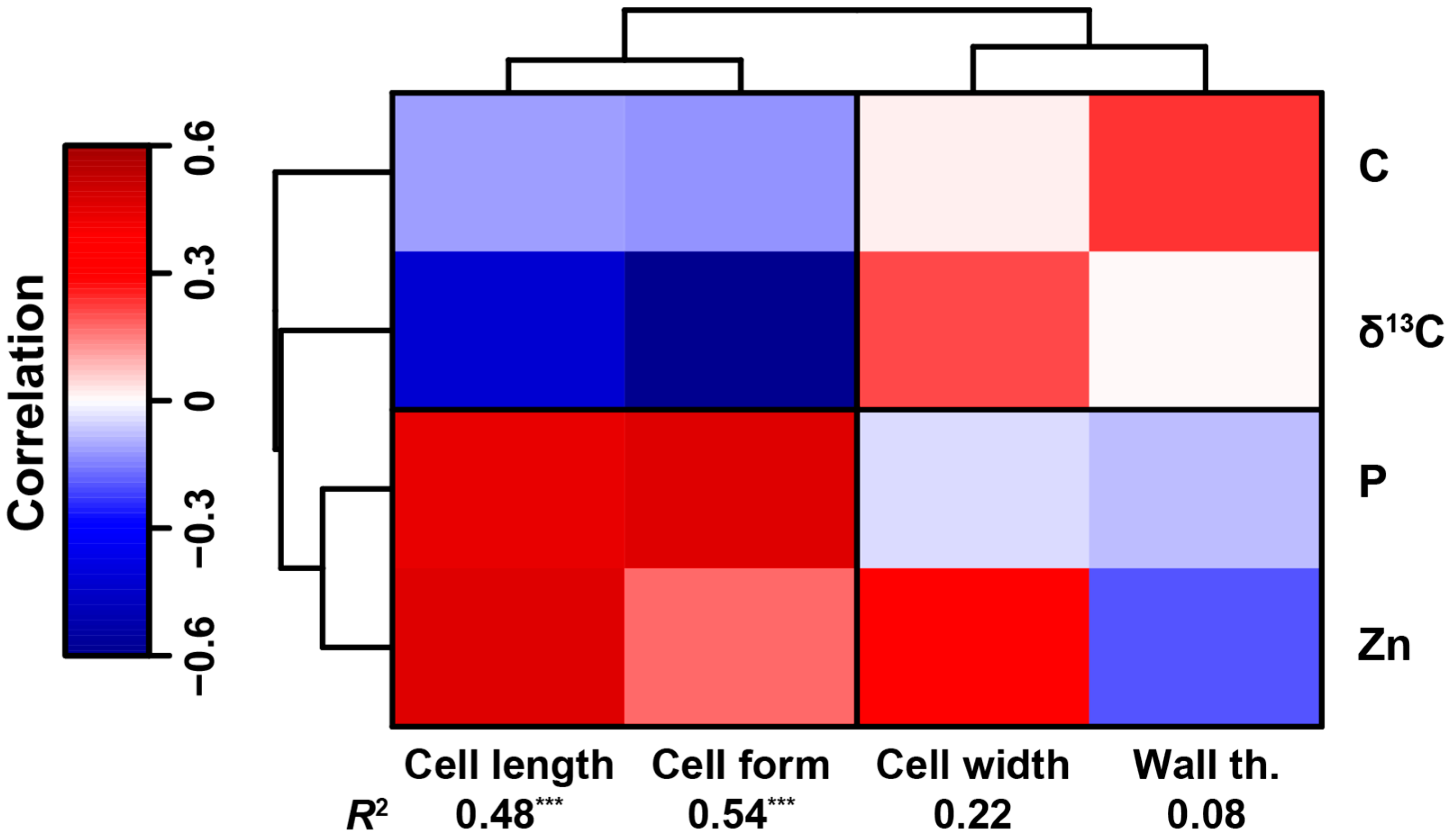
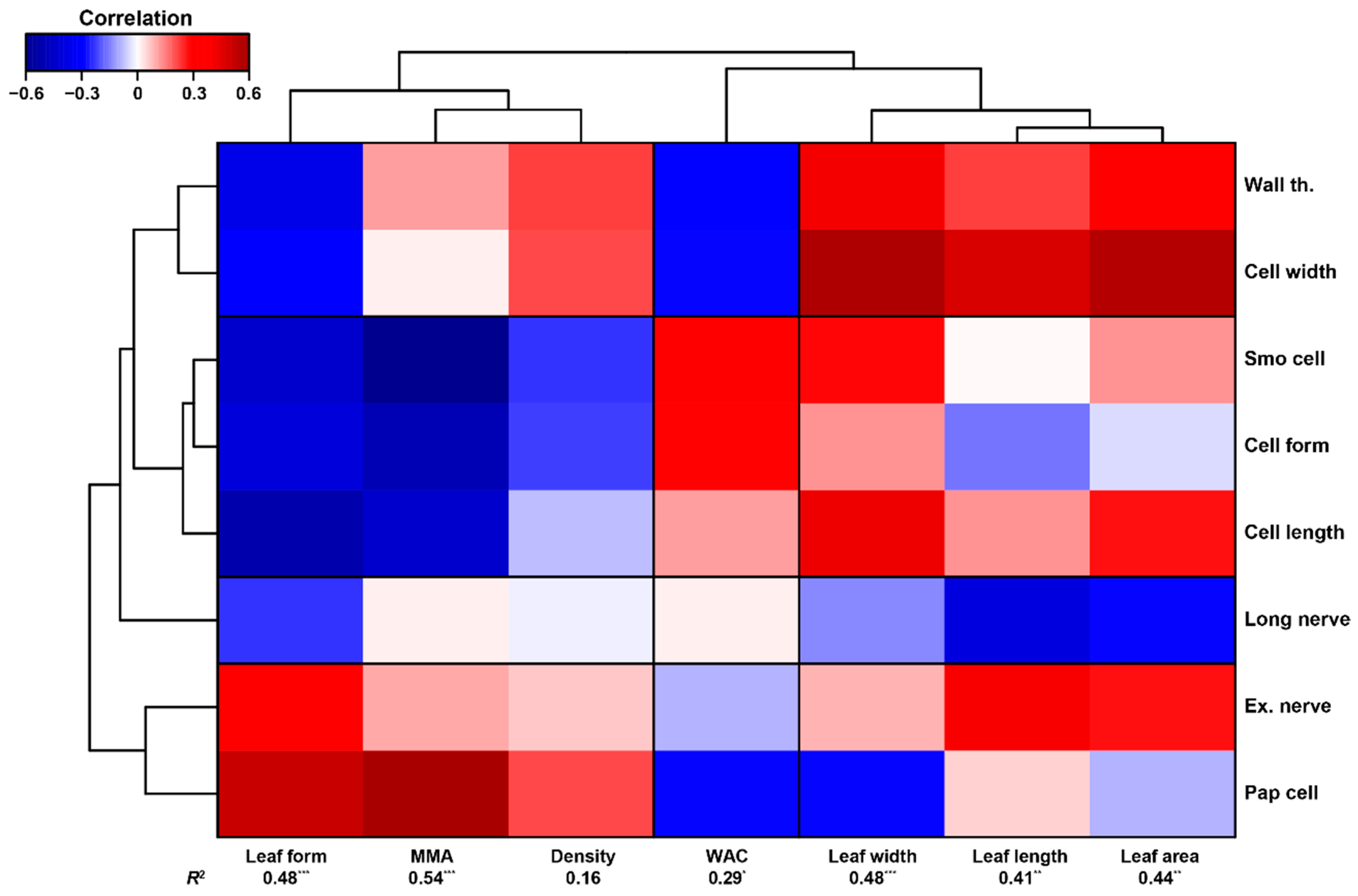
3.2. Cell Traits and Their Relationship with Moss Elemental Composition and Macroscopic Traits
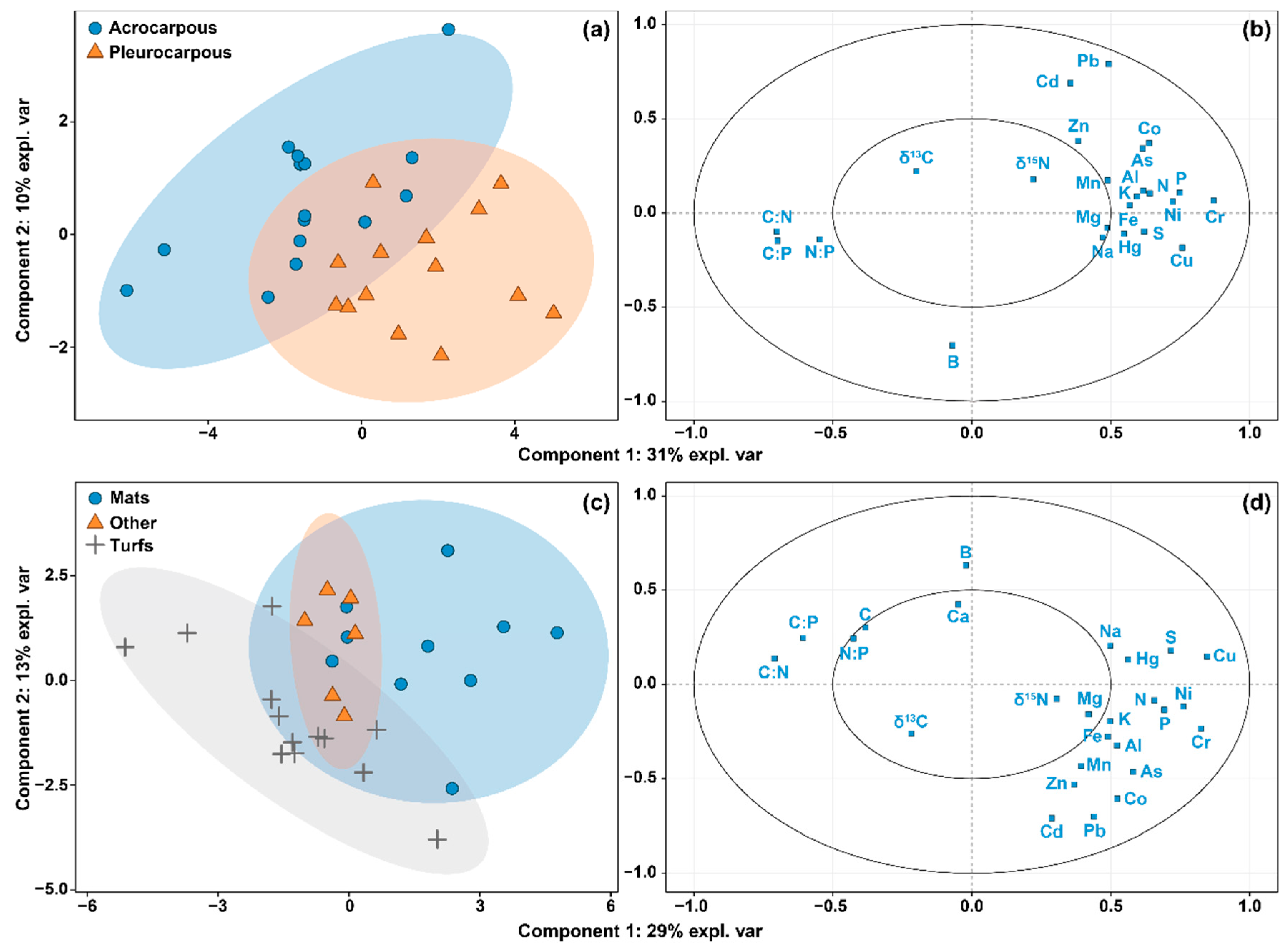
3.3. Relationship between Moss Elemental Composition, Morphological Traits, and Growth Forms
4. Discussion
4.1. Elementome Differences between Species
4.2. Moss Elemental Composition Controls Micro- and Macroscopic Morphological Traits across Species
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Porley, R.; Hodgetts, N.G. Mosses and Liverworts, 1st ed.; HarperCollins Publishers: London, UK, 2005; ISBN 0-00-220212-3. [Google Scholar]
- Fernández-Martínez, M.; Corbera, J.; Domene, X.; Sayol, F.; Sabater, F.; Preece, C. Nitrate pollution reduces bryophyte diversity in Mediterranean springs. Sci. Total Environ. 2020, 705, 135823. [Google Scholar] [CrossRef] [PubMed]
- Say, P.J.; Whitton, B.A. Accumulation of heavy metals by aquatic mosses. 1: Fontinalis antipyretica Hedw. Hydrobiologia 1983, 100, 245–260. [Google Scholar] [CrossRef]
- Martins, R.J.E.; Pardo, R.; Boaventura, R.A.R. Cadmium(II) and zinc(II) adsorption by the aquatic moss Fontinalis antipyretica: Effect of temperature, pH and water hardness. Water Res. 2004, 38, 693–699. [Google Scholar] [CrossRef]
- Rao, D.N. Responses of Bryophytes to Air Pollution. In Bryophyte Ecology; Springer: Dordrecht, The Netherlands, 1982; pp. 445–471. [Google Scholar]
- Morgan, S.; Lee, J.; Ashenden, T. Effects of Nitrogen-Oxides on Nitrate Assimilation in Bryophytes. New Phytol. 1992, 120, 89–97. [Google Scholar] [CrossRef]
- Soares, A.; Pearson, J. Short-term physiological responses of mosses to atmospheric ammonium and nitrate. Water Air Soil Pollut. 1997, 93, 225–242. [Google Scholar] [CrossRef]
- Huang, J.; Liu, W.; Li, S.; Song, L.; Lu, H.; Shi, X.; Chen, X.; Hu, T.; Liu, S.; Liu, T. Ecological stoichiometry of the epiphyte community in a subtropical forest canopy. Ecol. Evol. 2019. [Google Scholar] [CrossRef]
- Steinman, A.D. The influence of phosphorus enrichment on lotic bryophytes. Freshw. Biol. 1994, 31, 53–63. [Google Scholar] [CrossRef]
- Waite, M.; Sack, L. Does global stoichiometric theory apply to bryophytes? Tests across an elevation × soil age ecosystem matrix on Mauna Loa, Hawaii. J. Ecol. 2011, 99, 122–134. [Google Scholar] [CrossRef]
- Hájek, M.; Plesková, Z.; Syrovátka, V.; Peterka, T.; Laburdová, J.; Kintrová, K.; Jiroušek, M.; Hájek, T. Patterns in moss element concentrations in fens across species, habitats, and regions. Perspect. Plant Ecol. Evol. Syst. 2014, 16, 203–218. [Google Scholar] [CrossRef]
- García-Álvaro, M.A.; Martínez-Abaigar, J.; Núñez-Olivera, E.; Beaucourt, N. Element concentrations and enrichment ratios in the aquatic moss Rhynchostegium riparioides along the River Iregua (La Rioja, northern Spain). Bryologist 2000, 103, 518–533. [Google Scholar] [CrossRef]
- Güsewell, S. N:P ratios in terrestrial plants: Variation and functional significance. New Phytol. 2004, 164, 243–266. [Google Scholar] [CrossRef] [PubMed]
- Sterner, R.W.; Elser, J.J. Ecological Stoichiometry: The Biology of Elements from Molecules to the Biosphere; Princeton University Press: Princeton, NJ, USA, 2002; ISBN 0691074909. [Google Scholar]
- Peñuelas, J.; Fernández-Martínez, M.; Ciais, P.; Jou, D.; Piao, S.; Obersteiner, M.; Vicca, S.; Janssens, I.A.; Sardans, J. The bioelements, the elementome, and the biogeochemical niche. Ecology 2019, 100, e02652. [Google Scholar] [CrossRef] [PubMed]
- Sardans, J.; Peñuelas, J. Potassium: A neglected nutrient in global change. Glob. Ecol. Biogeogr. 2015, 24, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Díaz, S.; Kattge, J.; Cornelissen, J.H.C.; Wright, I.J.; Lavorel, S.; Dray, S.; Reu, B.; Kleyer, M.; Wirth, C.; Colin Prentice, I.; et al. The global spectrum of plant form and function. Nature 2016, 529, 167–171. [Google Scholar] [CrossRef]
- Sardans, J.; Vallicrosa, H.; Zuccarini, P.; Farré-Armengol, G.; Fernández-Martínez, M.; Peguero, G.; Gargallo-Garriga, A.; Ciais, P.; Janssens, I.A.; Obersteiner, M.; et al. Empirical support for the biogeochemical niche hypothesis in forest trees. Nat. Ecol. Evol. 2021, 5, 184–194. [Google Scholar] [CrossRef] [PubMed]
- Leal, M.C.; Seehausen, O.; Matthews, B. The Ecology and Evolution of Stoichiometric Phenotypes. Trends Ecol. Evol. 2017, 32, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, M.; Llusià, J.; Filella, I.; Niinemets, Ü.; Arneth, A.; Wright, I.J.; Loreto, F.; Peñuelas, J. Nutrient-rich plants emit a less intense blend of volatile isoprenoids. New Phytol. 2018, 220, 773–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Martínez, M.; Sardans, J.; Sayol, F.; LaMontagne, J.M.; Bogdziewicz, M.; Collalti, A.; Hacket-Pain, A.; Vacchiano, G.; Espelta, J.M.; Peñuelas, J.; et al. Reply to: Nutrient scarcity cannot cause mast seeding. Nat. Plants 2020, 6, 763–765. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Waite, M.; Sack, L. How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiian species of contrasting light habitats. New Phytol. 2010, 185, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Bader, M.Y.; Feng, D.; Bao, W. The ‘plant economic spectrum’ in bryophytes, a comparative study in subalpine forest. Am. J. Bot. 2017, 104, 261–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Martínez, M.; Berloso, F.; Corbera, J.; Garcia-Porta, J.; Sayol, F.; Preece, C.; Sabater, F. Towards a moss sclerophylly continuum: Evolutionary history, water chemistry and climate control traits of hygrophytic mosses. Funct. Ecol. 2019, 33, 2273–2289. [Google Scholar] [CrossRef]
- Löbel, S.; Mair, L.; Lönnell, N.; Schröder, B.; Snäll, T. Biological traits explain bryophyte species distributions and responses to forest fragmentation and climatic variation. J. Ecol. 2018, 106, 1700–1713. [Google Scholar] [CrossRef] [Green Version]
- Hill, M.O.; Preston, C.D.; Bosanquet, S.D.S.; Roy, D.B. BRYOATT: Attributes of British and Irish Bryophytes; Centre for Ecology and Hydrology: Bailrigg, UK, 2007; ISBN 9781855312364. [Google Scholar]
- Laine, A.M.; Lindholm, T.; Nilsson, M.; Kutznetsov, O.; Jassey, V.E.J.; Tuittila, E.S. Functional diversity and trait composition of vascular plant and Sphagnum moss communities during peatland succession across land uplift regions. J. Ecol. 2021, 1774–1789. [Google Scholar] [CrossRef]
- Kangas, L.; Maanavilja, L.; Hájek, T.; Juurola, E.; Chimner, R.A.; Mehtätalo, L.; Tuittila, E.S. Photosynthetic traits of Sphagnum and feather moss species in undrained, drained and rewetted boreal spruce swamp forests. Ecol. Evol. 2014, 4, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, M.; Preece, C.; Corbera, J.; Cano, O.; Garcia-Porta, J.; Sardans, J.; Janssens, I.A.; Sabater, F.; Peñuelas, J. Bryophyte C:N:P stoichiometry, biogeochemical niches and elementome plasticity driven by environment and coexistence. Ecol. Lett. 2021, 24, 1375–1386. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Preece, C.; Corbera, J.; Cano, O.; Garcia-Porta, J.; Bogdziewicz, M.; Sardans, J.; Janssens, I.A.; Sabater, F.; Peñuelas, J. Nutrients control reproductive traits of hygrophytic bryophytes. Freshw. Biol. 2021, 66, 1436–1446. [Google Scholar] [CrossRef]
- Fernández-Martínez, M.; Margalef, O.; Sayol, F.; Asensio, D.; Bagaria, G.; Corbera, J.; Sabater, F.; Domene, X.; Preece, C. Sea spray influences water chemical composition of Mediterranean semi-natural springs. Catena 2019, 173, 414–423. [Google Scholar] [CrossRef]
- Martín-Vide, J. El Clima Geografia General dels Països Catalans. In Enciclopèdia Catalana; Carreras, C., Ed.; Enciclopèdia Catalana SAU: Barcelona, Spain, 1992; pp. 1–110. [Google Scholar]
- Le Cao, K.-A.; Rohart, F.; Gonzalez, I.; Dejean, S.; Gautier, B.; Bartolo, F.; Monget, P.; Coquery, J.; Yao, F.; Liquet, B.; et al. mixOmics: Omics Data Integration Project. Available online: http://mixomics.org/ (accessed on 15 June 2017).
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019; Volume 1, p. 409. [Google Scholar]
- Smith, A.J.E. The Moss Flora of Britain and Ireland; Cambridge University Press: Cambridge, UK, 1978. [Google Scholar]
- Guerra, J.; Cano, M.J.; Brugués, M. (Eds.) Hypnales; Sociedad Española de Briología: Murcia, Flora, 2018; Volume VI, ISBN 978-84-697-9126-4. [Google Scholar]
- Hofmann, P.; Clark, A.; Hoffmann, P.; Chatzinotas, A.; Harpole, W.S.; Dunker, S. Beyond nitrogen: Phosphorus—Estimating the minimum niche dimensionality for resource competition between phytoplankton. Ecol. Lett. 2021. [Google Scholar] [CrossRef]
- Rivas-Ubach, A.; Sardans, J.; Peŕez-Trujillo, M.; Estiarte, M.; Penũelas, J. Strong relationship between elemental stoichiometry and metabolome in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 4181–4186. [Google Scholar] [CrossRef] [Green Version]
- Shipley, B.; Lechowicz, M.J.; Wright, I.; Reich, P.B. Fundamental trade-offs generating the worldwide leaf economics spectrum. Ecology 2006, 87, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Boquete, M.T.; Lang, I.; Weidinger, M.; Richards, C.L.; Alonso, C. Patterns and mechanisms of heavy metal accumulation and tolerance in two terrestrial moss species with contrasting habitat specialization. Environ. Exp. Bot. 2021, 182, 104336. [Google Scholar] [CrossRef]
- Petschinger, K.; Adlassnig, W.; Sabovljevic, M.S.; Lang, I. Lamina cell shape and cell wall thickness are useful indicators for metal tolerance—An example in bryophytes. Plants 2021, 10, 274. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Martínez, M.; Corbera, J.; Cano-Rocabayera, O.; Sabater, F.; Preece, C. Do Bryophyte Elemental Concentrations Explain Their Morphological Traits? Plants 2021, 10, 1581. https://doi.org/10.3390/plants10081581
Fernández-Martínez M, Corbera J, Cano-Rocabayera O, Sabater F, Preece C. Do Bryophyte Elemental Concentrations Explain Their Morphological Traits? Plants. 2021; 10(8):1581. https://doi.org/10.3390/plants10081581
Chicago/Turabian StyleFernández-Martínez, Marcos, Jordi Corbera, Oriol Cano-Rocabayera, Francesc Sabater, and Catherine Preece. 2021. "Do Bryophyte Elemental Concentrations Explain Their Morphological Traits?" Plants 10, no. 8: 1581. https://doi.org/10.3390/plants10081581
APA StyleFernández-Martínez, M., Corbera, J., Cano-Rocabayera, O., Sabater, F., & Preece, C. (2021). Do Bryophyte Elemental Concentrations Explain Their Morphological Traits? Plants, 10(8), 1581. https://doi.org/10.3390/plants10081581






