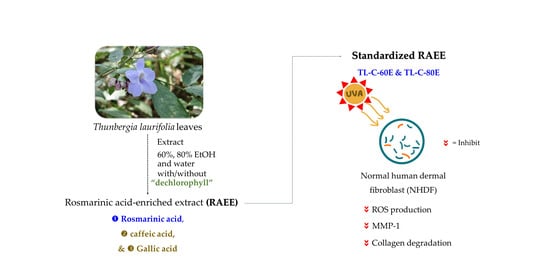
Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Contents and In Vitro Antioxidant Activity of T. laurifolia Extracts
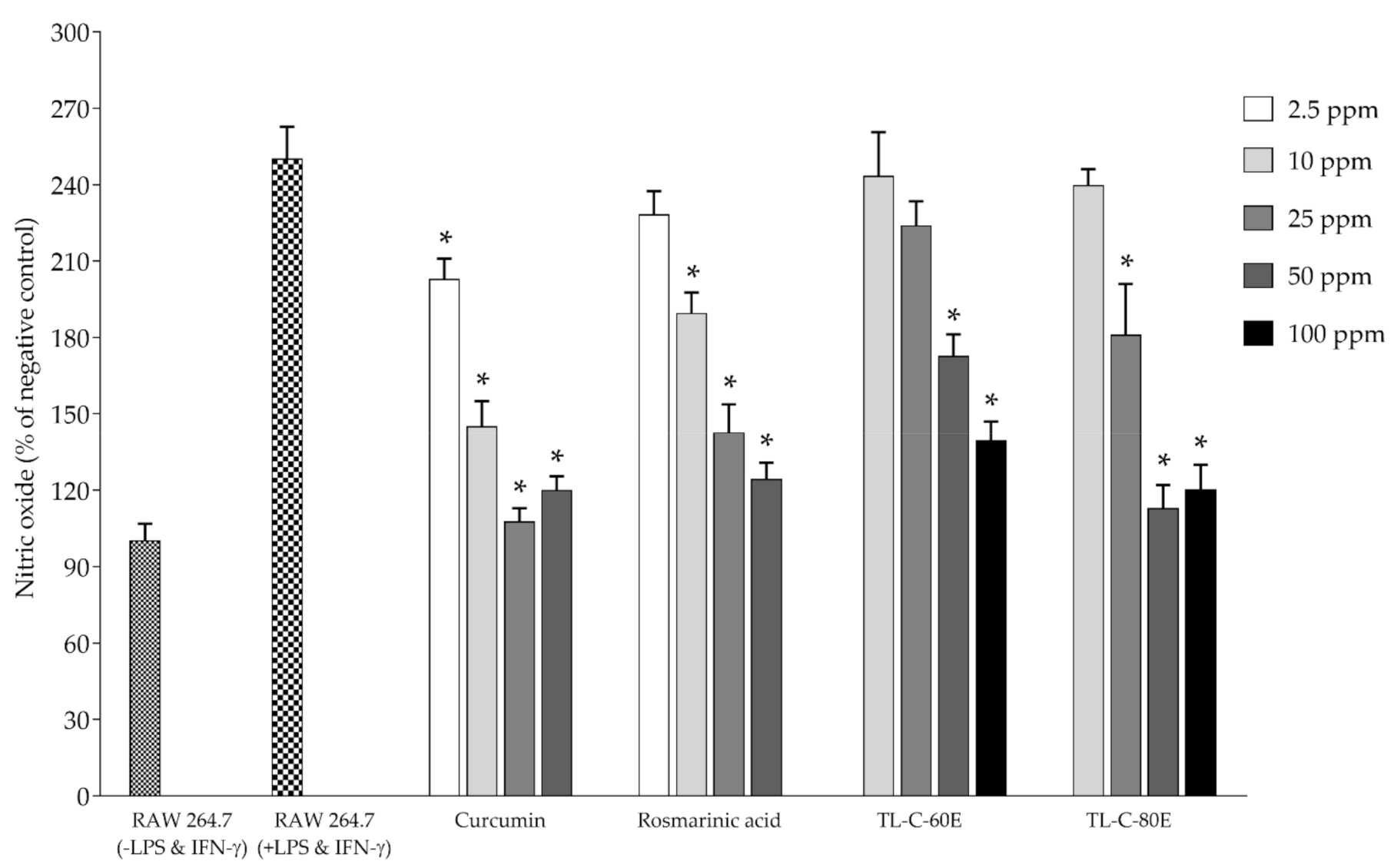
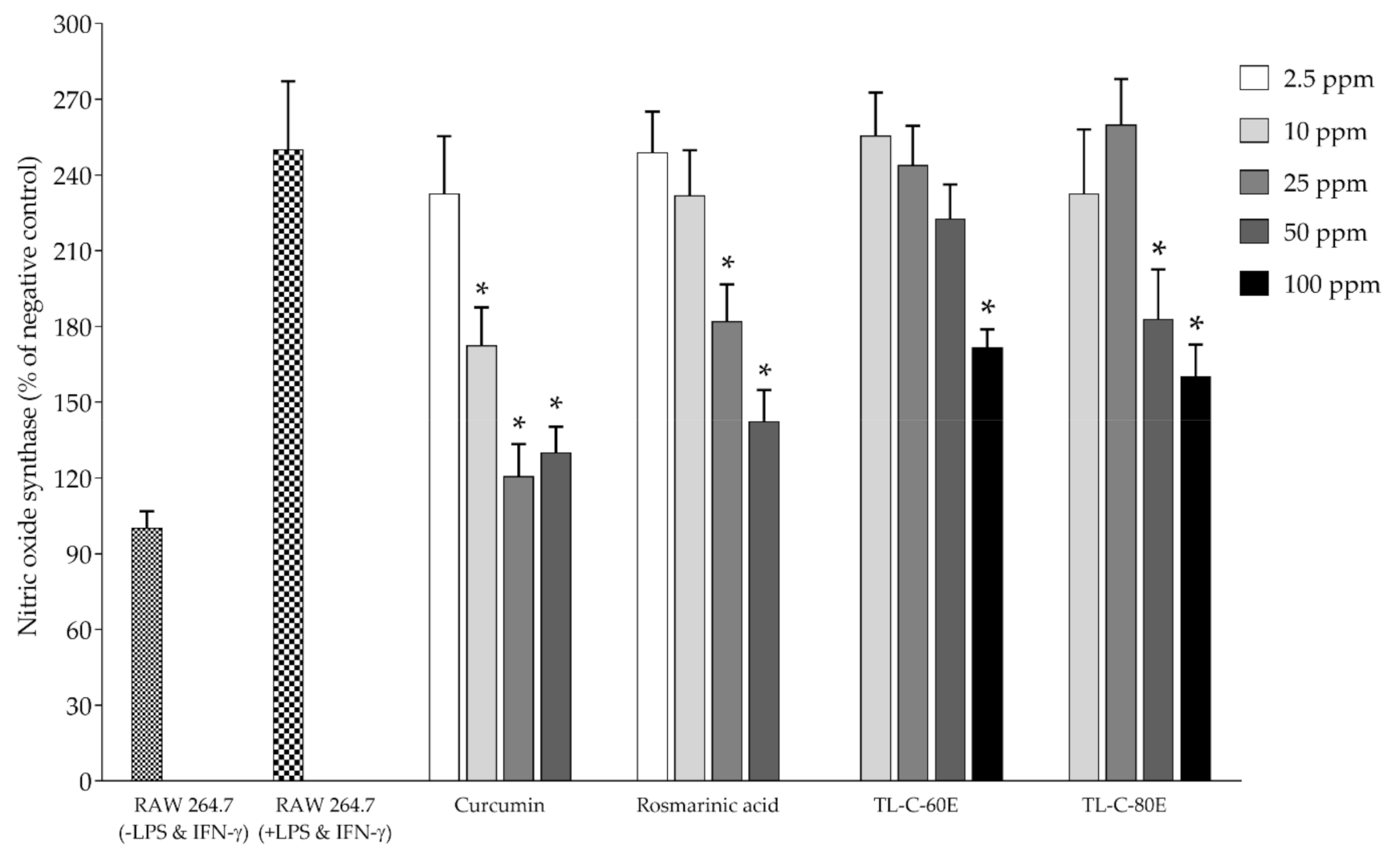
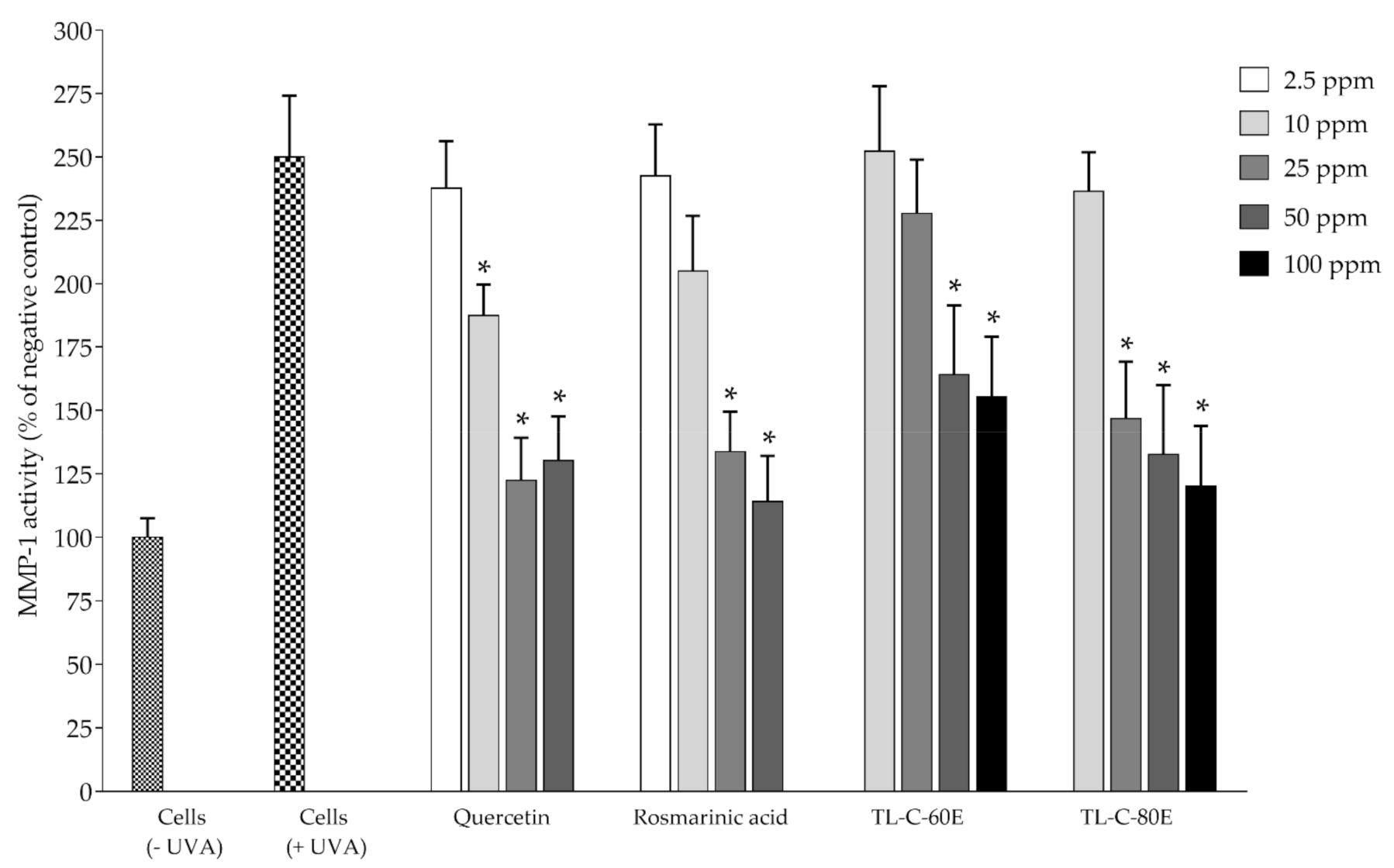
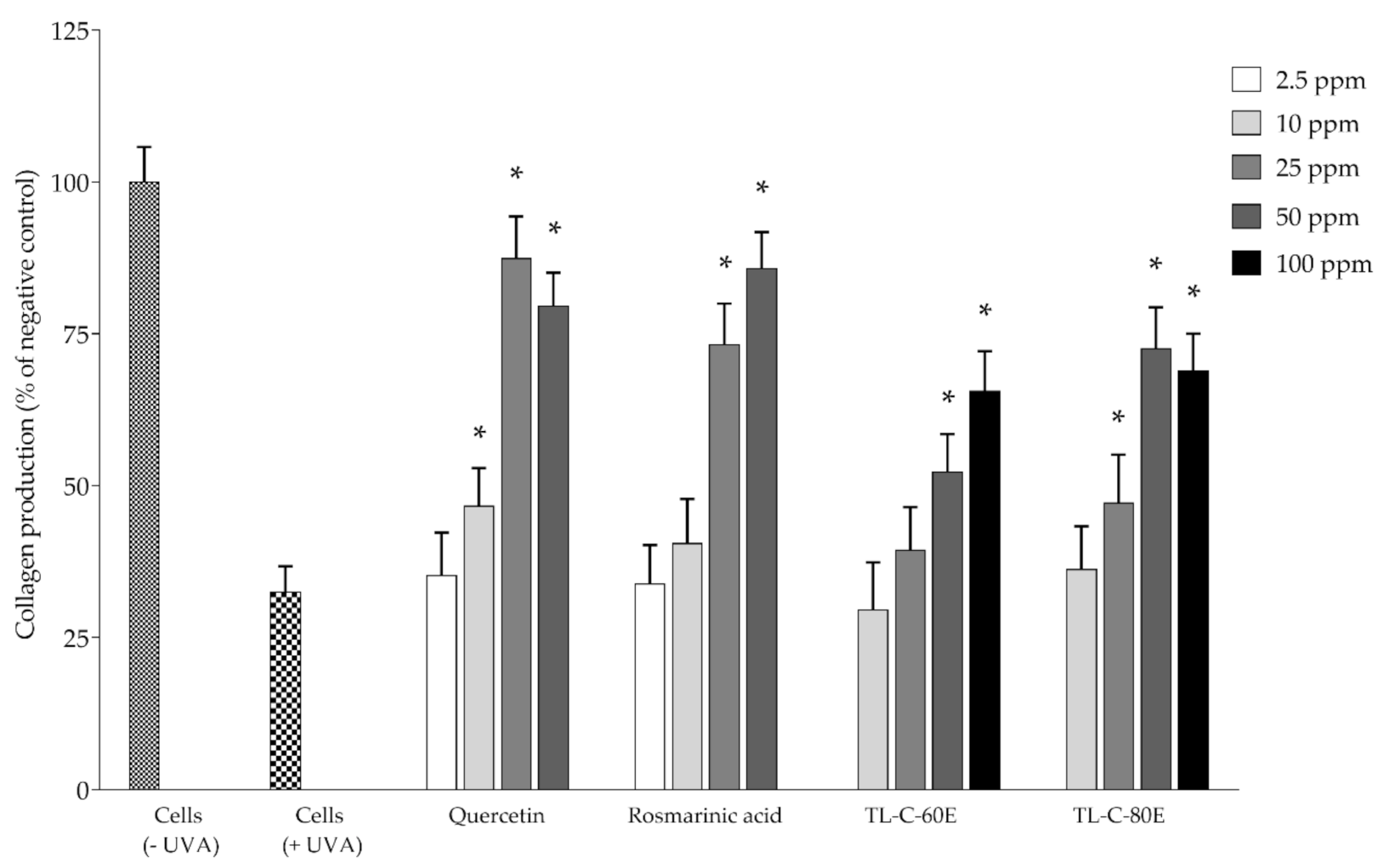
2.2. Antioxidant and Biological Activities in Cell-Based Studies
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Thunbergia laurifolia Leaf Sample and Preparation of Rosmarinic Acid-Enriched Extract (RAEE)
3.3. Chromatographic Analysis of Phenolic and Flavonoid Compounds
3.4. Determination of In Vitro Antioxidant Activity
3.4.1. Scavenging Effects on Nitric Oxide
3.4.2. Scavenging Effects on Superoxide Anion
3.4.3. Inhibition Effect on Lipid Peroxidation
3.5. Determination of Antioxidant and Biological Activities in Cell-Based Studies
3.5.1. Determination on Inhibition Effect on Intracellular ROS Production
3.5.2. Determination of the Effect on Nitric Oxide and Inducible Nitric Oxide Synthase (iNOS) Production
3.5.3. Determination on Matrix Metalloproteinase-1 (MMP-1) and Collagen Production
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Palipoch, S.; Punsawad, C.; Suwannalert, P. Thunbergia laurifolia, a new choice of natural antioxidant to prevent oxidative stress-related pathology: A review. J. Med. Plants Res. 2013, 7, 698–701. [Google Scholar]
- Ichihashi, M.; Ahmed, N.U.; Budiyanto, A.; Wu, A.; Bito, T.; Ueda, M.; Osawa, T. Preventive effect of antioxidant on ultraviolet-induced skin cancer in mice. J. Dermatol. Sci. 2000, 23, S45–S50. [Google Scholar] [CrossRef]
- Huang, A.H.; Chien, A.L. Photoaging: A review of current literature. Curr. Dermatol. Rep. 2020, 9, 22–29. [Google Scholar] [CrossRef]
- Pandel Mikuš, R.; Poljšak, B.; Godic, A.; Dahmane, R. Skin photoaging and the role of antioxidants in its prevention. Int. Sch. Res. Not. Dermatol. 2013, 2013, 930164. [Google Scholar] [CrossRef] [PubMed]
- Kosai, P.; Jiraungkoorskul, K. Review of antidiabetic activity of “Rang Jeud” Thunbergia laurifolia. J. Appl. Pharm. Sci. 2015, 5, 99–103. [Google Scholar]
- Chan, E.W.C.; Eng, S.Y.; Tan, Y.P.; Wong, Z.C. Phytochemistry and pharmacological properties of Thunbergia laurifolia: A review. Pharmacogn. J. 2011, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Thongsaard, W.; Marsden, C.A. A herbal medicine used in the treatment of addiction mimics the action of amphetamine on in vitro rat striatal dopamine release. Neurosci. Lett. 2002, 329, 129–132. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Mario, G. Antioxidant activity and cytotoxicity of Rang Chuet (Thunbergia laurifolia Lindl.) extracts. As. J. Food Ag-Ind. 2008, 1, 116–128. [Google Scholar]
- Tangpong, J.; Satarug, S. Alleviation of lead poisoning in the brain with aqueous leaf extract of the Thunbergia laurifolia (Linn.). Toxicol. Lett. 2010, 198, 83–88. [Google Scholar] [CrossRef]
- Junsi, M.; Siripongvutikorn, S.; Yupanqui, C.; Usawakesmanee, W. Efficacy of Thunbergia laurifolia (Rang Jued) aqueous leaf extract for specific biological activities using RAW 264.7 macrophage cells as test model. Int. Food Res. J. 2017, 24, 2317–2329. [Google Scholar]
- Pramyothin, P.; Chirdchupunsare, H.; Rungsipipat, A.; Chaichantipyuth, C. Hepatoprotective activity of Thunbergia laurifolia Linn extract in rats treated with ethanol: In vitro and in vivo studies. J. Ethnopharmacol. 2005, 102, 408–411. [Google Scholar] [CrossRef]
- Oonsivilai, R.; Cheng, C.; Bomser, J.; Ferruzzi, M.G.; Ningsanond, S. Phytochemical profiling and phase II enzyme-inducing properties of Thunbergia laurifolia Lindl. (RC) extracts. J. Ethnopharmacol. 2007, 114, 300–306. [Google Scholar] [CrossRef]
- Suwanchaikasem, P.; Chaichantipayuth, C.; Sukrong, S. Antioxidant-guided Isolation of rosmarinic acid, a major constituent from Thunbergia laurifolia, and Its use as a bioactive marker for standardization. Chiang Mai J. Sci. 2014, 41, 117–127. [Google Scholar]
- Ruangpayungsak, N.; Sithisarn, P.; Rojsanga, P. High performance liquid chromatography fingerprinting and chemometric analysis of antioxidant quality of Thunbergia laurifolia leaves. J. Liq. Chromatogr. Rel. Technol. 2018, 41, 713–721. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Yamasaki, K. Iridoid glucosides from Thunbergia laurifolia. Phytochemistry 2002, 60, 769–771. [Google Scholar] [CrossRef]
- Chao, C.Y.; Mong, M.C.; Chan, K.C.; Yin, M.C. Anti-glycative and anti-inflammatory effects of caffeic acid and ellagic acid in kidney of diabetic mice. Mol. Nutr. Food Res. 2010, 54, 388–395. [Google Scholar] [CrossRef]
- Sato, Y.; Itagaki, S.; Kurokawa, T.; Ogura, J.; Kobayashi, M.; Hirano, T.; Sugawara, M.; Iseki, K. In vitro and in vivo antioxidant properties of chlorogenic acid and caffeic acid. Int. J. Pharm. 2011, 403, 136–138. [Google Scholar] [CrossRef]
- Boonyarikpunchai, W.; Sukrong, S.; Towiwat, P. Antinociceptive and anti-inflammatory effects of rosmarinic acid isolated from Thunbergia laurifolia Lindl. Pharmacol. Biochem. Behav. 2014, 124, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Sultana, K.W.; Chatterjee, S.; Roy, A.; Chandra, I. An overview of ethnopharmacological and phytochemical properties of Thunbergia. J. Med. Aromat. Plants 2015, 4, 1–6. [Google Scholar]
- Junsi, M.; Siripongvutikorn, S.; Yupanqui, C.; Usawakesmanee, W. Phenolic and flavonoid compounds in aqueous extracts of Thunbergia laurifolia leaves and their effect on the toxicity of the carbamate insecticide methomyl to murine macrophage cells. Funct. Foods Health Dis. 2017, 7, 529. [Google Scholar] [CrossRef]
- Phaisan, S.; Yusakul, G.; Sakdamas, A.; Taluengjit, N.; Sakamoto, S.; Putalun, W. A green and effective method using oils to remove chlorophyll from Chromolaena odorata (L.) R.M. King & H. Rob. Songklanakarin J. Sci. Technol. 2020, 42, 1084–1090. [Google Scholar]
- Chan, E.; Eng, S.; Tan, Y.; Wong, Z.; Lye, P.; Tan, L. Antioxidant and sensory properties of Thai herbal teas with emphasis on Thunbergia laurifolia Lindl. Chiang Mai J. Sci. 2012, 39, 599–609. [Google Scholar]
- Pukumpuang, W.; Thongwai, N.; Tragoolpua, Y. Total phenolic contents, antibacterial and antioxidant activities of some Thai medicinal plant extracts. J. Med. Plants Res. 2012, 6, 4953–4960. [Google Scholar] [CrossRef]
- Chivapat, S.; Chavalittumrong, P.; Attawish, A.; Bansiddhi, J.; Padungpat, S. Chronic toxicity of Thunbergia laurifolia Lindl. extract. J. Thai Trad. Altern. Med. 2010, 7, 17–25. [Google Scholar]
- Huang, N.; Hauck, C.; Yum, M.-Y.; Rizshsky, L.; Widrlechner, M.P.; McCoy, J.-A.; Murphy, P.A.; Dixon, P.M.; Nikolau, B.J.; Birt, D.F. Rosmarinic acid in Prunella vulgaris ethanol extract inhibits lipopolysaccharide-induced prostaglandin E2 and nitric oxide in RAW 264.7 mouse macrophages. J. Agric. Food Chem. 2009, 57, 10579–10589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mairuae, N.; Cheepsunthorn, P.; Buranrat, B. Antioxidant activity and inhibitory effect on nitric oxide production of Rang Chuet (Thunbergia laurifolia Lindl.) leaf extracts in lipopolysaccharide-stimulated BV2 microglial cells. Pharmacogn. Mag. 2020, 16, 573–577. [Google Scholar] [CrossRef]
- Papi, S.; Ahmadizar, F.; Hasanvand, A. The role of nitric oxide in inflammation and oxidative stress. Immunopathol. Persa 2019, 5, e08. [Google Scholar] [CrossRef]
- Gantner, B.N.; LaFond, K.M.; Bonini, M.G. Nitric oxide in cellular adaptation and disease. Redox Biol. 2020, 34, 101550. [Google Scholar] [CrossRef]
- Pittayapruek, P.; Meephansan, J.; Prapapan, O.; Komine, M.; Ohtsuki, M. Role of matrix metalloproteinases in photoaging and photocarcinogenesis. Int. J. Mol. Sci. 2016, 17, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quan, T.; Qin, Z.; Xia, W.; Shao, Y.; Voorhees, J.J.; Fisher, G.J. Matrix-degrading metalloproteinases in photoaging. J. Investig. Dermatol. Symp. Proc. 2009, 14, 20–24. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.; Pierrard, C.; Lejeune, F.; Hilaire, P.; Breton, L.; Bernerd, F. Photoprotective effect of a water-soluble extract of Rosmarinus officinalis L. against UV-induced matrix metalloproteinase-1 in human dermal fibroblasts and reconstructed skin. Eur. J. Dermatol. 2008, 18, 128–135. [Google Scholar] [PubMed]
- Murata, T.; Sasaki, K.; Sato, K.; Yoshizaki, F.; Yamada, H.; Mutoh, H.; Umehara, K.; Miyase, T.; Warashina, T.; Aoshima, H.; et al. Matrix metalloproteinase-2 inhibitors from Clinopodium chinense var. parviflorum. J. Nat. Prod. 2009, 72, 1379–1384. [Google Scholar] [CrossRef] [PubMed]
- Ao, C.; Li, A.; Elzaawely, A.; Tawata, S. MMP-13 inhibitory activity of thirteen selected plant species from Okinawa. Int. J. Pharmacol. 2008, 4, 202–207. [Google Scholar] [CrossRef] [Green Version]
- Eo, S.H.; Kim, S.J. Rosmarinic acid induces rabbit articular chondrocyte differentiation by decreases matrix metalloproteinase-13 and inflammation by upregulating cyclooxygenase-2 expression. J. Biomed. Sci. 2017, 24, 75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matwiejczuk, N.; Galicka, A.; Zaręba, I.; Brzóska, M.M. The Protective effect of rosmarinic acid against unfavorable influence of methylparaben and propylparaben on collagen in human skin fibroblasts. Nutrients 2020, 12, 1282. [Google Scholar] [CrossRef]
- Sreejayan; Rao, M.N. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997, 49, 105–107. [Google Scholar]
- Kidarn, S.; Saenjum, C.; Hongwiset, D.; Phrutivorapongkul, A. Furanocoumarins from Kaffir lime and their inhibitory effects on inflammatory mediator production. Cogent Chem. 2018, 4, 1529259. [Google Scholar] [CrossRef]
- Zou, Y.; Lu, Y.; Wei, D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J. Agric. Food Chem. 2004, 52, 5032–5039. [Google Scholar] [CrossRef] [PubMed]
- Saenjum, C.; Chaiyasut, C.; Chansakaow, S.; Suttajit, M.; Sirithunyalug, B. Antioxidant and anti-inflammatory activities of gamma-oryzanol rich extracts from Thai purple rice bran. J. Med. Plants Res. 2012, 6, 1070–1077. [Google Scholar]
- Phromnoi, K.; Suttajit, M.; Saenjum, C. Polyphenols and rosmarinic acid contents, antioxidant and anti-inflammatory activities of different solvent fractions from Nga-Mon (Perilla frutescens) leaf. J. Pharm. Nutr. Sci. 2019, 9, 1–7. [Google Scholar]
- Fadel, O.; El Kirat, K.; Morandat, S. The natural antioxidant rosmarinic acid spontaneously penetrates membranes to inhibit lipid peroxidation in situ. Biochim. Biophys. Acta 2011, 1808, 2973–2980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Gamaro, G.D.; Suyenaga, E.; Borsoi, M.; Lermen, J.; Pereira, P.; Ardenghi, P. Effect of rosmarinic and caffeic acids on inflammatory and nociception process in rats. Int. Sch. Res. Not. Pharmacol. 2011, 2011, 451682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.H.; Li, H.J.; Wu, N.L.; Hsiao, C.Y.; Lin, C.N.; Chang, H.H.; Hung, C.F. Photoprotective effects of cycloheterophyllin against UVA-induced damage and oxidative stress in human dermal fibroblasts. PLoS ONE 2016, 11, e0161767. [Google Scholar] [CrossRef] [PubMed]
- Ruangsuriya, J.; Charumanee, S.; Jiranusornkul, S.; Sirisa-Ard, P.; Sirithunyalug, B.; Sirithunyalug, J.; Pattananandecha, T.; Saenjum, C. Depletion of β-sitosterol and enrichment of quercetin and rutin in Cissus quadrangularis Linn fraction enhanced osteogenic but reduced osteoclastogenic marker expression. BMC Complement. Med. Ther. 2020, 20, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
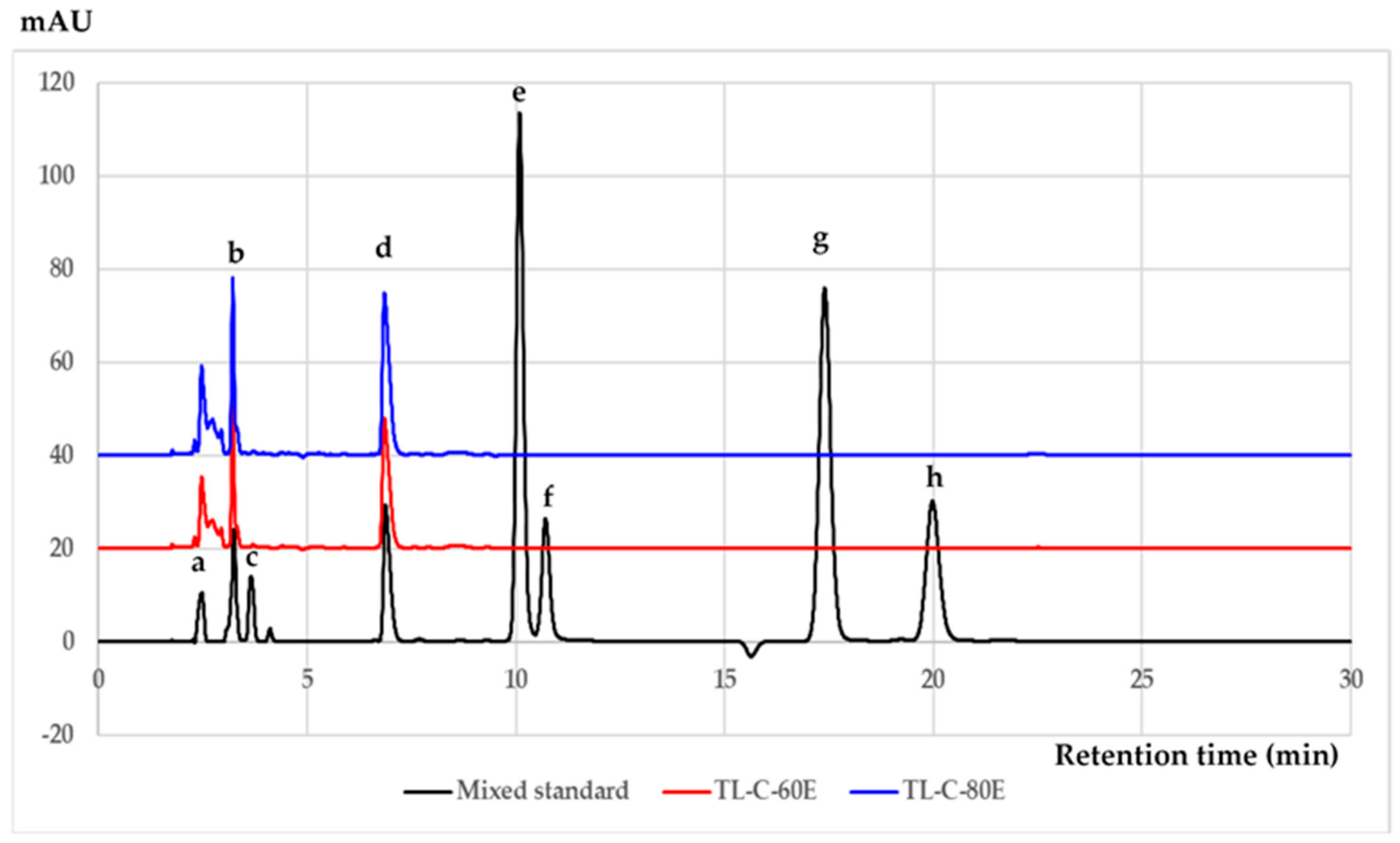

| Compounds | Precision (% RSD) | Linear Range (μg/mL) | Correlation Coefficient | LOD (μg/mL) | LOQ (μg/mL) | |||
|---|---|---|---|---|---|---|---|---|
| Retention Time | Peak Area | |||||||
| Intra-Day | Inter-Day | Intra-Day | Inter-Day | |||||
| Gallic acid | 1.09 | 1.28 | 0.85 | 0.97 | 5–100 | 0.9998 | 0.24 | 0.83 |
| Caffeic acid | 1.02 | 1.15 | 0.89 | 1.02 | 5–100 | 0.9992 | 0.40 | 1.35 |
| Rutin | 1.25 | 1.42 | 1.06 | 1.13 | 10–100 | 0.9994 | 0.30 | 1.01 |
| Rosmarinic acid | 1.01 | 1.18 | 0.81 | 0.92 | 5–100 | 0.9998 | 0.19 | 0.62 |
| Luteolin | 0.93 | 1.03 | 0.78 | 0.90 | 5–100 | 0.9998 | 0.11 | 0.36 |
| Quercetin | 1.11 | 1.32 | 0.98 | 1.09 | 5–75 | 0.9997 | 0.13 | 0.45 |
| Apigenin | 0.75 | 0.92 | 0.62 | 0.74 | 5–100 | 0.9995 | 0.25 | 0.82 |
| Kaempferol | 0.65 | 0.78 | 0.58 | 0.65 | 10–100 | 0.9996 | 0.33 | 1.07 |
| Samples | Phytochemical Content (mg/g Extract) | |
|---|---|---|
| Rosmarinic Acid | Caffeic Acid | |
| TL−W | 147.3 ± 10.4 f | 120.8 ± 13.4 e |
| TL−60E | 177.6 ± 12.3 d,e | 158.2 ± 10.7 c,d |
| TL−80E | 183.2 ± 10.6 d | 161.9 ± 12.6 b,c |
| TL−C−W | 221.8 ± 17.7 c | 147.2 ± 11.4 d |
| TL−C−60E | 260.5 ± 16.6 b | 186.8 ± 12.7 a |
| TL−C−80E | 292.3 ± 18.3 a | 195.4 ± 16.5 a |
| Samples/Positive Control | IC50 (ppm) | ||
|---|---|---|---|
| Nitric Oxide | Superoxide Anion | Lipid Peroxidation | |
| TL−W | 43.84 ± 1.87 f,g | 35.55 ± 0.82 f | 72.28 ± 3.23 g,h |
| TL−60E | 38.57 ± 1.63 d,e | 31.73 ± 1.39 d,e | 65.94 ± 2.95 g |
| TL−80E | 40.56 ± 1.68 f | 29.47 ± 1.48 d | 52.72 ± 2.84 e |
| TL−C−W | 36.37 ± 1.56 d | 28.24 ± 1.57 d | 57.85 ± 2.73 f |
| TL−C−60E | 26.59 ± 1.42 c | 18.53 ± 1.23 c | 39.29 ± 1.77 d |
| TL−C−80E | 25.18 ± 1.26 c | 20.58 ± 1.06 c | 33.89 ± 1.85 c |
| Rosmarinic acid | 16.72 ± 0.92 b | 8.34 ± 0.45 b | 30.64 ± 1.32 b |
| Curcumin | 9.14 ± 0.68 a | ND | ND |
| L-ascorbic acid | ND | 6.91 ± 0.26 a | ND |
| α-Tocopherol | ND | ND | 14.32 ± 0.93 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pattananandecha, T.; Apichai, S.; Julsrigival, J.; Ungsurungsie, M.; Samuhasaneetoo, S.; Chulasiri, P.; Kwankhao, P.; Pitiporn, S.; Ogata, F.; Kawasaki, N.; et al. Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves. Plants 2021, 10, 1648. https://doi.org/10.3390/plants10081648
Pattananandecha T, Apichai S, Julsrigival J, Ungsurungsie M, Samuhasaneetoo S, Chulasiri P, Kwankhao P, Pitiporn S, Ogata F, Kawasaki N, et al. Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves. Plants. 2021; 10(8):1648. https://doi.org/10.3390/plants10081648
Chicago/Turabian StylePattananandecha, Thanawat, Sutasinee Apichai, Jakaphun Julsrigival, Malyn Ungsurungsie, Suched Samuhasaneetoo, Pat Chulasiri, Pakakrong Kwankhao, Supaporn Pitiporn, Fumihiko Ogata, Naohito Kawasaki, and et al. 2021. "Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves" Plants 10, no. 8: 1648. https://doi.org/10.3390/plants10081648
APA StylePattananandecha, T., Apichai, S., Julsrigival, J., Ungsurungsie, M., Samuhasaneetoo, S., Chulasiri, P., Kwankhao, P., Pitiporn, S., Ogata, F., Kawasaki, N., & Saenjum, C. (2021). Antioxidant Activity and Anti-Photoaging Effects on UVA-Irradiated Human Fibroblasts of Rosmarinic Acid Enriched Extract Prepared from Thunbergia laurifolia Leaves. Plants, 10(8), 1648. https://doi.org/10.3390/plants10081648