Anti-Inflammatory Effects of Weigela subsessilis Callus Extract via Suppression of MAPK and NF-κB Signaling
Abstract
1. Introduction
2. Results and Discussion
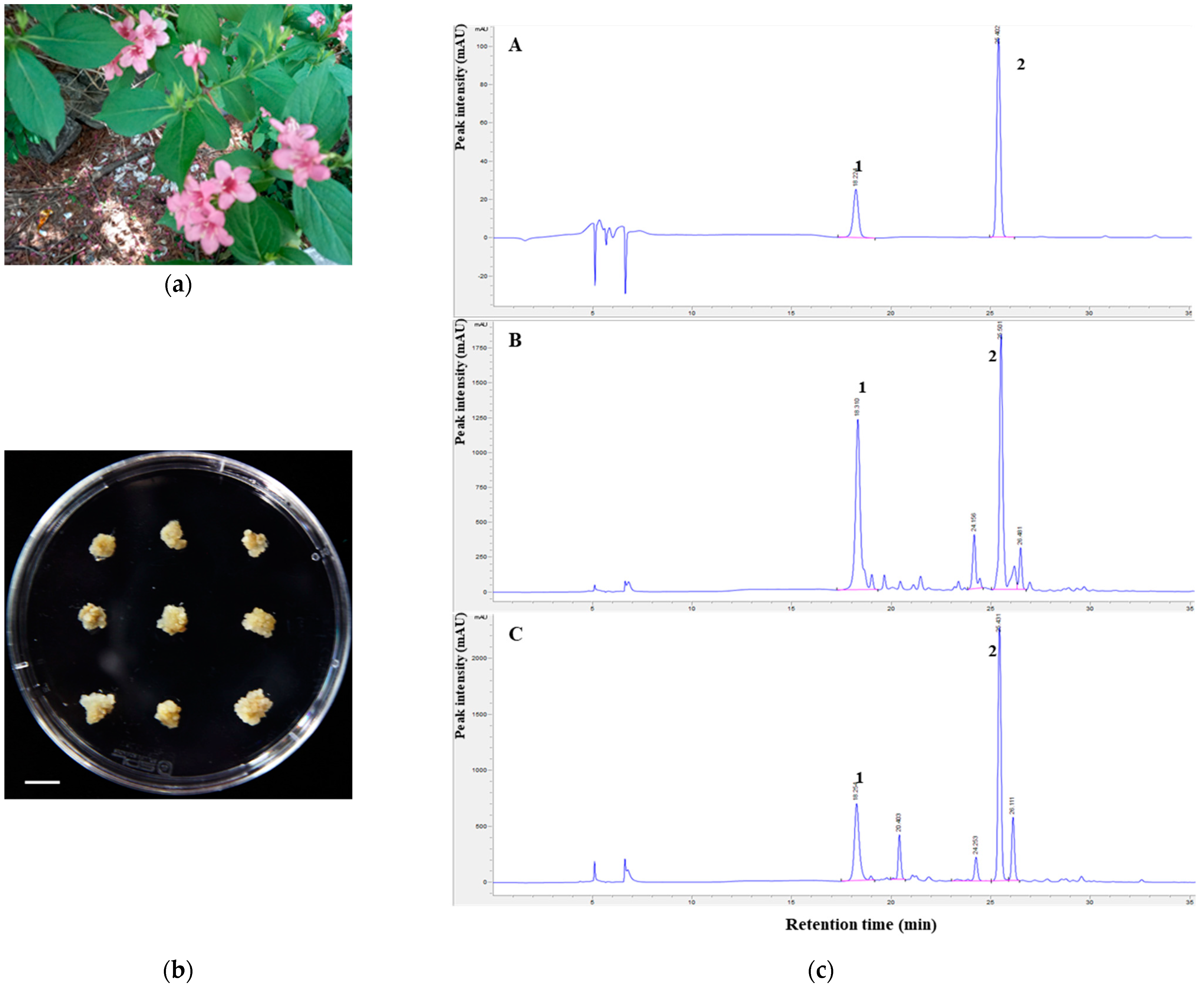
2.1. In Vitro Proliferation of W. subsessilis Callus
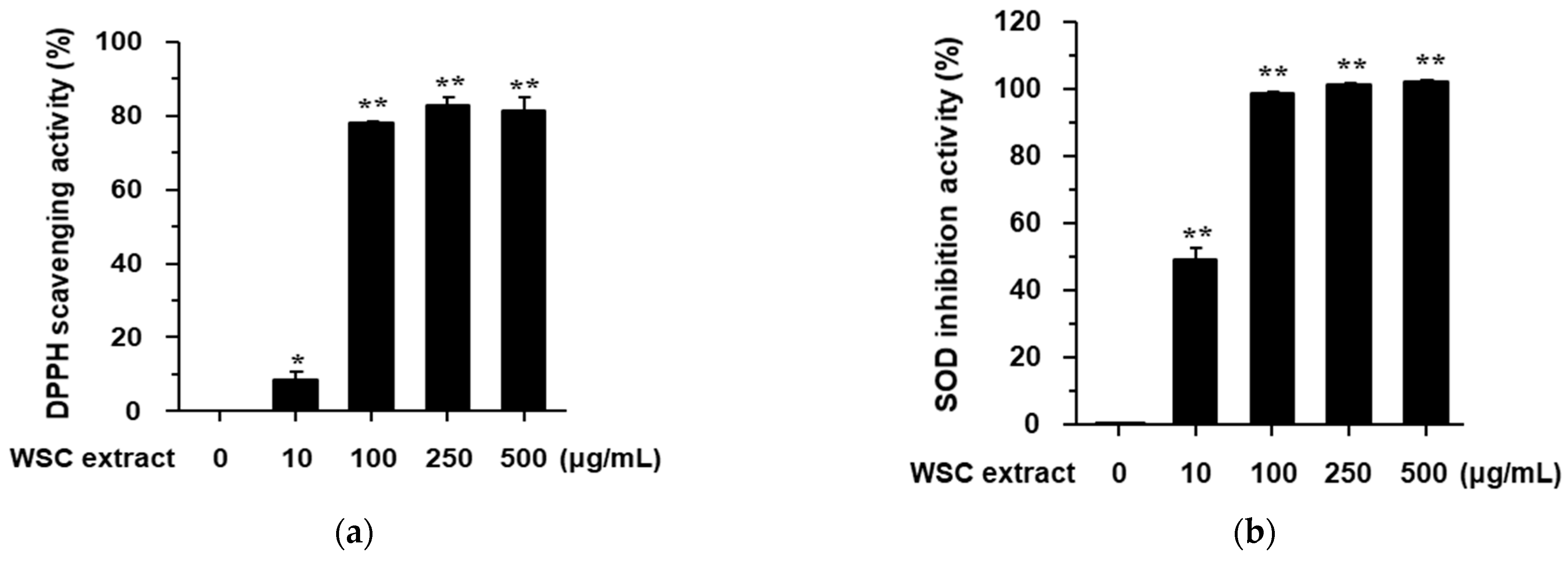
2.2. Antioxidant Effect and Viability of W. subsessilis Callus Extract in RAW264.7 Cells
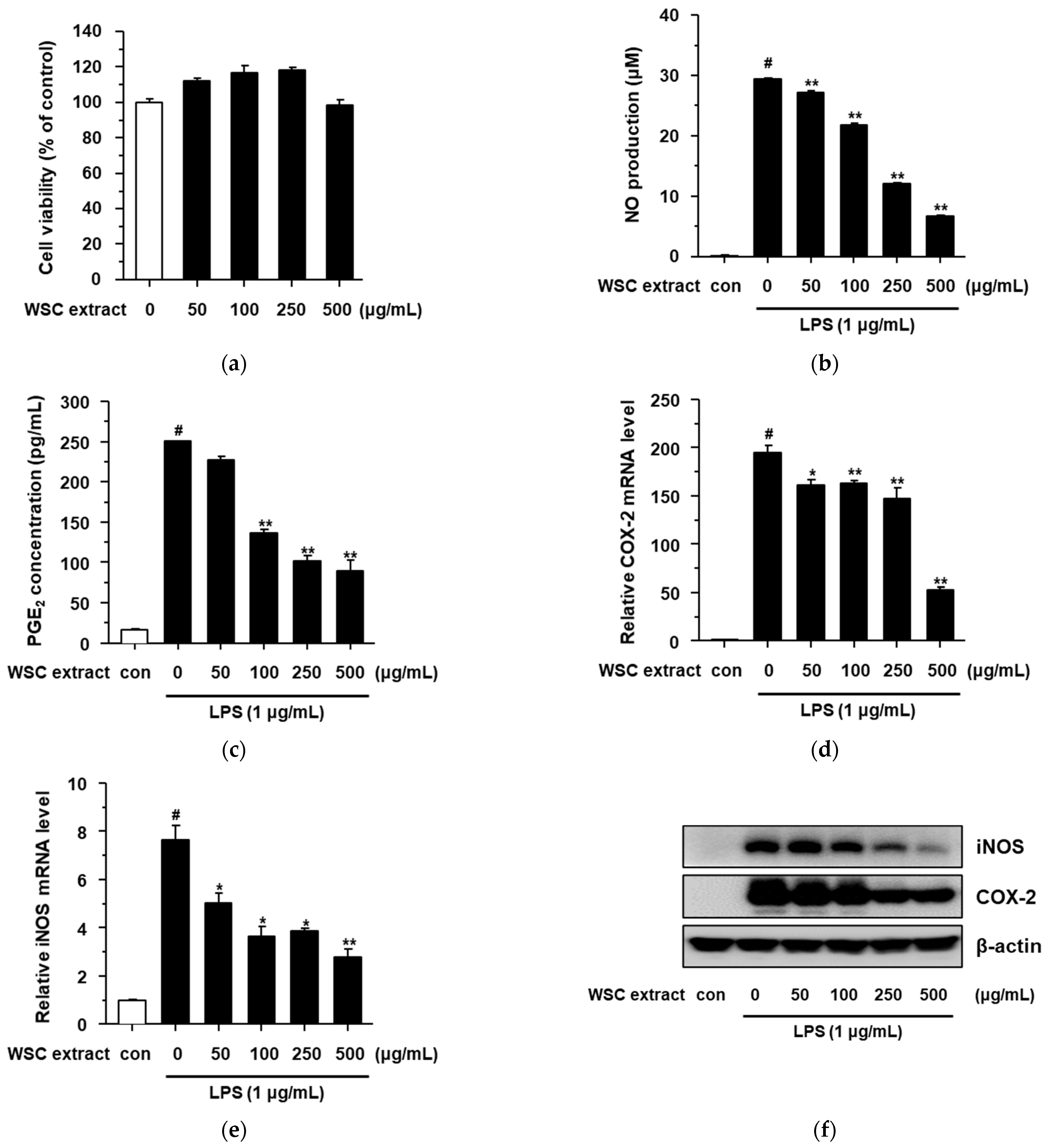
2.3. Anti-Inflammatory Effect of the WSC Extract on Lipopolysaccharide-Stimulated RAW264.7 Cells
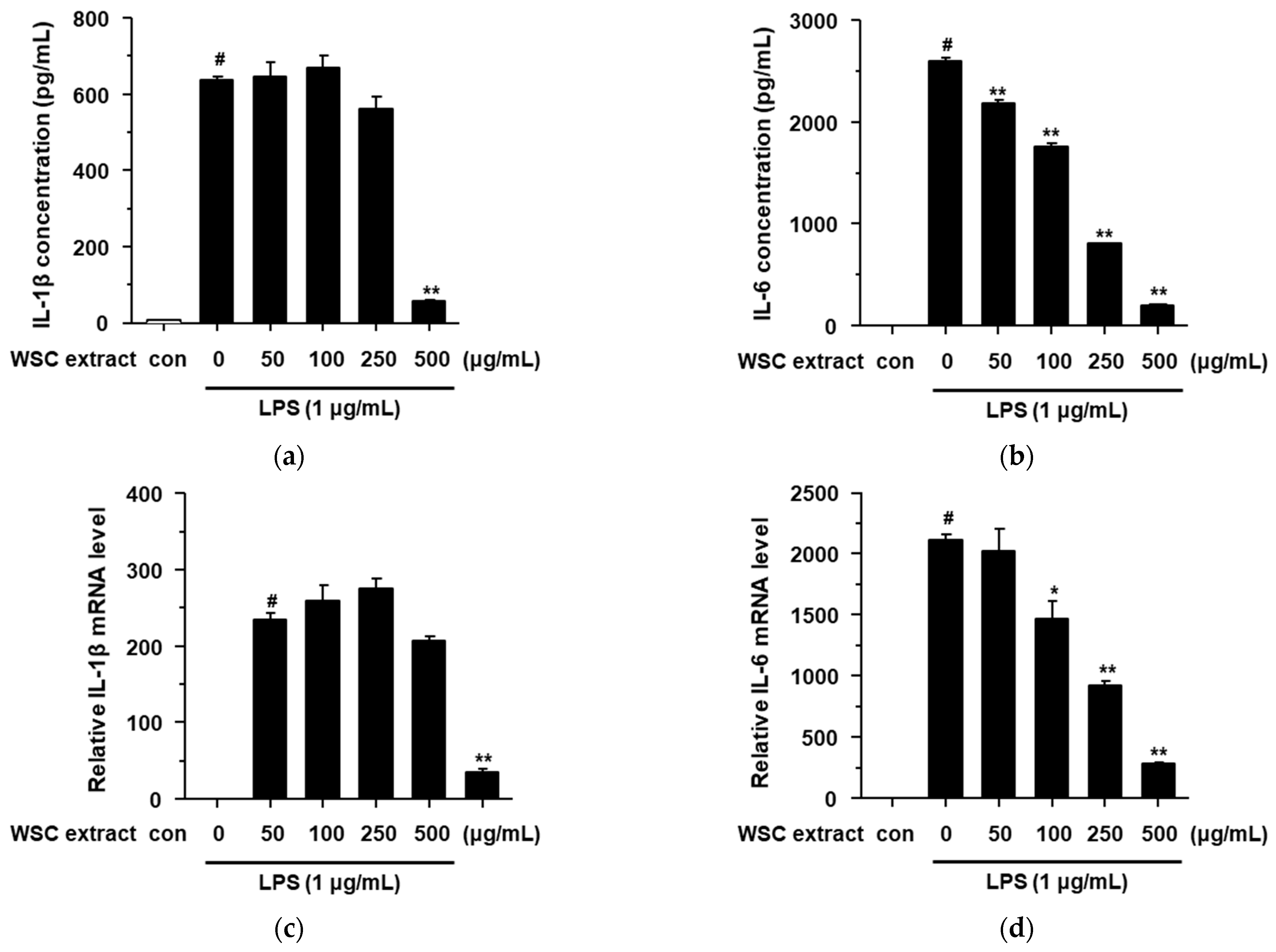
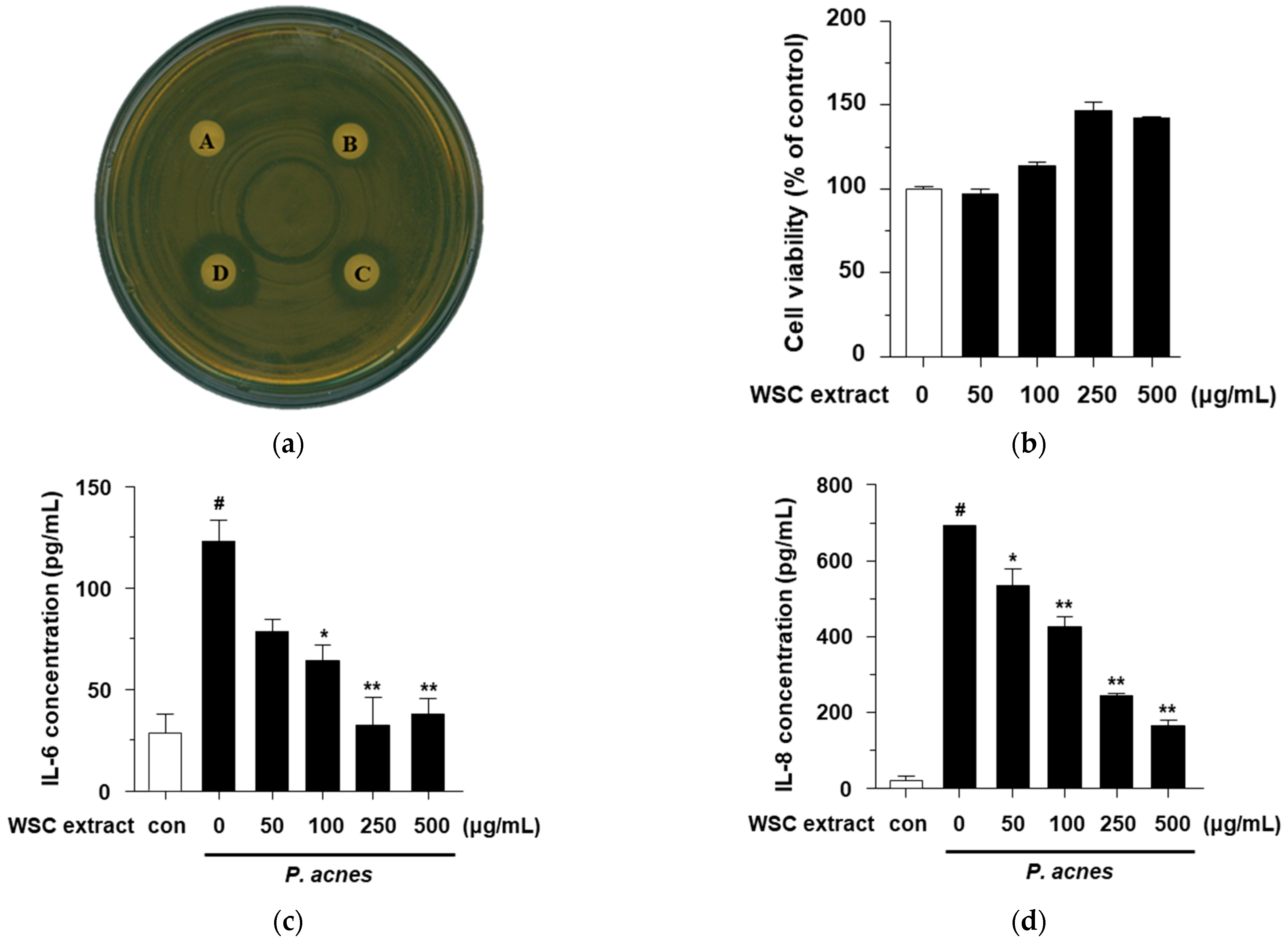
2.4. Effects of the WSC Extract on LPS-Stimulated Pro-Inflammatory Cytokines in RAW264.7 Cells
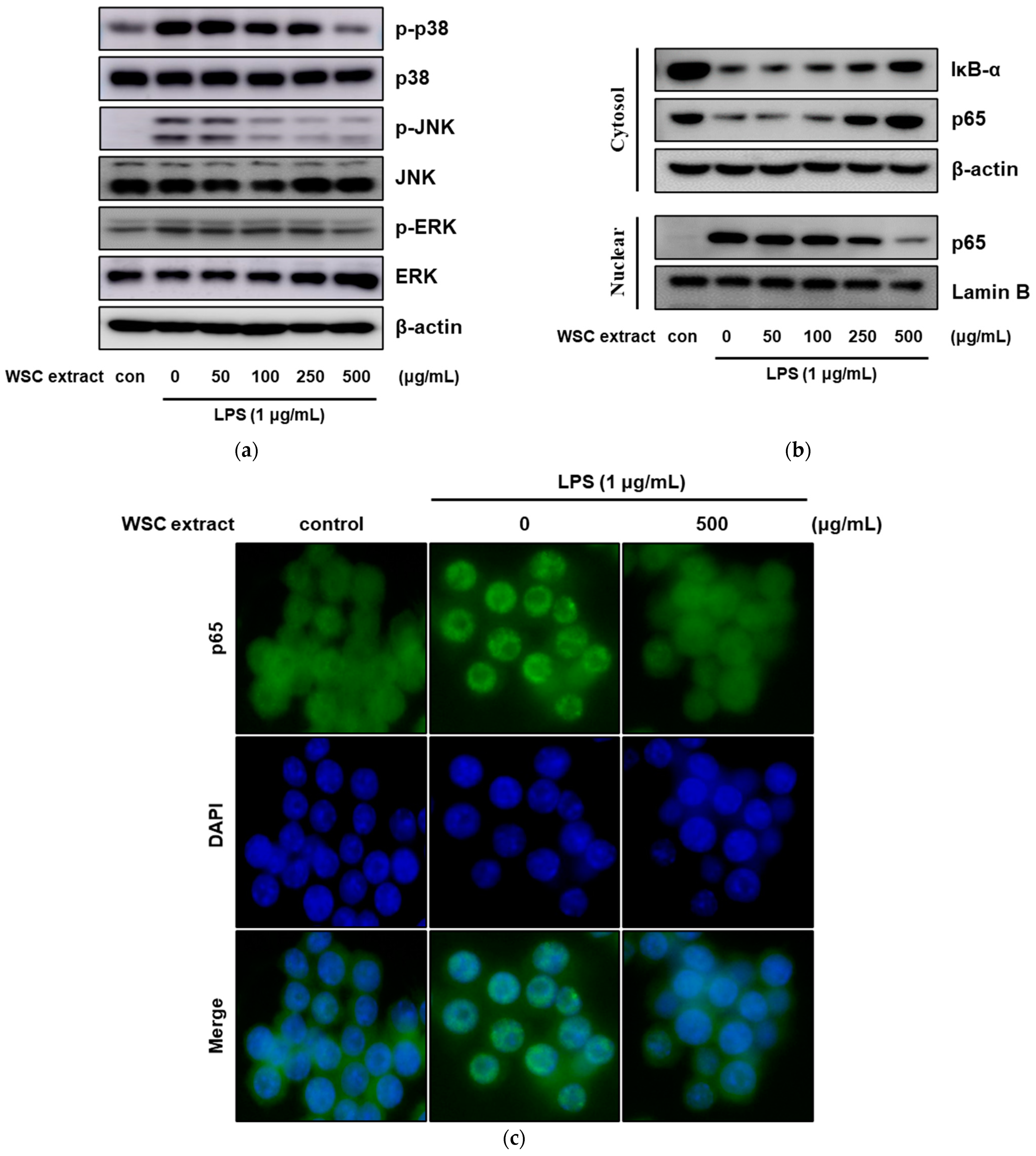
2.5. Effect of the WSC Extract on Mitogen-Activated Protein Kinase Signaling and Nuclear Translocation of NF-κB in LPS-Treated RAW264.7 Cells
2.6. Antibacterial and Anti-Inflammatory Effects of the WSC Extract
3. Materials and Methods
3.1. Callus Induction and Proliferation of W. subsessilis
3.2. HPLC Apparatus and Conditions
3.3. Preparation of the WSC Extract
3.4. Cell Culture and Viability
3.5. Antibacterial Activity
3.6. DPPH Assay
3.7. SOD Assay
3.8. Nitric Oxide Production Assay
3.9. Determination of Inflammatory Cytokine and PGE2 Level
3.10. Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
3.11. Western Blot Analyses
3.12. Immunofluorescence Staining
3.13. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Won, Y.M.; Seong, Z.K.; Kim, J.L.; Kim, H.S.; Song, H.H.; Kim, D.Y.; Kim, J.H.; Oh, S.R.; Cho, H.W.; Cho, J.H.; et al. Triterpene glycosides with stimulatory activity on melanogenesis from the aerial parts of Weigela subsessilis. Arch. Pharm. Res. 2015, 38, 1541–1551. [Google Scholar] [CrossRef]
- Thuong, P.T.; Na, M.; Su, N.D.; Seong, R.S.; Lee, Y.M.; Sok, D.E.; Bae, K. Inhibitory effect of coumarins from Weigela subsessilis on low density lipoprotein oxidation. Biol. Pharm. Bull. 2005, 28, 1095–1097. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Thuong, P.T. Stimulation of glucose uptake by triterpenoids from Weigela subsessilis. Phytother. Res. 2010, 24, 49–53. [Google Scholar] [CrossRef]
- Yoo, Y.C.; Lee, G.W.; Cho, Y.H. Antioxidant and anti-inflammatory effects of extracts from the flowers of Weigela subsessilis on RAW 264.7 macrophages. J. Life Sci. 2016, 26, 338–345. [Google Scholar] [CrossRef][Green Version]
- Quilantang, N.G.; Lee, J.S.; Ryu, S.H.; Park, S.H.; Byun, J.S.; Chun, J.S.; Jacinto, S.; Lee, S. Inhibitory effects of Synurus excelsus and Weigela subsessilis on aldose reductase and HPLC-UV analysis of scopolin, scopoletin, and quercetin. J. Appl. Biol. Chem. 2018, 61, 135–139. [Google Scholar] [CrossRef]
- Thakur, A.; Singla, R.; Jaitak, V. Coumarins as anticancer agents: A review on synthetic strategies, mechanism of action and SAR studies. Eur. J. Med. Chem. 2015, 101, 476–495. [Google Scholar] [CrossRef]
- Bansal, Y.; Sethi, P.; Bansal, G. Coumarin: A potential nucleus for anti-inflammatory molecules. Med. Chem. Res. 2013, 22, 3049–3060. [Google Scholar] [CrossRef]
- de Souza, S.M.; Delle Monache, F.; Smânia, A., Jr. Antibacterial activity of coumarins. Z. Naturforsch. C J. Biosci. 2005, 60, 693–700. [Google Scholar] [CrossRef]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Na, M.; Thuong, P.T.; Hwang, I.H.; Bae, K.; Kim, B.Y.; Osada, H.; Ahn, J.S. Protein tyrosine phosphatase 1B inhibitory activity of 24-norursane triterpenes isolated from Weigela subsessilis. Phytother. Res. 2010, 24, 1716–1719. [Google Scholar] [CrossRef]
- Chang, C.S. Flavonoid chemistry of Weigela (Caprifoliaceae) in Korea. J. Plant Res. 1997, 110, 275–281. [Google Scholar] [CrossRef]
- Blois, M. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Santos, R.B.; Abranches, R.; Fischer, R.; Sack, M.; Holland, T. Putting the spotlight back on plant suspension cultures. Front. Plant Sci. 2016, 7, 297. [Google Scholar] [CrossRef]
- Nielsen, E.; Temporiti, M.E.E.; Cella, R. Improvement of phytochemical production by plant cells and organ culture and by genetic engineering. Plant Cell Rep. 2019, 38, 1199–1215. [Google Scholar] [CrossRef] [PubMed]
- Espinosa-Leal, C.A.; Puente-Garza, C.A.; García-Lara, S. In vitro plant tissue culture: Means for production of biological active compounds. Planta 2018, 248, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.C.; Wogan, G.N. Growth and viability of macrophages continuously stimulated to produce nitric oxide. Proc. Natl. Acad. Sci. USA 1997, 94, 11875–11880. [Google Scholar] [CrossRef]
- Lin, C.Y.; Lee, C.H.; Chang, Y.W.; Wang, H.M.; Chen, C.Y.; Chen, Y.H. Pheophytin a inhibits inflammation via suppression of LPS-induced nitric oxide synthase-2, prostaglandin E2, and interleukin-1β of macrophages. Int. J. Mol. Sci. 2014, 15, 22819–22834. [Google Scholar] [CrossRef]
- Boscá, L.; Zeini, M.; Través, P.G.; Hortelano, S. Nitric oxide and cell viability in inflammatory cells: A role for NO in macrophage function and fate. Toxicology 2005, 208, 249–258. [Google Scholar] [CrossRef] [PubMed]
- Boje, K.M. Nitric oxide neurotoxicity in neurodegenerative diseases. Front. Biosci. 2004, 9, 763–776. [Google Scholar] [CrossRef]
- Kwon, D.H.; Cha, H.J.; Choi, E.O.; Leem, S.H.; Kim, G.Y.; Moon, S.K.; Chang, Y.C.; Yun, S.J.; Hwang, H.J.; Kim, B.W.; et al. Schisandrin A suppresses lipopolysaccharide-induced inflammation and oxidative stress in RAW 264.7 macrophages by suppressing the NF-κB, MAPKs and PI3K/Akt pathways and activating Nrf2/HO-1 signaling. Int. J. Mol. Med. 2018, 41, 264–274. [Google Scholar] [CrossRef]
- Cavaillon, J.M.; Adib-Conquy, M. Monocytes/macrophages and sepsis. Crit. Care Med. 2005, 33, S506–S509. [Google Scholar] [CrossRef]
- Zhao, B.B.; Guo, H.J.; Liu, Y.; Luo, X.Y.; Yang, S.X.; Wang, Y.T.; Leng, X.; Mo, C.F.; Zou, Q. K313, a novel benzoxazole derivative, exhibits anti-inflammatory properties via inhibiting GSK3β activity in LPS-induced RAW264.7 macrophages. J. Cell. Biochem. 2018, 119, 5382–5390. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, Y.; Xu, J.; Feng, J.; He, X. Propacin, a coumarinolignoid isolated from durian, inhibits the lipopolysaccharide-induced inflammatory response in macrophages through the MAPK and NF-κB pathways. Food Funct. 2020, 11, 596–605. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Kim, D.W.; Jo, H.S.; Cho, S.B.; Park, J.H.; Lee, C.H.; Choi, Y.J.; Yeo, E.J.; Park, S.Y.; Kim, S.T.; et al. Tat-biliverdin reductase A inhibits inflammatory response by regulation of MAPK and NF-κB pathways in Raw 264.7 cells and edema mouse model. Mol. Immunol. 2015, 63, 355–366. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin inhibits LPS-induced inflammation via TLR4 abrogation mediated by the NF-kB and MAPK signaling pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Kaisho, T.; Akira, S. Toll-like receptors and their signaling mechanism in innate immunity. Acta Odontol. Scand. 2001, 59, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Contassot, E.; French, L.E. New insights into acne pathogenesis: Propionibacterium acnes activates the inflammasome. J. Investig. Dermatol. 2014, 134, 310–313. [Google Scholar] [CrossRef]
- Nagy, I.; Pivarcsi, A.; Kis, K.; Koreck, A.; Bodai, L.; McDowell, A.; Seltmann, H.; Patrick, S.; Zouboulis, C.C.; Kemény, L. Propionibacterium acnes and lipopolysaccharide induce the expression of antimicrobial peptides and proinflammatory cytokines/chemokines in human sebocytes. Microbes Infect. 2006, 8, 2195–2205. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R.P. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence (5′ to 3′) | |
|---|---|---|
| COX-2 | Forward | CTCTACAACAACTCCATCCT |
| (mouse) | Reverse | ATTCTGCAGCCATTTCCTTC |
| iNOS | Forward | GTCCTACACCACACCAAACT |
| (mouse) | Reverse | AATCTCTGCCTATCCGTCTC |
| IL-1β | Forward | ACCTGTGTCTTTCCCGTGG |
| (mouse) | Reverse | TCATCTCGGAGCCTGTAGTG |
| IL-6 | Forward | TGTCTATACCACTTCACAAGTCGGAG |
| (mouse) | Reverse | GCACAACTCTTTTCTCATTTCCA |
| β-actin | Forward | CGGTTCCGATGCCCTGAGGCTCTT |
| (human) | Reverse | CGTCACACTTCATGATGGAATTGA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, H.-J.; Jie, E.Y.; Park, I.-S.; Kim, S.-J.; Ahn, W.S.; Jeong, S.-I.; Kim, S.W.; Jung, C.-H. Anti-Inflammatory Effects of Weigela subsessilis Callus Extract via Suppression of MAPK and NF-κB Signaling. Plants 2021, 10, 1635. https://doi.org/10.3390/plants10081635
Lim H-J, Jie EY, Park I-S, Kim S-J, Ahn WS, Jeong S-I, Kim SW, Jung C-H. Anti-Inflammatory Effects of Weigela subsessilis Callus Extract via Suppression of MAPK and NF-κB Signaling. Plants. 2021; 10(8):1635. https://doi.org/10.3390/plants10081635
Chicago/Turabian StyleLim, Hyeon-Ji, Eun Yee Jie, In-Sun Park, Sang-Jun Kim, Woo Seok Ahn, Seung-Il Jeong, Suk Weon Kim, and Chan-Hun Jung. 2021. "Anti-Inflammatory Effects of Weigela subsessilis Callus Extract via Suppression of MAPK and NF-κB Signaling" Plants 10, no. 8: 1635. https://doi.org/10.3390/plants10081635
APA StyleLim, H.-J., Jie, E. Y., Park, I.-S., Kim, S.-J., Ahn, W. S., Jeong, S.-I., Kim, S. W., & Jung, C.-H. (2021). Anti-Inflammatory Effects of Weigela subsessilis Callus Extract via Suppression of MAPK and NF-κB Signaling. Plants, 10(8), 1635. https://doi.org/10.3390/plants10081635






