Single and Combined Salinity and Heat Stresses Impact Yield and Dead Pericarp Priming Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Growth Conditions and Exposure to Stress
2.2. Plant Hormone Analysis
2.3. Nutrient Analysis
2.4. Germination Assays
2.5. Priming Experiments
2.6. Bacterial Growth Assay
2.7. Statistical Analysis
3. Results
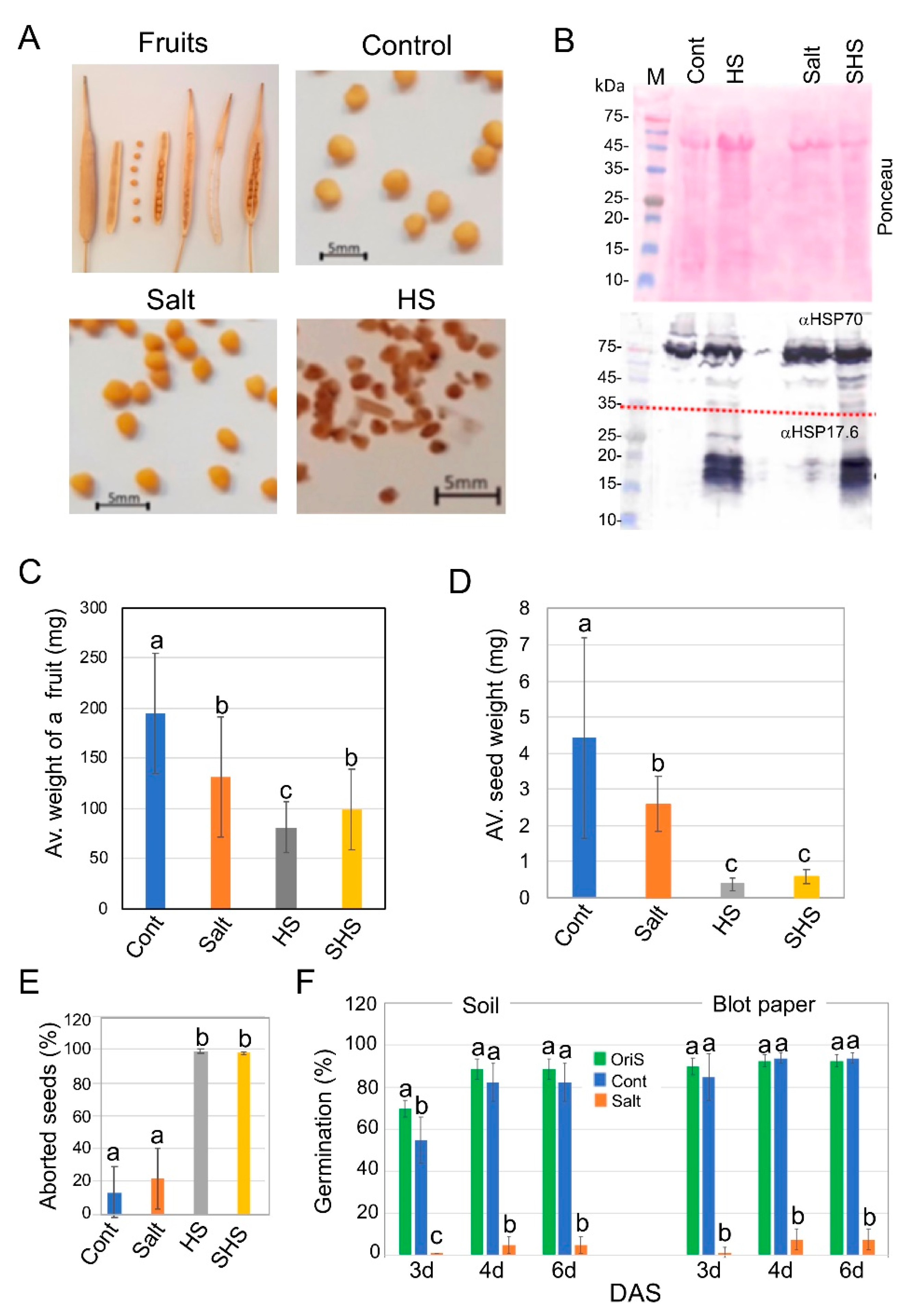
3.1. Exposure to Salinity and Heat Stress Has a Dramatic Impact on Progeny Seed Production of Brassica juncea
3.2. The Effect of Pericarp Extracts on Seed Germination
3.3. Single and Combined Stresses Altered Phytohormone Accumulation in Dead Pericarps
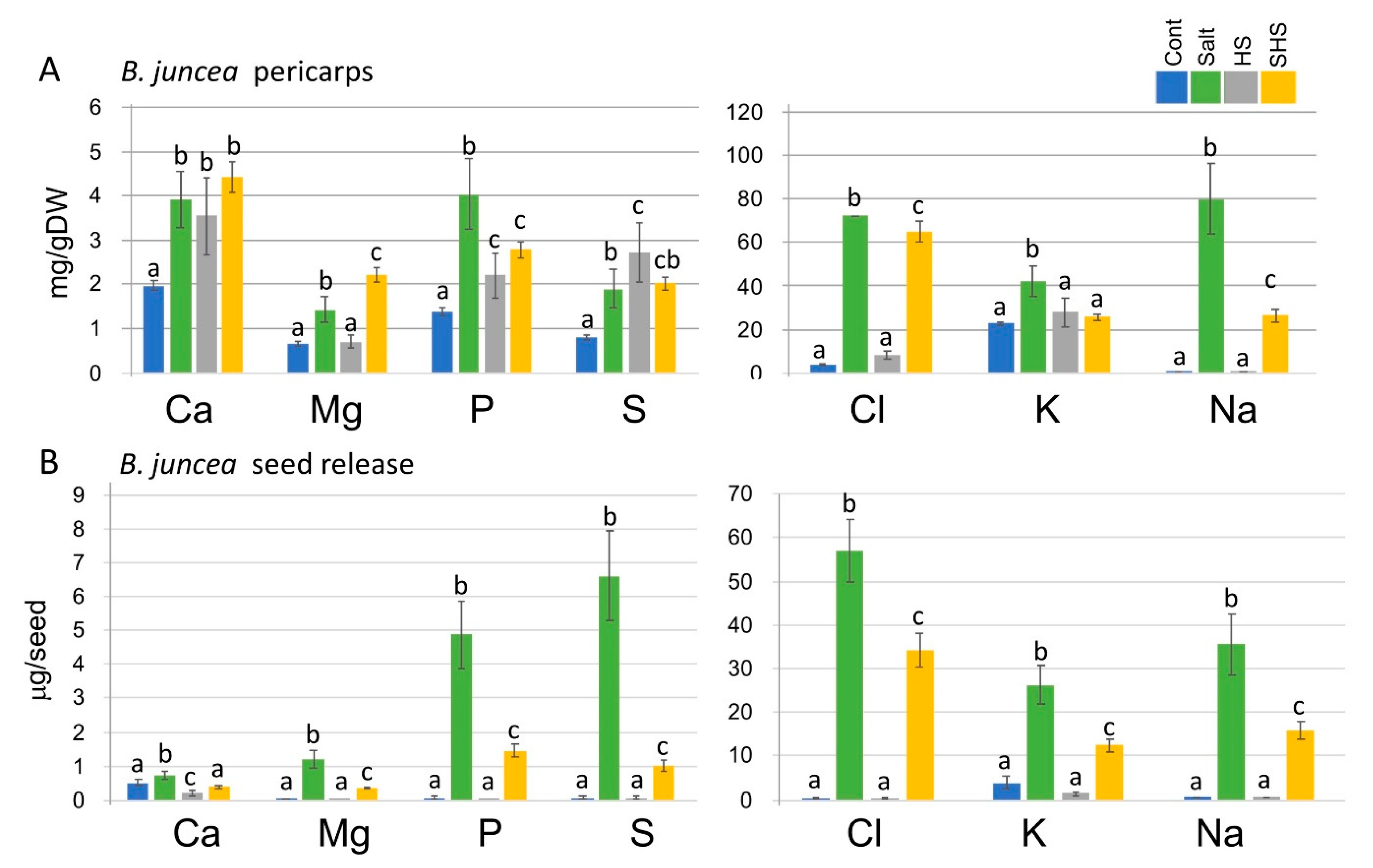
3.4. Single and Combined Stresses Altered Nutrient Levels Extracted from Pericarps or Secreted from Seeds
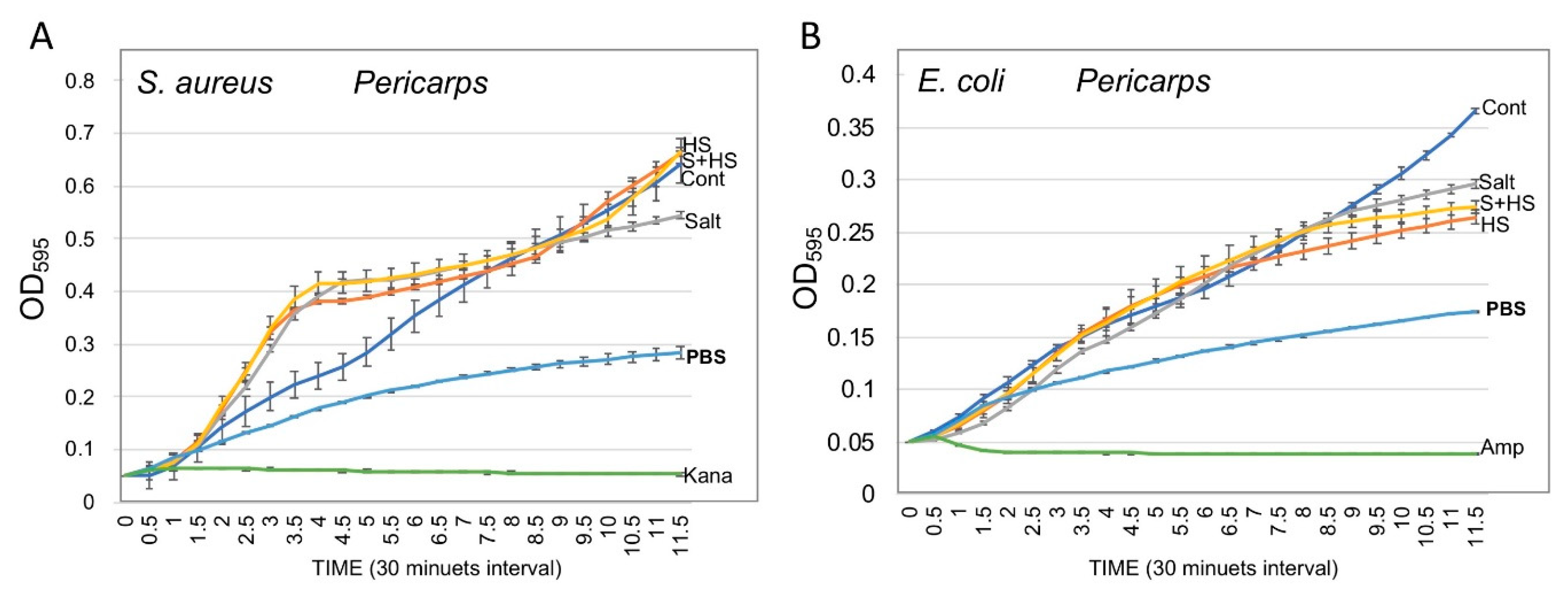
3.5. Pericarps Possess Bacterial Growth Promoting Substances
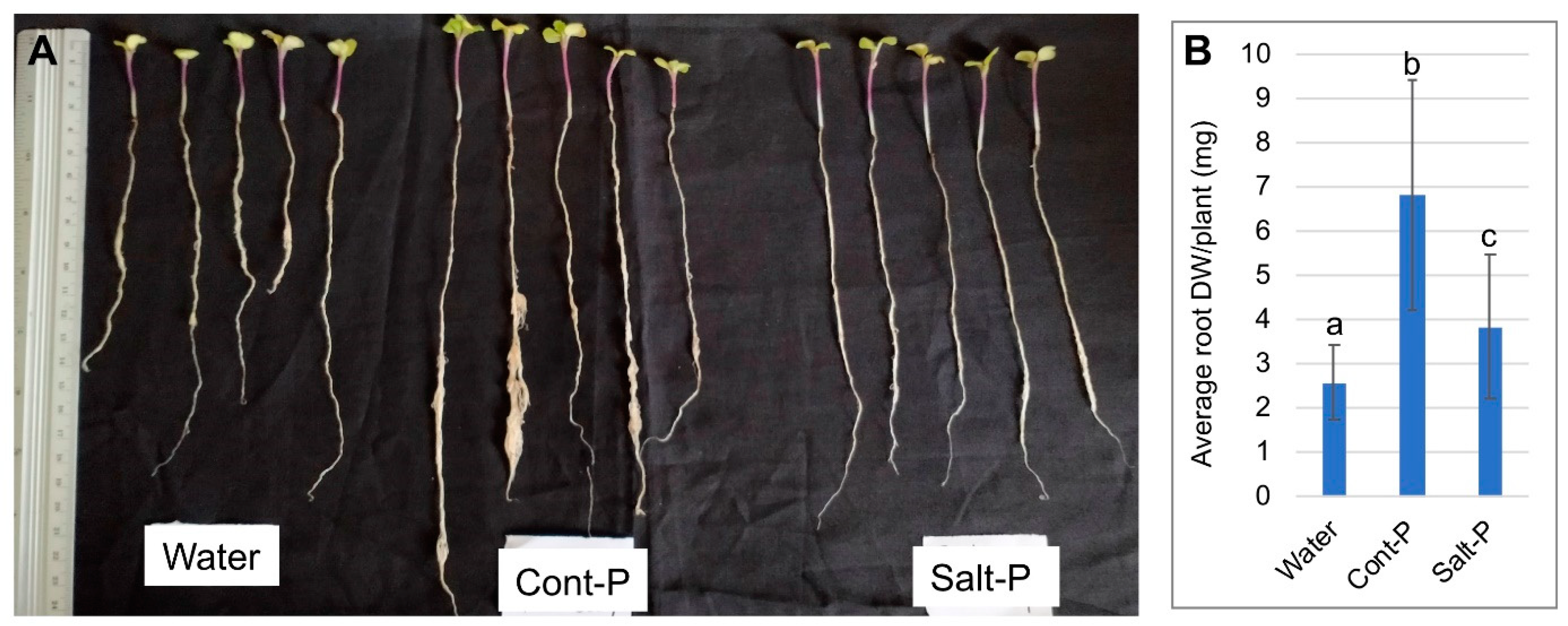
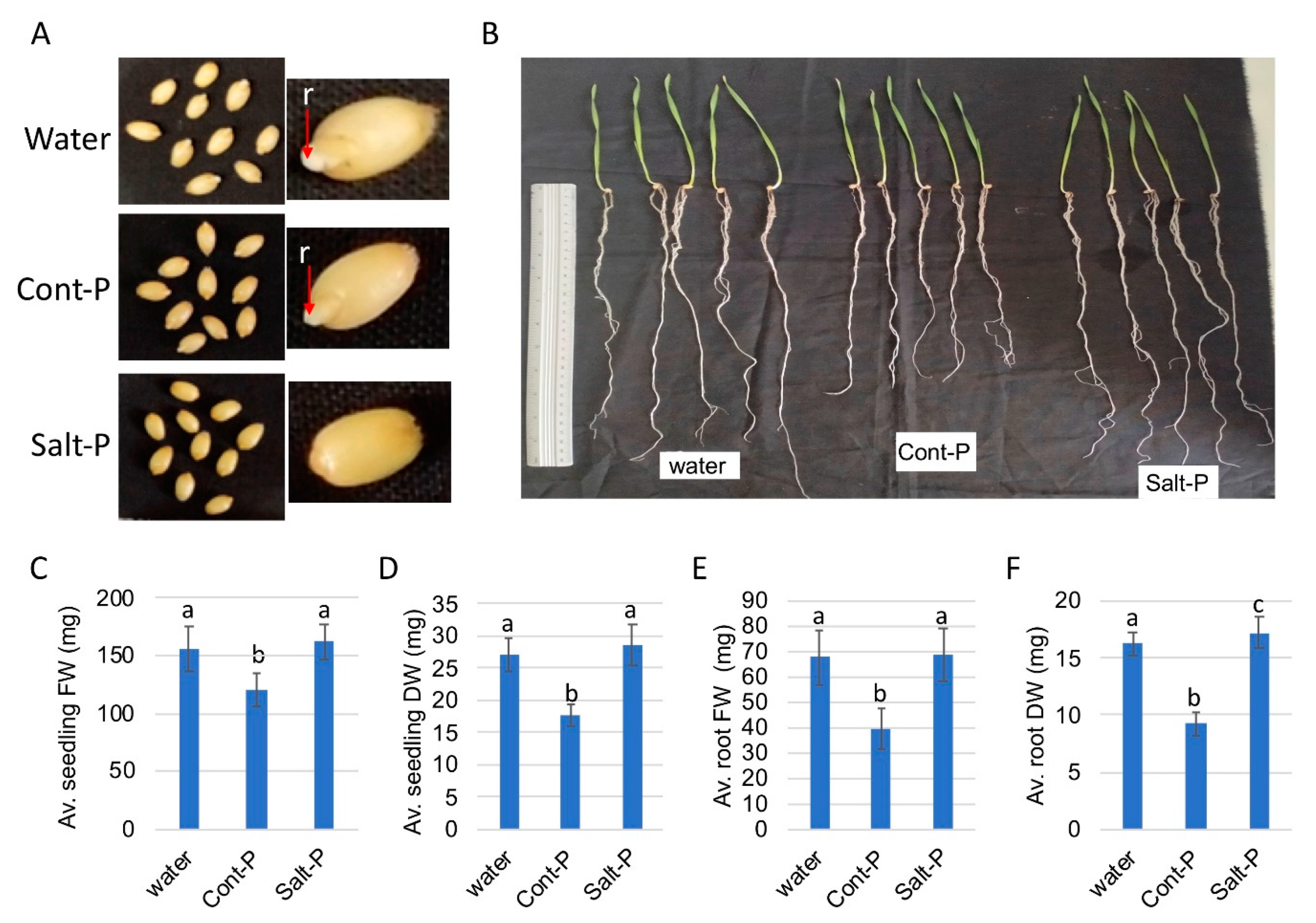
3.6. Priming Capacity of Dead Pericarps
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lobell, D.B.; Field, C.B. Global scale climate-crop yield relationships and the impacts of recent warming. Environ. Res. Lett. 2017, 2, 014002. [Google Scholar] [CrossRef]
- Brisson, N.; Gate, P.; Gouache, D.; Charmet, G.; Francois-Xavier, O.; Huarda, F. Why are wheat yields stagnating in Europe? A comprehensive data analysis for France. Field Crop. Res. 2010, 119, 201–212. [Google Scholar] [CrossRef]
- Francini, A.; Sebastiani, L. Abiotic stress effects on performance of horticultural crops. Horticulturae 2019, 5, 67. [Google Scholar] [CrossRef] [Green Version]
- Lesk, C.; Rowhani, P.; Ramankutty, N. Influence of extreme weather disasters on global crop production. Nature 2016, 529, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Mueller, V. Climate-induced cross-border migration and change in demographic structure. Popul. Environ. 2019, 41, 98–125. [Google Scholar] [CrossRef]
- Corwin, D.L. Climate change impacts on soil salinity in agricultural areas. Eur. J. Soil Sci. 2020, 72, 842–862. [Google Scholar] [CrossRef]
- Arnell, N.W.; Lowe, J.A.; Challinor, A.J.; Osborn, T.J. Global and regional impacts of climate change at different levels of global temperature increase. Clim. Chang. 2019, 155, 377–391. [Google Scholar] [CrossRef] [Green Version]
- Diffenbaugh, N.S.; Pal, J.S.; Giorgi, F.; Gao, X. Heat stress intensification in the Mediterranean climate change hotspot. Geophys. Res. Lett. 2007, 34, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Cohen, I.; Zandalinas, S.I.; Huck, C.; Fritschi, F.B.; Mittler, R. Meta-analysis of drought and heat stress combination impact on crop yield and yield components. Physiol. Plant. 2021, 171, 66–76. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Narayanan, S.; Erdayani, E.; Prasad, P.V.V. Effects of high temperature stress during anthesis and grain filling periods on photosynthesis, lipids and grain yield in wheat. BMC Plant Biol. 2020, 20, 268. [Google Scholar] [CrossRef] [PubMed]
- Fenner, M. The effects of the parent environment on seed germinability. Seed Sci. Res. 1991, 1, 75–84. [Google Scholar] [CrossRef]
- Li, Y.P.; Ye, W.; Wang, M.; Yan, X.D. Climate change and drought: A risk assessment of crop-yield impacts. Clim. Res. 2009, 39, 31–46. [Google Scholar] [CrossRef]
- Penfield, S.; MacGregor, D.R. Effects of environmental variation during seed production on seed dormancy and germination. J. Exp. Bot. 2017, 68, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Kissoudis, C.; Yan, Z.; Visser, R.G.F.; van der Linden, G. Plant behaviour under combined stress: Tomato responses to combined salinity and pathogen stress. Plant J. 2018, 93, 781–793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017, 90, 839–855. [Google Scholar] [CrossRef] [Green Version]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-Kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morphological traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [Green Version]
- Singiri, J.R.; Swetha, B.; Sikron-Persi, N.; Grafi, G. Differential response to single and combined salt and heat stresses: Impact on accumulation of proteins and metabolites in dead pericarps of Brassica juncea. Int. J. Mol. Sci. 2021, 22, 7076. [Google Scholar] [CrossRef] [PubMed]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Suzuki, N.; Rivero, R.M.; Shulaev, V.; Blumwald, E.; Mittler, R. Abiotic and biotic stress combinations. New Phytol. 2014, 203, 32–43. [Google Scholar] [CrossRef]
- Sattar, A.; Sher, A.; Ijaz, M.; Ul-Allah, S.; Rizwan, M.S.; Hussain, M.; Jabran, K.; Cheema, M.A. Terminal drought and heat stress alter physiological and biochemical attributes in flag leaf of bread wheat. PLoS ONE 2020, 15, e0232974. [Google Scholar] [CrossRef]
- Khadka, J.; Raviv, B.; Swetha, B.; Grandhi, R.; Singiri, J.R.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Huang, Z.; Grafi, G. Maternal environment alters dead pericarp biochemical properties of the desert annual plant Anastatica hierochuntica L. PLoS ONE 2020, 15, e0237045. [Google Scholar] [CrossRef]
- Raviv, B.; Khadka, J.; Swetha, B.; Singiri, J.R.; Grandhi, R.; Shapira, E.; Novoplansky, N.; Gutterman, Y.; Galis, I.; Sternberg, M.; et al. Extreme drought alters progeny dispersal unit properties of winter wild oat (Avena sterilis L.). Planta 2020, 252, 77. [Google Scholar] [CrossRef]
- Godwin, J.; Raviv, B.; Grafi, G. Dead pericarps of dry fruits function as long-term storage for active hydrolytic enzymes and other substances that affect germination and microbial growth. Plants 2017, 6, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raviv, B.; Aghajanyan, L.; Granot, G.; Makover, V.; Frenkel, O.; Gutterman, Y.; Grafi, G. The dead seed coat functions as a long-term storage for active hydrolytic enzymes. PLoS ONE 2017, 12, e0181102. [Google Scholar] [CrossRef] [Green Version]
- Raviv, B.; Granot, G.; Chalifa-Caspi, V.; Grafi, G. The dead, hardened floral bracts of dispersal units of wild wheat function as storage for active hydrolases and in enhancing seedling vigor. PLoS ONE 2017, 12, e0177537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raviv, B.; Godwin, J.; Granot, G.; Grafi, G. The dead can nurture: Novel insights into the function of dead organs enclosing embryos. Int. J. Mol. Sci. 2018, 19, 2455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grafi, G. Dead but Not Dead End: Multifunctional Role of Dead Organs Enclosing Embryos in Seed Biology. Int. J. Mol. Sci. 2020, 21, 8024. [Google Scholar] [CrossRef]
- Vandvik, V.; Klanderud, K.; Meineri, E.; Måren, I.E.; Töpper, J. Seed banks are biodiversity reservoirs: Species-area relationships above versus below ground. Oikos 2015, 125, 218–228. [Google Scholar] [CrossRef] [Green Version]
- Huang, Z.; Liu, S.; Bradford, K.J.; Huxman, T.E.; Venable, D.L. The contribution of germination functional traits to population dynamics of a desert plant community. Ecology 2016, 97, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Santner, A.; Calderon-Villalobos, L.I.; Estelle, M. Plant hormones are versatile chemical regulators of plant growth. Nat. Chem. Biol. 2009, 5, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Finkelstein, R.; Reeves, W.; Ariizumi, T.; Steber, C. Molecular aspects of seed dormancy. Annu. Rev. Plant Biol. 2008, 59, 387–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rhaman, M.S.; Imran, S.; Rauf, F.; Khatun, M.; Baskin, C.C.; Murata, Y.; Hasanuzzaman, M. Seed priming with phytohormones: An effective approach for the mitigation of abiotic stress. Plants 2021, 10, 37. [Google Scholar] [CrossRef]
- Barnabás, B.; Jäger, K.; Fehér, A. The effect of drought and heat stress on reproductive processes in cereals. Plant Cell Environ. 2008, 31, 11–38. [Google Scholar] [CrossRef] [PubMed]
- Mickelbart, M.V.; Hasegawa, P.M.; Bailey-Serres, J. Genetic mechanisms of abiotic stress tolerance that translate to crop yield stability. Nat. Rev. Genet. 2015, 16, 237–251. [Google Scholar] [CrossRef] [PubMed]
- He, M.; He, C.Q.; Ding, N.Z. Abiotic stresses: General defenses of land plants and chances for engineering multistress tolerance. Front. Plant Sci. 2018, 9, 1771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vries, J.; de Vries, S.; Curtis, B.A.; Zhou, H.; Penny, S.; Feussner, K.; Pinto, D.M.; Steinert, M.; Cohen, A.M.; von Schwartzenberg, K.; et al. Heat stress response in the closest algal relatives of land plants reveals conserved stress signaling circuits. Plant J. 2020, 103, 1025–1048. [Google Scholar] [CrossRef]
- Martínez-Lüscher, J.; Chen, C.C.L.; Brillante, L.; Kurtural, S.K. Mitigating heat wave and exposure damage to “cabernet sauvignon” wine grape with partial shading under two irrigation amounts. Front. Plant Sci. 2020, 11, 579192. [Google Scholar] [CrossRef]
- Gupta, S.K. Brassicas. In Technological Innovations in Major World Oil Crops, Volume 1, Breeding; Gupta, S.K., Ed.; Springer: New York, NY, USA, 2012; pp. 53–83. [Google Scholar]
- Shapiro, M.B. Soils of Israel. Eurasian Soil Sci. 2006, 39, 1170–1175. [Google Scholar] [CrossRef]
- Matsuura, T.; Mori, I.C.; Himi, E.; Hirayama, T. Plant hormone profiling in developing seeds of common wheat (Triticum aestivum L.). Breed. Sci. 2019, 69, 601–610. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, K.; Sawada, H.; Kohno, Y.; Matsuura, T.; Mori, I.C.; Terao, T.; Ioki, M.; Tamaoki, M. Ozone-induced rice grain yield loss is triggered via a change in panicle morphology that is controlled by ABERRANT PANICLE ORGANIZATION1 gene. PLoS ONE 2015, 10, e0123308. [Google Scholar] [CrossRef] [Green Version]
- Patton, T.; Barrett, J.; Brennan, J.; Moran, N. Use of a spectrophotometric bioassay for determination of microbial sensitivity to manuka honey. J. Microbiol. Methods 2006, 64, 84–95. [Google Scholar] [CrossRef]
- Young, L.W.; Wilen, R.W.; Bonham-Smith, P.C. High temperature stress of Brassica napus during flowering reduces micro- and megagametophyte fertility, induces fruit abortion, and disrupts seed production. J. Exp. Bot. 2004, 55, 485–495. [Google Scholar] [CrossRef] [Green Version]
- Angadi, S.V.; Cutforth, H.W.; Miller, P.R.; McConkey, B.G.; Entz, M.H.; Brandt, A.; Olkmar, K.M. Response of three Brassica species to high temperature stress during reproductive growth. Can. J. Plant Sci. 2000, 80, 693–701. [Google Scholar] [CrossRef] [Green Version]
- Morrison, M.J.; Stewart, D.W. Heat stress during flowering in summer Brassica. Crop Sci. 2002, 42, 797–803. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, B.; Piao, S.; Wang, X.; Lobell, D.B.; Huang, Y.; Huang, M.; Yao, Y.; Bassu, S.; Ciais, P.; et al. Temperature increase reduces global yields of major crops in four independent estimates. Proc. Natl. Acad. Sci. USA 2017, 114, 9326–9331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferris, R.; Ellis, R.; Wheeler, T.; Hadley, P. Effect of high temperature stress at anthesis on grain yield and biomass of field-grown crops of wheat. Ann. Bot. 1998, 82, 631–639. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.; Paulsen, G. Yield components of wheat grown under high temperature stress during reproductive growth. Crop Sci. 1999, 39, 1841–1846. [Google Scholar] [CrossRef]
- Rezaei, E.E.; Webber, H.; Gaiser, T.; Naab, J.; Ewert, F. Heat stress in cereals: Mechanisms and modelling. Eur. J. Agron. 2015, 64, 98–113. [Google Scholar] [CrossRef]
- Tashiro, T.; Wardlaw, I.F. The response to high temperature shock and humidity changes prior to and during the early stages of grain development in wheat. Aust. J. Plant Physiol. 1990, 17, 551–561. [Google Scholar] [CrossRef]
- Wheeler, T.R.; Crawford, P.Q.; Ellis, R.H.; Porter, J.R.; Prasad, P.W. Temperature variability and the yield of annual crops. Agric. Ecosyst. Environ. 2000, 82, 159–167. [Google Scholar] [CrossRef]
- Molina, M.O.; Sánchez, E.; Gutiérrez, C. Future heat waves over the Mediterranean from an Euro-CORDEX regional climate model ensemble. Sci. Rep. 2020, 10, 8801. [Google Scholar] [CrossRef] [PubMed]
- Vishal, B.; Kumar, P.P. Regulation of seed germination and abiotic stresses by gibberellins and abscisic acid. Front. Plant Sci. 2018, 9, 838. [Google Scholar] [CrossRef] [PubMed]
- Evenari, M. Germination inhibitors. Bot. Rev. 1949, 15, 153–194. [Google Scholar] [CrossRef]
- Koo, Y.M.; Heo, A.Y.; Choi, H.W. Salicylic acid as a safe plant protector and growth regulator. Plant Pathol. J. 2020, 36, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Marthandan, V.; Geetha, R.; Kumutha, K.; Renganathan, V.G.; Karthikeyan, A.; Ramalingam, J. Seed priming: A feasible strategy to enhance drought tolerance in crop plants. Int. J. Mol. Sci. 2020, 21, 8258. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swetha, B.; Singiri, J.R.; Novoplansky, N.; Grandhi, R.; Srinivasan, J.; Khadka, J.; Galis, I.; Grafi, G. Single and Combined Salinity and Heat Stresses Impact Yield and Dead Pericarp Priming Activity. Plants 2021, 10, 1627. https://doi.org/10.3390/plants10081627
Swetha B, Singiri JR, Novoplansky N, Grandhi R, Srinivasan J, Khadka J, Galis I, Grafi G. Single and Combined Salinity and Heat Stresses Impact Yield and Dead Pericarp Priming Activity. Plants. 2021; 10(8):1627. https://doi.org/10.3390/plants10081627
Chicago/Turabian StyleSwetha, Bupur, Jeevan R. Singiri, Nurit Novoplansky, Rohith Grandhi, Jansirani Srinivasan, Janardan Khadka, Ivan Galis, and Gideon Grafi. 2021. "Single and Combined Salinity and Heat Stresses Impact Yield and Dead Pericarp Priming Activity" Plants 10, no. 8: 1627. https://doi.org/10.3390/plants10081627
APA StyleSwetha, B., Singiri, J. R., Novoplansky, N., Grandhi, R., Srinivasan, J., Khadka, J., Galis, I., & Grafi, G. (2021). Single and Combined Salinity and Heat Stresses Impact Yield and Dead Pericarp Priming Activity. Plants, 10(8), 1627. https://doi.org/10.3390/plants10081627







