Effect of Long-Term Storage on Mycobiota of Barley Grain and Malt
Abstract
:1. Introduction
2. Results
2.1. Endogenous Mycobiota of Barley
2.2. Endogenous Mycobiota of Malt
2.3. Toxigenicity of Penicillium Species
3. Discussion
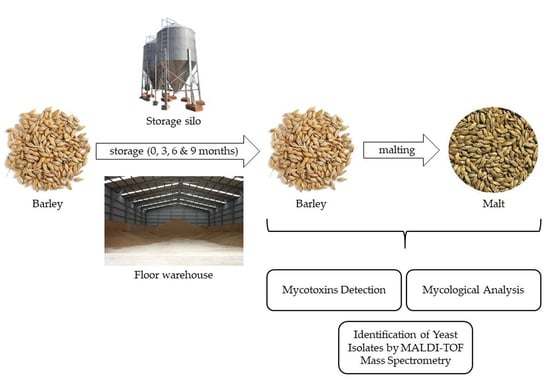
4. Materials and Methods
4.1. Samples
4.2. Micro-Malting Procedure
4.3. Mycological Analysis of Barley and Malt
4.4. Mycotoxins Detection
4.5. Mass Spectrometry Identification of Yeast Isolates
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Magan, N.; Aldred, D. Managing microbial spoilage in cereal and baking products. In Food Spoilage Microorganisms; Blackburn, C.d.W., Ed.; Woodhead Publishing: Cambridge, UK, 2006; pp. 194–212. ISBN 978-1-85573-966-6. [Google Scholar]
- Van Nierop, S.N.E.; Rautenbach, M.; Axcell, B.C.; Cantrell, I.C. The impact of microorganisms on barley and malt quality—A review. J. Am. Soc. Brew. Chem. 2006, 64, 69–78. [Google Scholar] [CrossRef]
- Bullerman, L.B.; Bianchini, A. Food safety issues and the microbiology of cereals and cereal products. In Microbiologically Safe Foods; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009; pp. 315–335. [Google Scholar]
- Niessen, L. Fungal contamination of barley and malt. In Brewing Microbiology: Current Research, Omics and Microbial Ecology; Bokulich, N.A., Bamforth, C.W., Eds.; Caister Academic Press: Poole, UK, 2017; pp. 197–244. ISBN 978-1-910190-62-3. [Google Scholar]
- Piacentini, K.; Rocha, L.; Savi, G.; Carnielli-Queiroz, L.; De Carvalho Fontes, L.; Correa, B. Assessment of toxigenic fusarium species and their mycotoxins in brewing barley grains. Toxins 2019, 11, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noots, I.; Delcour, J.A.; Michiels, C.W. From field barley to malt: Detection and specification of microbial activity for quality aspects. Crit. Rev. Microbiol. 1999, 25, 121–153. [Google Scholar] [CrossRef] [PubMed]
- Laitila, A.; Kotaviita, E.; Peltola, P.; Home, S.; Wilhelmson, A. Indigenous microbial community of barley greatly influences grain germination and malt quality. J. Inst. Brew. 2007, 113, 9–20. [Google Scholar] [CrossRef]
- Magan, N.; Lacey, J. Effects of gas composition and water activity on growth of field and storage fungi and their interactions. Trans. Br. Mycol. Soc. 1984, 82, 305–314. [Google Scholar] [CrossRef]
- Christensen, C.M.; Meronuck, R.A. Quality Maintenance in Stored Grains and Seeds; University of Minessota Press: Minneapolis, MN, USA, 1986. [Google Scholar]
- Pitt, J.I.; Hocking, A.D. Fungi and Food Spoilage; Springer: Boston, MA, USA, 2009; ISBN 978-0-387-92206-5. [Google Scholar]
- Hill, R.A.; Lacey, J. The microflora of ripening barley grain and the effects of pre harvest fungicide application. Ann. Appl. Biol. 1983, 102, 455–465. [Google Scholar] [CrossRef]
- Flannigan, B. The microbiota of barley and malt. In Brewing Microbiology; Springer: Boston, MA, USA, 2003; pp. 113–180. [Google Scholar]
- Petters, H.I.; Flannigan, B.; Austin, B. Quantitative and qualitative studies of the microflora of barley malt production. J. Appl. Bacteriol. 1988, 65, 279–297. [Google Scholar] [CrossRef]
- Justé, A.; Malfliet, S.; Waud, M.; Crauwels, S.; De Cooman, L.; Aerts, G.; Marsh, T.L.; Ruyters, S.; Willems, K.; Busschaert, P.; et al. Bacterial community dynamics during industrial malting, with an emphasis on lactic acid bacteria. Food Microbiol. 2014, 39, 39–46. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Bamforth, C.W. The microbiology of malting and brewing. Microbiol. Mol. Biol. Rev. 2013, 77, 157–172. [Google Scholar] [CrossRef] [Green Version]
- Fleurat-Lessard, F. Integrated management of the risks of stored grain spoilage by seedborne fungi and contamination by storage mould mycotoxins—An update. J. Stored Prod. Res. 2017, 71, 22–40. [Google Scholar] [CrossRef]
- Raulio, M.; Wilhelmson, A.; Salkinoja-Salonen, M.; Laitila, A. Ultrastructure of biofilms formed on barley kernels during maltingwith and without starter culture. Food Microbiol. 2009, 26, 437–443. [Google Scholar] [CrossRef]
- Kumar Dikkala, P.; Hymavathi, T.V.; Roberts, P.; Sujatha, M. Effect of Heat Treatment and Gamma Irradiation on the Total Bacterial Count of Selected Millet Grains (Jowar, Bajra and Foxtail). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1293–1300. [Google Scholar] [CrossRef] [Green Version]
- Vegi, A.; Schwarz, P.; Wolf-Hall, C.E. Quantification of Tri5 gene, expression, and deoxynivalenol production during the malting of barley. Int. J. Food Microbiol. 2011, 150, 150–156. [Google Scholar] [CrossRef]
- Oliveira, P.M.; Mauch, A.; Jacob, F.; Waters, D.M.; Arendt, E.K. Fundamental study on the influence of Fusarium infection on quality and ultrastructure of barley malt. Int. J. Food Microbiol. 2012, 156, 32–43. [Google Scholar] [CrossRef]
- Laitila, A.; Sarlin, T.; Raulio, M.; Wilhelmson, A.; Kotaviita, E.; Huttunen, T.; Juvonen, R. Yeasts in malting, with special emphasis on Wickerhamomyces anomalus (synonym Pichia anomala). Antonie Van Leeuwenhoek 2011, 99, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulou, A.; Wheaton, L.; Muller, R. The control of selected micro-organisms during the malting process. J. Inst. Brew. 2000, 106, 179–188. [Google Scholar] [CrossRef]
- Beccari, G.; Caproni, L.; Tini, F.; Uhlig, S.; Covarelli, L. Presence of Fusarium species and other toxigenic fungi in malting barley and multi-mycotoxin analysis by liquid chromatography–High-resolution mass spectrometry. J. Agric. Food Chem. 2016, 64, 4390–4399. [Google Scholar] [CrossRef]
- Krasauskas, A. Fungi in malting barley grain and malt production. Biologija 2017, 63. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, L.K.; Cook, D.J.; Edwards, S.G.; Ray, R.V. The prevalence and impact of Fusarium head blight pathogens and mycotoxins on malting barley quality in UK. Int. J. Food Microbiol. 2014, 179, 38–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastanjević, K.; Krstanović, V.; Mastanjević, K.; Šarkanj, B. Malting and brewing industries encounter Fusarium spp. Related problems. Fermentation 2018, 4, 3. [Google Scholar] [CrossRef] [Green Version]
- Bíliková, J.; Hudec, K. Occurrence of Fusarium head blight of barley in Slovakia. J. Cent. Eur. Agric. 2014, 15, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Escrivá, L.; Oueslati, S.; Font, G.; Manyes, L. Alternaria mycotoxins in food and feed: An overview. J. Food Qual. 2017, 2017, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Birck, N.M.M.; Lorini, I.; Scussel, V.M. Fungus and mycotoxins in wheat grain at post harvest. In Proceedings of the 9th International Working Conference on Stored Product Protection, Campinas, São Paulo, Brazil, 15–18 October 2006; pp. 198–205. [Google Scholar]
- Filtenborg, J.C.; Frisvad, J.C.; Samson, R. Specific association of fungi to foods and influence of physical environmental factors. In Introduction to Food—And Airborne Fungi; Samson, R.A., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O., Eds.; Ponsen & Looyen: Wageningen, The Netherlands, 2002; pp. 306–320. [Google Scholar]
- Hashmi, F.M.H.; Ghaffar, A. Seed-borne mycoflora of wheat, sorghum and barley. Pak. J. Bot. 2006, 38, 185–192. [Google Scholar]
- Krnjaja, V.; Lukic, M.; Delic, N.; Tomic, Z.; Mandic, V.; Bijelic, Z.; Gogic, M. Mycobiota and mycotoxins in freshly harvested and stored maize. Biotechnol. Anim. Husb. 2015, 31, 291–302. [Google Scholar] [CrossRef]
- Dudoiu, R.; Cristea, S.; Lupu, C.; Popa, D.; Oprea, M. Micoflora associated with maize grains during storage period. Agrolife Sci. J. 2016, 5, 63–68. [Google Scholar]
- Bok, G.; Hallenberg, N.; Åberg, O. Mass occurrence of Penicillium corylophilum in crawl spaces, south Sweden. Build. Environ. 2009, 44, 2413–2417. [Google Scholar] [CrossRef]
- Piacentini, K.C.; Savi, G.D.; Pereira, M.E.V.; Scussel, V.M. Fungi and the natural occurrence of deoxynivalenol and fumonisins in malting barley (Hordeum vulgare L.). Food Chem. 2015, 187, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez Pereyra, M.L.; Rosa, C.A.R.; Dalcero, A.M.; Cavaglieri, L.R. Mycobiota and mycotoxins in malted barley and brewer’s spent grain from Argentinean breweries. Lett. Appl. Microbiol. 2011, 53, 649–655. [Google Scholar] [CrossRef]
- Lugauskas, A.; Raila, A.; Zvicevicius, E.; Railiene, M.; Novosinskas, H. Factors determining accumulation of mycotoxin producers in cereal grain during harvesting. Ann. Agric. Environ. Med. 2007, 14, 173–186. [Google Scholar] [PubMed]
- Przybylska-Balcerek, A.; Kurasiak-Popowska, D.; Buśko, M.; Szwajkowska-Michałek, L.; Stuper-Szablewska, K. Contamination of barley grain with microscopic fungi and their metabolites in Poland in the years 2015–2016. Eur. J. Med. Technol. 2020, 1, 21–29. [Google Scholar]
- Clarke, J.H.; Hill, S.T. Mycofloras of moist barley during sealed storage in farm and laboratory silos. Trans. Br. Mycol. Soc. 1981, 77, 557–565. [Google Scholar] [CrossRef]
- Bolechová, M.; Benešová, K.; Běláková, S.; Čáslavský, J.; Pospíchalová, M.; Mikulíková, R. Determination of seventeen mycotoxins in barley and malt in the Czech Republic. Food Control 2015, 47, 108–113. [Google Scholar] [CrossRef]
- Khodaei, D.; Javanmardi, F.; Khaneghah, A.M. The global overview of the occurrence of mycotoxins in cereals: A three-year survey. Curr. Opin. Food Sci. 2021, 39, 36–42. [Google Scholar] [CrossRef]
- Magnoli, C.; Violante, M.; Combina, M.; Palacio, G.; Dalcero, A. Mycoflora and ochratoxin-producing strains of Aspergillus section Nigri in wine grapes in Argentina. Lett. Appl. Microbiol. 2003, 37, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Samson, R.A.; Hoekstra, E.S.; Frisvad, J.C.; Filtenborg, O. Introduction to Food—And Airborne Fungi, 6th ed.; Centraalbureau voor Schimmelcultures (CBS): Ulrecht, The Netherlands, 2002; ISBN 90-70351-42-0. [Google Scholar]
- Samson, R.A.; Houbraken, J.; Thrane, U.; Frisvad, J.C.; Andersen, B. Food and Indoor Fungi; Centraalbureau voor Schimmelcultures (CBS): Ulrecht, The Netherlands, 2010; ISBN 978-90-70351-82-3. [Google Scholar]
- Samson, R.A.; Frisvad, J.C. Penicillium Subgenus Penicillium: New Taxonomic Schemes and Mycotoxins and Other Extrolites; Centraalbureau voor Schimmelcultures (CBS): Ulrecht, The Netherlands, 2004; Volume 449, ISBN 90-70351-53-6. [Google Scholar]
- Gautam, A.; Sharma, S.; Bhadauria, R. Detection of toxigenic fungi and mycotoxins in medicinally important powdered herbal drugs. Internet J. Microbiol. 2009, 7, 1–8. [Google Scholar]
- González, H.H.L.; Martinez, E.J.; Pacin, A.; Resnik, S.L. Relationship between Fusarium graminearum and Alternaria alternata contamination and deoxynivalenol occurrence on Argentinian durum wheat. Mycopathologia 1998, 144, 97–102. [Google Scholar] [CrossRef]
- Samson, R.A.; Hoekstra, E.S.; Lund, F.; Filtenborg, O.; Frisvad, J.C. Method for the detection, isolation and characterisation of food-borne fungi. In Introduction to Food—And Airborne Fungi; Samson, R.A., Hoekstra, E.S., Frisvad, J.C., Filtenborg, O., Eds.; Centraalbureau voor Schimmelcultures (CBS): Ulrecht, The Netherlands, 2002; pp. 283–297. [Google Scholar]
- Labuda, R.; Tancinová, D. Fungi recovered from Slovakian poultry feed mixtures and their toxinogenity. Ann. Agric. Environ. Med. 2006, 13, 193–200. [Google Scholar]
- Kačániová, M.; Kunová, S.; Sabo, J.; Ivanišová, E.; Žiarovská, J.; Felsöciová, S.; Terentjeva, M. Identification of Yeasts with Mass Spectrometry during Wine Production. Fermentation 2020, 6, 5. [Google Scholar] [CrossRef] [Green Version]
| Fungal Taxa/ Varieties | after Harvest | after 3 Months | after 6 Months | after 9 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | K | W | L | K | W | L | K | W | L | K | W | |
| Alternaria | 44 | 19 | 19 | 11 | 11 | 23 | 26 | 16 | 23 | 24 | 10 | 19 |
| Arthrinium | 4 | 9 | 4 | 11 | 3 | 2 | 7 | 1 | 3 | 4 | 4 | 1 |
| Aspergillus | 1 | 2 | 2 | 2 | 2 | 1 | ||||||
| Bipolaris | 3 | |||||||||||
| Cladosporium | 1 | 27 | 1 | 2 | 2 | 8 | 4 | 13 | ||||
| Epicoccum | 6 | 3 | 2 | 5 | 4 | 4 | 4 | 4 | 7 | 2 | 1 | |
| Fusarium | 15 | 4 | 7 | 1 | 10 | 4 | 2 | 1 | 1 | |||
| Geotrichum | 2 | 1 | ||||||||||
| Mucor | 1 | 1 | ||||||||||
| Penicillium | 2 | 1 | 2 | 5 | 2 | 5 | 4 | 5 | 5 | |||
| P. aurantiogriseum | 1 | 1 | ||||||||||
| P. brevicompactum | 1 | 1 | 2 | |||||||||
| P. crustosum | 1 | |||||||||||
| P. expansum | 3 | |||||||||||
| P. glabrum | 1 | |||||||||||
| P. griseofulvum | 1 | 1 | 2 | |||||||||
| P. chrysogenum | 2 | |||||||||||
| P. raistrickii | 1 | 1 | 1 | |||||||||
| Penicillium spp. | 1 | 2 | 3 | 2 | 3 | |||||||
| Rhizopus | 1 | 5 | 2 | 19 | 7 | 2 | 14 | 1 | 11 | |||
| Sordaria | 1 | 1 | ||||||||||
| Stemphylium | 1 | 3 | ||||||||||
| Mycelia sterilia | 2 | 2 | 2 | 3 | ||||||||
| Total | 72 | 38 | 32 | 65 | 40 | 41 | 58 | 44 | 47 | 52 | 39 | 41 |
| Fungal Taxa/Varieties | after Harvest | after 3 Months | after 6 Months | after 9 Months | from 3–9 Months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IF | RD | n | IF | RD | n | IF | RD | n | IF | RD | n | IF | RD | |
| Alternaria | 82 | 100 | 58 | 45 | 100 | 31 | 65 | 100 | 44 | 53 | 100 | 40 | 163 | 100 | 38 |
| Arthrinium | 17 | 100 | 12 | 16 | 100 | 11 | 11 | 100 | 7 | 9 | 100 | 7 | 36 | 100 | 8 |
| Aspergillus | 1 | 33 | <1 | 2 | 33 | 2 | 4 | 67 | 3 | 4 | 67 | 3 | 10 | 55 | 2 |
| Bipolaris | 3 | 33 | 2 | 3 | 11 | <1 | |||||||||
| Cladosporium | 1 | 33 | <1 | 28 | 67 | 19 | 4 | 67 | 3 | 25 | 100 | 19 | 57 | 78 | 13 |
| Epicoccum | 11 | 100 | 8 | 9 | 67 | 6 | 12 | 100 | 8 | 10 | 100 | 7 | 31 | 89 | 7 |
| Fusarium | 19 | 67 | 13 | 8 | 67 | 6 | 16 | 100 | 11 | 2 | 67 | 1.5 | 26 | 78 | 6 |
| Geotrichum | 2 | 33 | 1 | 1 | 33 | <1 | 3 | 22 | <1 | ||||||
| Mucor | 1 | 33 | <1 | 1 | 33 | <1 | 2 | 22 | <1 | ||||||
| Penicillium | 5 | 100 | 3 | 12 | 100 | 8 | 14 | 100 | 10 | 31 | 100 | 7 | |||
| P. aurantiogriseum | 2 | 67 | 17 | 2 | 22 | 6 | |||||||||
| P. brevicompactum | 1 | 33 | 8 | 3 | 67 | 21 | 4 | 33 | 13 | ||||||
| P. crustosum | 1 | 33 | 20 | 1 | 11 | 3 | |||||||||
| P. expansum | 3 | 33 | 21 | 3 | 11 | 10 | |||||||||
| P. glabrum | 1 | 33 | 8 | 1 | 11 | 3 | |||||||||
| P. griseofulvum | 1 | 33 | 20 | 3 | 67 | 25 | 4 | 33 | 13 | ||||||
| P. chrysogenum | 2 | 33 | 14 | 2 | 11 | 6 | |||||||||
| P. raistrickii | 2 | 67 | 17 | 1 | 33 | 7 | 3 | 33 | 10 | ||||||
| Penicillium spp. | 3 | 67 | 60 | 3 | 33 | 25 | 5 | 67 | 36 | 11 | 56 | 35 | |||
| Rhizopus | 6 | 67 | 4 | 28 | 100 | 19 | 17 | 100 | 11 | 11 | 33 | 8 | 56 | 78 | 13 |
| Sordaria | 1 | 33 | <1 | 1 | 33 | <1 | 1 | 11 | <1 | ||||||
| Stemphylium | 4 | 67 | 3 | 4 | 22 | <1 | |||||||||
| Mycelia sterilia | 4 | 67 | 3 | 2 | 33 | 1 | 3 | 33 | 2 | 5 | 22 | 1 | |||
| Total | 142 | 146 | 149 | 133 | 428 | ||||||||||
| Fungal Taxa/Varieties | after 3 Months | after 6 Months | after 9 Months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L | K | W | L | K | W | L | K | W | |
| Alternaria | 11 | 15 | 26 | 26 | 7 | 16 | 21 | 4 | 10 |
| Arthrinium | 11 | 4 | 1 | 1 | 10 | 13 | 12 | ||
| Aspergillus | 1 | 1 | |||||||
| Cladosporium | 3 | 1 | 1 | 3 | 2 | ||||
| Epicoccum | 3 | 5 | 4 | 3 | 4 | 1 | |||
| Fusarium | 6 | 6 | 8 | 2 | |||||
| Mucor | 1 | ||||||||
| Penicillium | 4 | 2 | 1 | 3 | 1 | 2 | 1 | 3 | |
| P. aurantiogriseum | 3 | ||||||||
| P. crustosum | 2 | ||||||||
| P. glabrum | 1 | ||||||||
| P. griseofulvum | 2 | 1 | 1 | ||||||
| P. hordei | 1 | ||||||||
| P. chrysogenum | 1 | 1 | |||||||
| P. raistrickii | 1 | 1 | 1 | ||||||
| Penicillium spp. | 1 | ||||||||
| Rhizopus | 2 | 6 | 1 | 3 | 1 | ||||
| Sordaria | 6 | 1 | 1 | ||||||
| Mycelia sterilia | 4 | 6 | 9 | 7 | |||||
| Total | 47 | 43 | 44 | 35 | 24 | 30 | 39 | 19 | 26 |
| Fungal Taxa/Varieties | after 3 Months | after 6 Months | after 9 Months | from 3–9 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IF | RD | n | IF | RD | n | IF | RD | N | IF | RD | |
| Alternaria | 52 | 100 | 39 | 49 | 100 | 55 | 35 | 100 | 42 | 136 | 100 | 44 |
| Arthrinium | 16 | 100 | 12 | 1 | 33 | 1 | 35 | 100 | 42 | 52 | 78 | 17 |
| Aspergillus | 1 | 33 | <1 | 1 | 33 | 1 | 2 | 22 | <1 | |||
| Cladosporium | 5 | 100 | 4 | 3 | 33 | 3 | 2 | 33 | 2 | 10 | 56 | 3 |
| Epicoccum | 8 | 67 | 6 | 7 | 67 | 8 | 5 | 67 | 6 | 20 | 67 | 5 |
| Fusarium | 20 | 100 | 15 | 2 | 33 | 2 | 22 | 44 | 7 | |||
| Mucor | 1 | 33 | <1 | 1 | 11 | <1 | ||||||
| Penicillium | 7 | 100 | 5 | 6 | 100 | 7 | 4 | 67 | 5 | 17 | 89 | 5 |
| P. aurantiogriseum | 3 | 33 | 50 | 3 | 11 | 18 | ||||||
| P. crustosum | 2 | 33 | 29 | 2 | 11 | 12 | ||||||
| P. glabrum | 1 | 33 | 14 | 1 | 11 | 6 | ||||||
| P. griseofulvum | 2 | 33 | 29 | 1 | 33 | 17 | 1 | 33 | 25 | 4 | 33 | 23 |
| P. hordei | 1 | 33 | 25 | 1 | 11 | 6 | ||||||
| P. chrysogenum | 1 | 33 | 14 | 1 | 33 | 25 | 2 | 22 | 12 | |||
| P. raistrickii | 2 | 67 | 33 | 1 | 33 | 25 | 3 | 33 | 18 | |||
| Penicillium spp. | 1 | 33 | 14 | 1 | 11 | 6 | ||||||
| Rhizopus | 8 | 67 | 6 | 4 | 67 | 5 | 1 | 33 | 1 | 13 | 56 | 4 |
| Sordaria | 6 | 33 | 4.5 | 2 | 67 | 2 | 8 | 33 | 3 | |||
| Mycelia sterilia | 10 | 67 | 7.5 | 16 | 67 | 18 | 26 | 44 | 8 | |||
| Total | 134 | 89 | 84 | 307 | ||||||||
| Fungal Taxa/Varieties | after Harvest | after 3 Months | after 6 Months | after 9 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| L | K | W | L | K | W | L | K | W | L | K | W | |
| Absidia | 2 | |||||||||||
| Alternaria | 11 | 3 | 36 | 5 | 1 | 16 | 22 | 3 | 36 | 3 | 16 | |
| Arthrinium | 4 | |||||||||||
| Aspergillus | 1 | |||||||||||
| Cladosporium | 3 | 9 | 4 | 2 | 3 | 2 | 2 | 1 | ||||
| Epicoccum | 10 | 5 | 4 | 3 | 1 | 2 | 4 | 2 | 2 | |||
| Fusarium | 7 | 2 | 9 | 2 | 4 | 1 | ||||||
| Geotrichum | 1 | |||||||||||
| Mucor | 1 | 1 | 4 | 16 | 1 | 3 | 3 | 2 | 1 | |||
| Penicillium | 2 | 1 | 2 | 4 | ||||||||
| P. griseofulvum | 1 | 1 | ||||||||||
| P. chrysogenum | 1 | |||||||||||
| P. raistrickii | 3 | |||||||||||
| Penicillium spp. | 1 | 1 | 1 | |||||||||
| Rhizopus | 1 | 1 | 7 | 8 | ||||||||
| Mycelia sterilia | 2 | 3 | 1 | 3 | 1 | 5 | 2 | 2 | ||||
| Total | 35 | 23 | 54 | 16 | 11 | 36 | 32 | 13 | 49 | 20 | 9 | 25 |
| Fungal Taxa | after Harvest | after 3 Months | after 6 Months | after 9 Months | from 3–9 Months | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IF | RD | n | IF | RD | n | IF | RD | n | IF | RD | n | IF | RD | |
| Absidia | 2 | 33 | 3 | 2 | 11 | <1 | |||||||||
| Alternaria | 50 | 100 | 45 | 22 | 100 | 35 | 61 | 100 | 65 | 19 | 67 | 35 | 102 | 89 | 48 |
| Arthrinium | 4 | 33 | 7 | 4 | 11 | 2 | |||||||||
| Aspergillus | 1 | 33 | 1 | 1 | 11 | <1 | |||||||||
| Cladosporium | 14 | 67 | 12 | 4 | 33 | 6 | 7 | 100 | 7 | 3 | 67 | 6 | 14 | 67 | 7 |
| Epicoccum | 19 | 100 | 17 | 3 | 33 | 5 | 7 | 100 | 7 | 4 | 67 | 7 | 14 | 67 | 7 |
| Fusarium | 18 | 100 | 16 | 6 | 67 | 9 | 1 | 33 | 1 | 7 | 33 | 3 | |||
| Geotrichum | 1 | 33 | <1 | ||||||||||||
| Mucor | 2 | 67 | 2 | 20 | 67 | 32 | 7 | 100 | 7 | 3 | 67 | 6 | 30 | 78 | 14 |
| Penicillium | 2 | 33 | 2 | 1 | 33 | 2 | 2 | 33 | 2 | 4 | 33 | 7 | 7 | 33 | 3 |
| P. griseofulvum | 1 | 33 | 1 | 33 | 1 | 11 | |||||||||
| P. chrysogenum | 1 | 33 | |||||||||||||
| P. raistrickii | 3 | 33 | 3 | 11 | |||||||||||
| Penicillium spp. | 1 | 33 | 1 | 33 | 1 | 33 | 3 | 33 | |||||||
| Rhizopus | 1 | 33 | 2 | 1 | 33 | 1 | 15 | 67 | 28 | 17 | 44 | 8 | |||
| Mycelia sterilia | 6 | 100 | 5 | 4 | 67 | 6 | 7 | 67 | 7 | 2 | 33 | 4 | 13 | 56 | 6 |
| Total | 112 | 63 | 94 | 54 | 211 | ||||||||||
| Fungal Taxa/Varieties | after 3 Months | after 6 Months | after 9 Months | ||||||
|---|---|---|---|---|---|---|---|---|---|
| L | K | W | L | K | W | L | K | W | |
| Alternaria | 25 | 4 | 22 | 6 | 5 | 10 | 14 | ||
| Arthrinium | 4 | ||||||||
| Aspergillus | 1 | ||||||||
| Cladosporium | 10 | 1 | 1 | 2 | |||||
| Epicoccum | 4 | 2 | 2 | 2 | 3 | ||||
| Fusarium | 1 | ||||||||
| Geotrichum | 3 | ||||||||
| Mucor | 1 | 2 | 6 | 2 | 8 | ||||
| Penicillium | 1 | 8 | 5 | 9 | |||||
| P. aurantiogriseum | 1 | ||||||||
| P. brevicompactum | 2 | ||||||||
| P. corylophilum | 1 | 3 | |||||||
| P. hordei | 1 | ||||||||
| P. polonicum | 3 | ||||||||
| Penicillium spp. | 5 | 7 | |||||||
| Rhizopus | 1 | 17 | 4 | ||||||
| Mycelia sterilia | 2 | 1 | 2 | 1 | |||||
| Total | 43 | 18 | 32 | 8 | 6 | 12 | 26 | 25 | 22 |
| Fungal Taxa | after 3 Months | after 6 Months | after 9 Months | from 3–9 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | IF | RD | n | IF | RD | n | IF | RD | n | IF | RD | |
| Alternaria | 51 | 100 | 55 | 21 | 100 | 81 | 14 | 33 | 19 | 86 | 78 | 45 |
| Arthrinium | 4 | 33 | 5 | 4 | 11 | 2 | ||||||
| Aspergillus | 1 | 33 | 1 | 1 | 11 | <1 | ||||||
| Cladosporium | 11 | 67 | 12 | 1 | 33 | 4 | 2 | 33 | 3 | 14 | 44 | 7 |
| Epicoccum | 6 | 67 | 6 | 7 | 100 | 10 | 13 | 56 | 7 | |||
| Fusarium | 1 | 33 | 4 | 1 | 11 | <1 | ||||||
| Geotrichum | 3 | 33 | 3 | 3 | 11 | 2 | ||||||
| Mucor | 9 | 100 | 10 | 10 | 67 | 14 | 19 | 56 | 10 | |||
| Penicillium | 9 | 67 | 10 | 14 | 67 | 19 | 23 | 44 | 12 | |||
| P. aurantiogriseum | 1 | 33 | 11 | 1 | 11 | 4 | ||||||
| P. brevicompactum | 2 | 33 | 14 | 2 | 11 | 9 | ||||||
| P. corylophilum | 4 | 67 | 44 | 4 | 22 | 17 | ||||||
| P. hordei | 1 | 33 | 11 | 1 | 11 | 4 | ||||||
| P. polonicum | 3 | 33 | 33 | 3 | 11 | 13 | ||||||
| Penicillium spp. | 12 | 67 | 86 | 12 | 22 | 52 | ||||||
| Rhizopus | 1 | 33 | 1 | 21 | 67 | 29 | 22 | 33 | 12 | |||
| Mycelia sterilia | 3 | 67 | 3 | 3 | 67 | 11 | 6 | 44 | 3 | |||
| Total | 93 | 26 | 73 | 192 | ||||||||
| Time of Storage | Varieties | Yeast log CFU/g | Fungi log CFU/g | Species |
|---|---|---|---|---|
| 0 | Laudis | 1.3 × 10 B | 3.6 × 10 C | Cladosporium, Fusarium, Mucor, Mycelia sterilia, Ogataea polymorpha, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 3.2 × 10 B | 3.6 × 10 B | Aspergillus, Cladosporium, Fusarium, Candida vini, Filobasidium oeirense, Candida spp., Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 9.1 × 10 A | 1.3 × 10 B | Fusarium, Penicillium chrysogenum, Mucor, Mycelia sterilia, Diutina catenulata, Candida saitoana, Sporobolomyces roseus | |
| Storage silo | ||||
| 3 months | Laudis | 1.8 × 10 b,AB | 8.5 × 10 a,A | Aspergillus, Fusarium, Ulocladium, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 8.9 × 10 b,A | 9.0 × 10 a,A | Aspergillus, Eurotium (now Aspergillus), Mucor, Penicillium aurantiogriseum, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.4 × 10 a,B | <1 × 10 | Cladosporium, P. citrinum, P. griseofulvum, Candida saitoana, Sporobolomyces roseus | |
| 6 months | Laudis | 2.4 × 10 a,A | 5.4 × 10 b,B | Alternaria, Arthrinium, Aspergillus, Fusarium, Penicillium griseofulvum, Ulocladium, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 9.7 × 10 a,A | 1.1 × 10 c,C | Aspergillus, Eurotium (now Aspergillus), P. aurantiogriseum, P. citrinum, P. polonicum, Mycelia sterilia, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.6 × 10 a,B | 8.6 × 10 b,A | Cladosporium, P. canescens, P. glabrum, P. polonicum, P. raistrickii, Mycelia sterilia, Candida saitoana, Sporobolomyces roseus | |
| 9 months | Laudis | 1.2 × 10 b,B | 3.5 × 10 c,C | Alternaria, Arthrinium, Cladosporium, Penicillium spp., Mycelia sterilia, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 7.9 × 10 c,A | 3.6 × 10 b,B | Alternaria, Arthrinium, Aspergillus, Cladosporium, Rhizopus, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.3 × 10 a,B | 7.7 × 10 a,A | Aspergillus, Cladosporium, Rhizopus, Candida saitoana, Sporobolomyces roseus | |
| Floor warehouse | ||||
| 3 months | Laudis | 2.4 × 10 a,A | 1.0 × 10 c,D | Alternaria, Arthrinium, Cladosporium, Epicoccum, Fusarium, Giberella (now Fusarium), Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 7.7 × 10 a,A | <1 × 10 | Arthrinium, Aspergillus, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.0 × 10 b,B | 2.3 × 10 b,B | Alternaria, Aspergillus, Mycelia sterilia, Penicillium aurantiogriseum, P. polonicum, P. griseofulvum, Candida saitoana, Sporobolomyces roseus | |
| 6 months | Laudis | 1.4 × 10 b,B | 1.8 × 10 b,D | Alternaria, Arthrinium, Cladosporium, Epicoccum, Mycelia sterilia, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 2.8 × 10 b,B | - | Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.1 × 10 b,B | 6.8 × 10 a,A | Alternaria, Cladosporium, Mycelia sterilia, Penicillium aurantiogriseum, P. polonicum, Candida saitoana, Sporobolomyces roseus | |
| 9 months | Laudis | 1.0 × 10 b,B | 2.2 × 10 a,D | Aspergillus, Penicillium brevicompactum, P. chrysogenum, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 2.2 × 10 a,B | - | Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 2.7 × 10 a,B | - | Candida saitoana, Sporobolomyces roseus | |
| Time of Storage | Varieties | Yeastlog CFU/g | Fungilog CFU/g | Species |
|---|---|---|---|---|
| 0 | Laudis | 4.1 × 10 A | 1.8 × 10 C | Aspergillus, Cladosporium, Fusarium, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 2.2 × 10 A | 1.1 × 10 B | Aspergillus, Cladosporium, Mucor, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 4.5 × 10 B | 2.2 × 10 C | Alternaria, Aspergillus, Cladosporium, Fusarium, Penicillium griseofulvum, Candida saitoana, Sporobolomyces roseus | |
| Silo | ||||
| 3 months | Laudis | 1.7 × 10 a,B | 1.6 × 10 b,C | Alternaria, Cladosporium, Penicillium corylophilum, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 2.3 × 10 a,A | 1.0 × 10 b,B | Alternaria, Aspergillus, Mucor, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.8 × 10 a,C | 1.7 × 10 b,C | Alternaria, Penicillium griseofulvum, Ulocladium, Candida saitoana, Sporobolomyces roseus | |
| 6 months | Laudis | 2.0 × 10 a,B | 1.7 × 10 b,C | Alternaria, Cladosporium, Penicillium crustosum, Rhodotorula mucilaginosa, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 2.4 × 10 a,A | 1.3 × 10 b,B | Alternaria, Penicillium corylophilum, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.8 × 10 a,C | 2.2 × 10 b,C | Alternaria, Mucor, Candida saitoana, Sporobolomyces roseus | |
| 9 months | Laudis | 1.6 × 10 a,B | 3.3 × 10 a,B | Alternaria, Cladosporium, Mucor, Mycelia sterilia, Rhodotorula mucilaginosa, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 1.4 × 10 b,AB | 2.2 × 10 a,B | Alternaria, Mucor, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 1.3 × 10 a,C | 5.4 × 10 a,B | Alternaria, Cladosporium, Candida saitoana, Sporobolomyces roseus | |
| Floor warehouse | ||||
| 3 months | Laudis | 2.1 × 10 a,B | 1.8 × 10 b,C | Alternaria, Epicoccum, Penicillium corylophilum, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 1.7 × 10 ab,AB | 1.1 × 10 b,B | Alternaria, Absidia, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 6.8 × 10 a,A | 1.9 × 10 b,C | Alternaria, Cladosporium, Candida saitoana, Sporobolomyces roseus | |
| 6 months | Laudis | 1.8 × 10 a,B | 2.7 × 10 b,B | Alternaria, Mucor, P. aurantiogriseum, Rhodotorula mucilaginosa, Rhodotorula spp., Issatchenkia orientalis |
| Kangoo | 2.5 × 10 a,A | 8.1 × 10 a,A | Alternaria, Mucor, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 2.2 × 10 b,BC | 7.7 × 10 a,A | Penicillium griseofulvum, Rhizopus, Candida saitoana, Sporobolomyces roseus | |
| 9 months | Laudis | 1.0 × 10 b,B | 8.3 × 10 a,A | Penicillium crustosum, Rhodotorula mucilaginosa |
| Kangoo | 1.0 × 10 b,B | 1.8 × 10 b,B | Mucor, Rhizopus, Rhodotorula spp., Wickerhamomyces anomalus | |
| Wintmalt | 3.1 × 10 b,B | 1.3 × 10 b,C | P. chrysogenum, P. spp., Candida saitoana, Sporobolomyces roseus | |
| Species | P | G | RC | CPA | PA |
|---|---|---|---|---|---|
| Toxigenicity from barley by the plate dilution method | |||||
| P. griseofulvum | 5 */6 ** | 6/6 | 0/6 | 6/6 | |
| P. chrysogenum | 3/3 | ||||
| P. raistrickii | 5/5 | ||||
| Toxigenicity from malt by the plate dilution method | |||||
| P. chrysogenum | 0/2 | ||||
| Toxigenicity from endogenous mycobiota of barley | |||||
| P. crustosum | 0/2 | 2/2 | |||
| P. hordei | 1/1 | ||||
| P. expansum | 3/3 | 3/3 | 0/3 | ||
| P. griseofulvum | 6/8 | 7/8 | 0/8 | 7/8 | |
| P. chrysogenum | 0/3 | ||||
| P. raistrickii | 8/8 | ||||
| Toxigenicity from endogenous mycobiota of malt | |||||
| P. griseofulvum | 1/2 | 2/2 | 0/2 | 2/2 | |
| P. chrysogenum | 0/1 | ||||
| P. raistrickii | 3/3 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felšöciová, S.; Kowalczewski, P.Ł.; Krajčovič, T.; Dráb, Š.; Kačániová, M. Effect of Long-Term Storage on Mycobiota of Barley Grain and Malt. Plants 2021, 10, 1655. https://doi.org/10.3390/plants10081655
Felšöciová S, Kowalczewski PŁ, Krajčovič T, Dráb Š, Kačániová M. Effect of Long-Term Storage on Mycobiota of Barley Grain and Malt. Plants. 2021; 10(8):1655. https://doi.org/10.3390/plants10081655
Chicago/Turabian StyleFelšöciová, Soňa, Przemysław Łukasz Kowalczewski, Tomáš Krajčovič, Štefan Dráb, and Miroslava Kačániová. 2021. "Effect of Long-Term Storage on Mycobiota of Barley Grain and Malt" Plants 10, no. 8: 1655. https://doi.org/10.3390/plants10081655
APA StyleFelšöciová, S., Kowalczewski, P. Ł., Krajčovič, T., Dráb, Š., & Kačániová, M. (2021). Effect of Long-Term Storage on Mycobiota of Barley Grain and Malt. Plants, 10(8), 1655. https://doi.org/10.3390/plants10081655