Copper Tolerance and Accumulation on Pelargonium graveolens L’Hér. Grown in Hydroponic Culture
Abstract
1. Introduction
2. Results
2.1. Visible Injury and Plant Growth
2.2. Effects on Plant Physiology Attributes
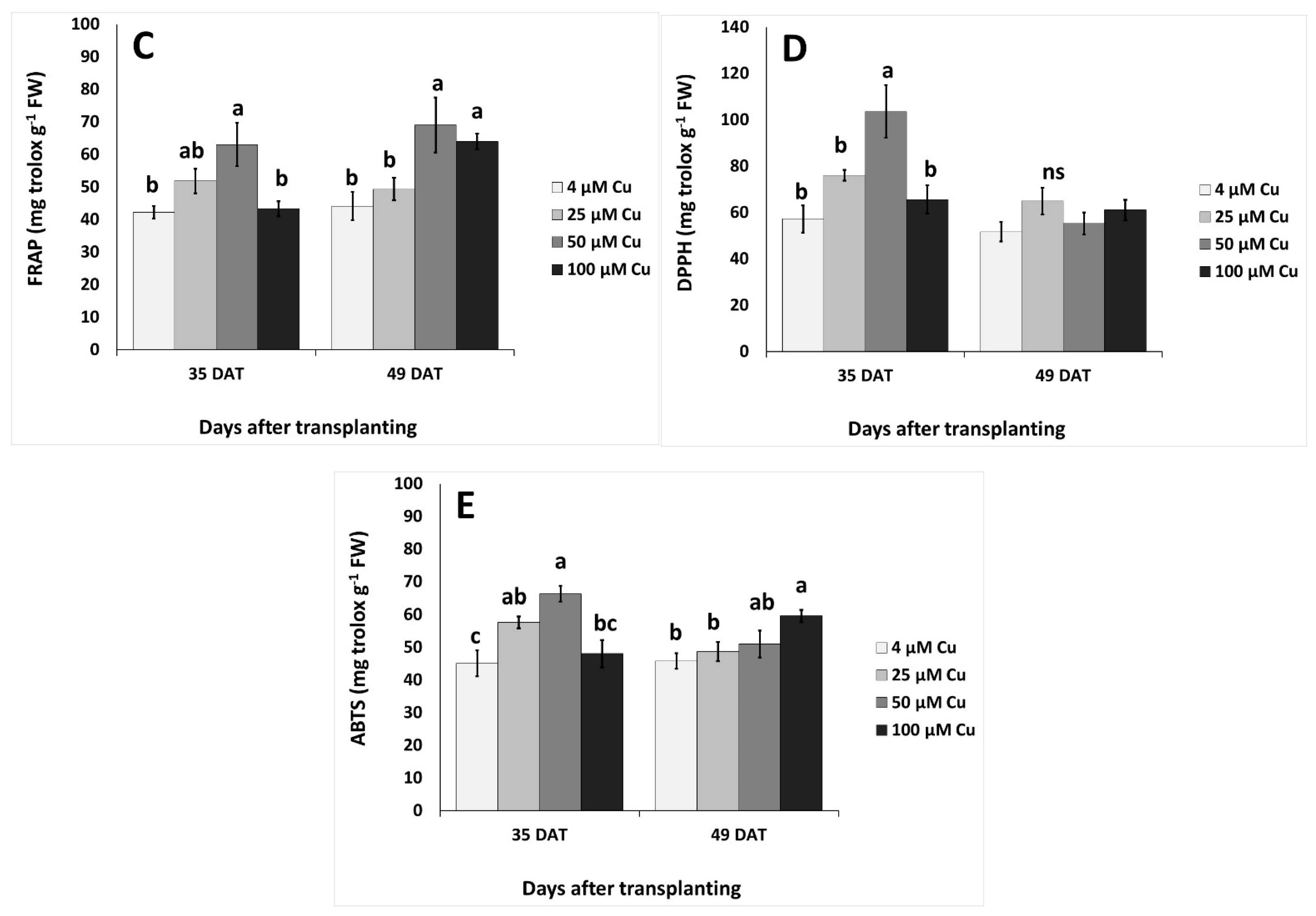
2.3. Effects on Total Phenols, Flavonoids and Antioxidant Activity
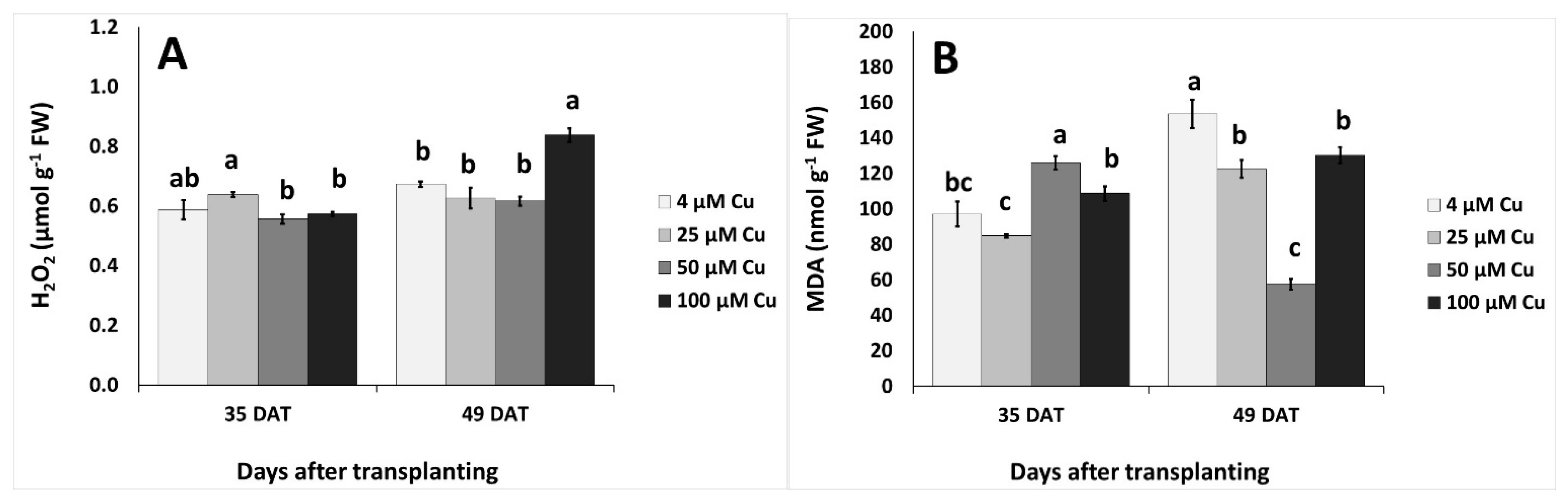
2.4. Plant Stress Indices
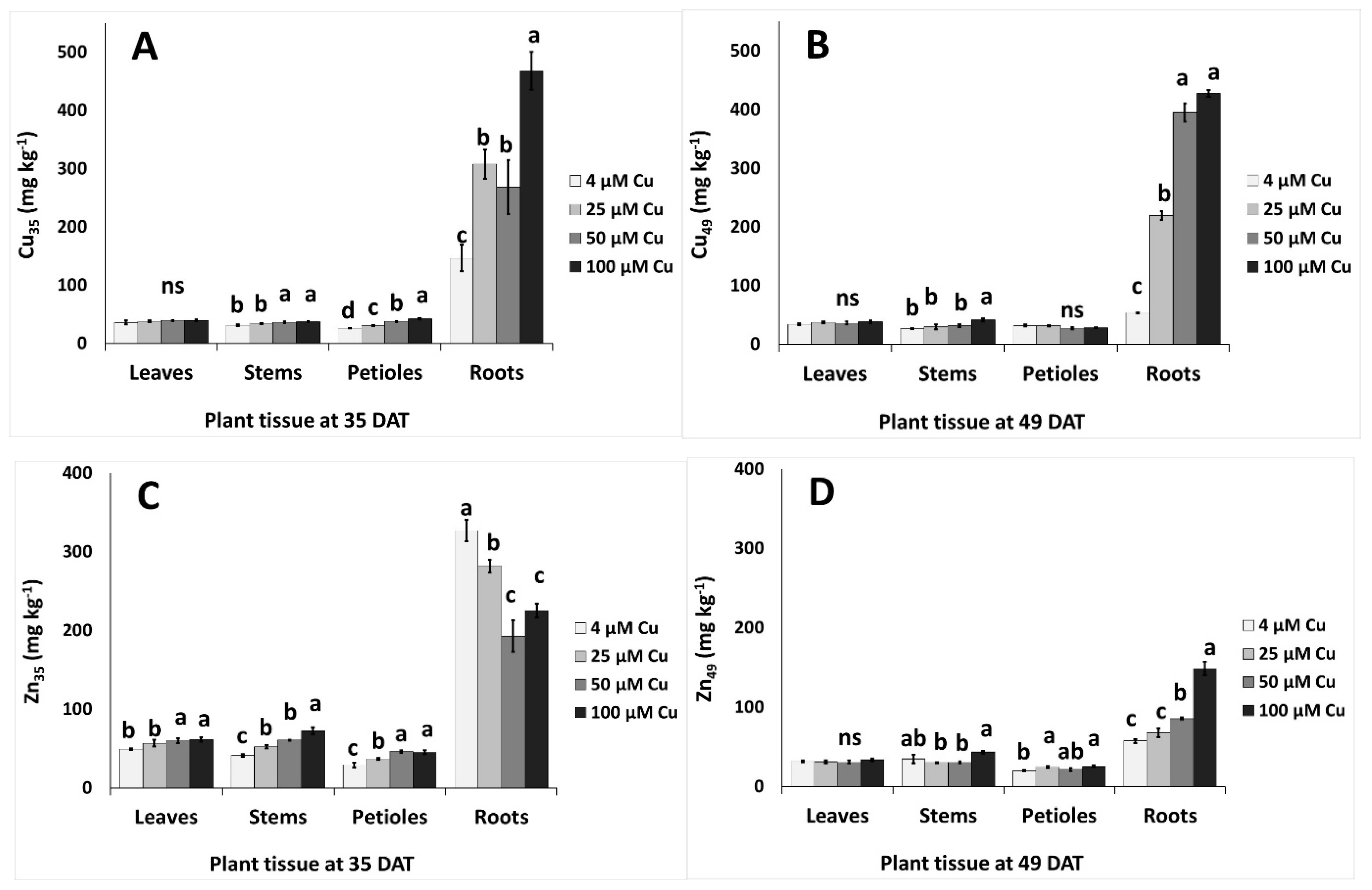
2.5. Copper Content in Plant Tissues
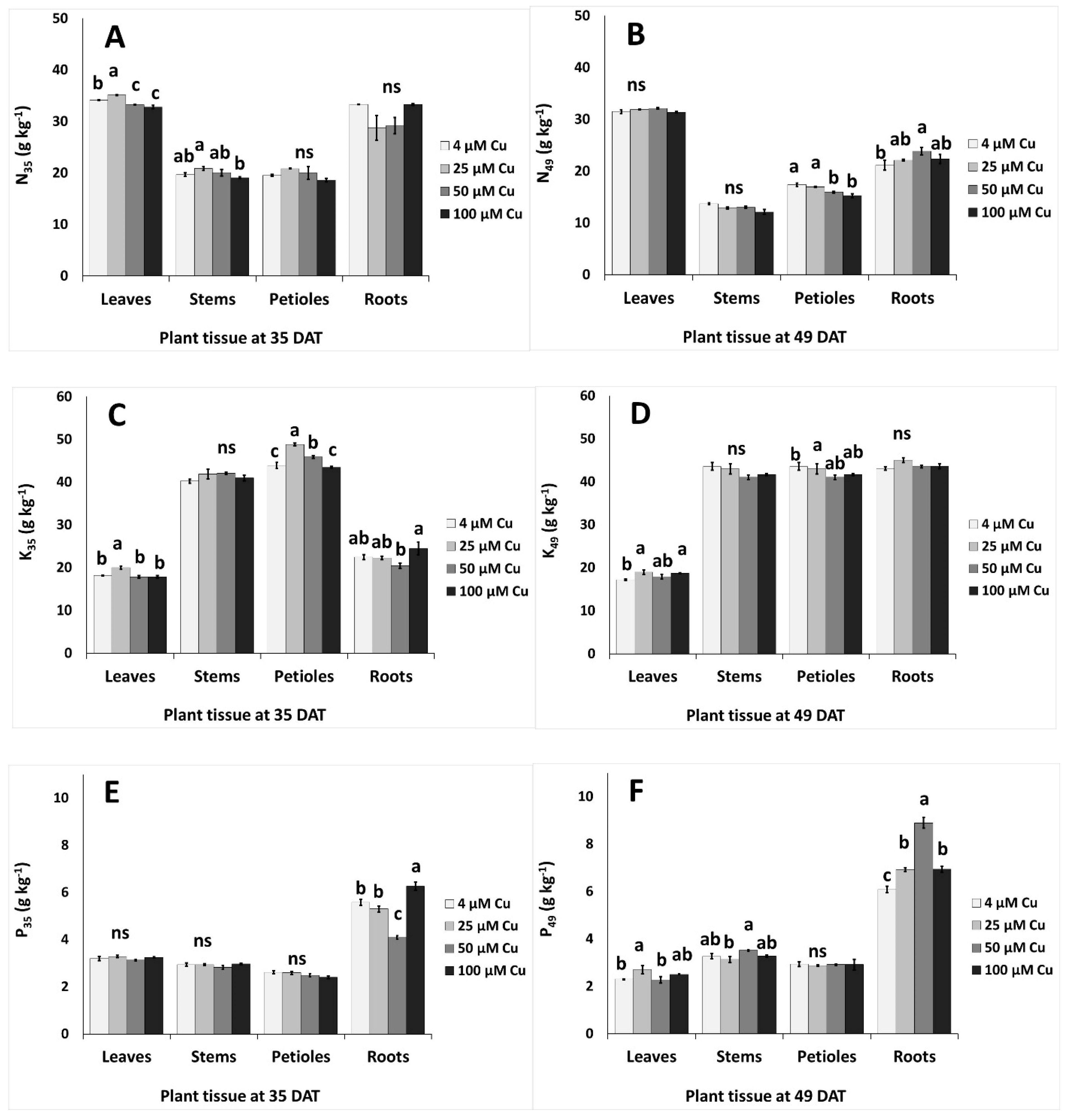
2.6. Responses of Other Nutrients
2.7. Regression Analysis
3. Discussion
4. Materials and Methods
4.1. Plant Material and Cultivation Conditions
4.2. Plant Growth and Physiological Measurements
4.3. Antioxidant Activity, Total Phenols and Total Flavonoids Content
4.4. Plant Stress Indicators
4.5. Nutrient Content
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gautam, S.; Anjani, K.; Srivastava, N. In vitro evaluation of excess copper affecting seedlings and their biochemical characteristics in Carthamus tinctorius L. (variety PBNS-12). Physiol. Mol. Biol. Plants 2016, 22, 121–129. [Google Scholar] [CrossRef]
- Amin, H.; Arain, B.A.; Jahangir, T.M.; Abbasi, A.R.; Mangi, J.; Abbasi, M.S.; Amin, F. Copper (Cu) tolerance and accumulation potential in four native plant species: A comparative study for effective phytoextraction technique. Geol. Ecol. Landsc. 2019, 5, 1–12. [Google Scholar] [CrossRef]
- MacKie, K.A.; Müller, T.; Kandeler, E. Remediation of copper in vineyards—A mini review. Environ. Pollut. 2012, 167, 16–26. [Google Scholar] [CrossRef]
- Adrees, M.; Ali, S.; Rizwan, M.; Ibrahim, M.; Abbas, F.; Farid, M.; Zia-ur-Rehman, M.; Irshad, M.K.; Bharwana, S.A. The effect of excess copper on growth and physiology of important food crops: A review. Environ. Sci. Pollut. Res. 2015, 22, 8148–8162. [Google Scholar] [CrossRef]
- Chen, J.; Shafi, M.; Li, S.; Wang, Y.; Wu, J.; Ye, Z.; Peng, D.; Yan, W.; Liu, D. Copper induced oxidative stresses, antioxidant responses and phytoremediation potential of Moso bamboo (Phyllostachys pubescens). Sci. Rep. 2015, 5, 13554. [Google Scholar] [CrossRef]
- Council of the European Union. Directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture (2018). Official Journal of the European Union, 4 July 2018; L 181, consolidated version. [Google Scholar]
- Kabata-Pendias, A.; Szteke, B. Copper: Trace Elements in Abiotic and Biotic Environments; CRC Press, Taylor & Francis Group: Abingdon, UK, 2015; p. 468. [Google Scholar]
- Conry, R.R. Copper: Inorganic and Coordination Chemistry. Encyclopedia of Inorganic and Bioinorganic Chemistry; John Wiley and Sons: Hoboken, NJ, USA, 2011. [Google Scholar]
- Wintz, H.; Fox, T.; Vulpe, C. Responses of plants to iron, zinc and copper deficiencies. Biochem. Soc. Trans. 2002, 30, 766–768. [Google Scholar] [CrossRef]
- Mahmood, T.; Islam, K.R. Response of rice seedlings to copper toxicity and acidity. J. Plant Nutr. 2006, 29, 943–957. [Google Scholar] [CrossRef]
- Li, S.; Zhang, G.; Gao, W.; Zhao, X.; Deng, C.; Lu, L. Plant growth, development and change in GSH level in Safflower (Carthamus tinctorius L.) exposed to copper and lead. Arch. Biol. Sci. 2015, 67, 385–396. [Google Scholar] [CrossRef]
- Rizwan, M.; Meunier, J.D.; Davidian, J.C.; Pokrovsky, O.S.; Bovet, N.; Keller, C. Silicon alleviates Cd stress of wheat seedlings (Triticum turgidum L. cv. Claudio) grown in hydroponics. Environ. Sci. Pollut. Res. 2016, 23, 1414–1427. [Google Scholar] [CrossRef]
- Saleem, M.H.; Kamran, M.; Zhou, Y.; Parveen, A.; Rehman, M.; Ahmar, S.; Malik, Z.; Mustafa, A.; Ahmad Anjum, R.M.; Wang, B.; et al. Appraising growth, oxidative stress and copper phytoextraction potential of flax (Linum usitatissimum L.) grown in soil differentially spiked with copper. J. Environ. Manag. 2020, 257. [Google Scholar] [CrossRef]
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 5th ed.; CRC Press: Boca Ratón, FL, USA, 2011. [Google Scholar]
- Yruela, I. Copper in plants: Acquisition, transport and interactions. Funct. Plant Biol. 2009, 36, 409–430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Liu, Q.; Qi, Y.; Duo, L. Responses of root growth and protective enzymes to copper stress in turfgrass. Acta Biol. Crac. Ser. Bot. 2010, 52, 7–11. [Google Scholar] [CrossRef]
- Liu, J.; Wang, J.; Lee, S.; Wen, R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE 2018, 13, e0203612. [Google Scholar] [CrossRef]
- Ku, H.-M.; Tan, C.-W.; Su, Y.-S.; Chiu, C.-Y.; Chen, C.-T.; Jan, F.-J. The effect of water deficit and excess copper on proline metabolism in Nicotiana benthamiana. Biol. Plant. 2011, 56, 337–343. [Google Scholar] [CrossRef]
- Monteoliva, M.I.; Rizzi, Y.S.; Cecchini, N.M.; Hajirezaei, M.R.; Alvarez, M.E. Context of action of Proline Dehydrogenase (ProDH) in the Hypersensitive Response of Arabidopsis. BMC Plant Biol. 2014, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Emamverdian, A.; Ding, Y.; Mokhberdoran, F.; Xie, Y. Heavy metal stress and some mechanisms of plant defense response. Sci. World J. 2015, 2015, 756120. [Google Scholar] [CrossRef]
- Mani, D.; Kumar, C. Biotechnological advances in bioremediation of heavy metals contaminated ecosystems: An overview with special reference to phytoremediation. Int. J. Environ. Sci. Technol. 2014, 11, 843–872. [Google Scholar] [CrossRef]
- Carolin, C.F.; Kumar, P.S.; Saravanan, A.; Joshiba, G.J.; Naushad, M. Efficient techniques for the removal of toxic heavy metals from aquatic environment: A review. J. Environ. Chem. Eng. 2017, 5, 2782–2799. [Google Scholar] [CrossRef]
- Derwich, E.; Benziane, Z.; Boukir, A. Chemical composition of leaf essential oil of Juniperus phoenicea and evaluation of its antibacterial activity. Int. J. Agric. Biol. 2010, 12, 199–204. [Google Scholar]
- Tripathy, V.; Basak, B.B.; Varghese, T.S.; Saha, A. Residues and contaminants in medicinal herbs—A review. Phytochem. Lett. 2015, 14, 67–78. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Handique, G.K.; Handique, A.K. Proline accumulation in lemongrass (Cymbopogon flexuosus Stapf.) due to heavy metal stress. J. Environ. Biol. 2009, 30, 299–302. [Google Scholar]
- Kováčik, J.; Klejdus, B.; Hedbavny, J.; Štork, F.; Bačkor, M. Comparison of cadmium and copper effect on phenolic metabolism, mineral nutrients and stress-related parameters in Matricaria chamomilla plants. Plant Soil 2009, 320, 231–242. [Google Scholar] [CrossRef]
- Ali, I.B.E.; Tajini, F.; Boulila, A.; Jebri, M.A.; Boussaid, M.; Messaoud, C.; Sebaï, H. Bioactive compounds from Tunisian Pelargonium graveolens (L’Hér.) essential oils and extracts: α-amylase and acethylcholinesterase inhibitory and antioxidant, antibacterial and phytotoxic activities. Ind. Crops Prod. 2020, 158, 112951. [Google Scholar] [CrossRef]
- Fiz, O.; Vargas, P.; Alarcón, M.; Aedo, C.; García, J.L.; Aldasoro, J.J. Phylogeny and historical biogeography of geraniaceae in relation to climate changes and pollination ecology. Syst. Bot. 2008, 33, 326–342. [Google Scholar] [CrossRef]
- Ćavar, S.; Maksimović, M.; Vidic, D.; Parić, A. Chemical composition and antioxidant and antimicrobial activity of essential oil of Artemisia annua L. from Bosnia. Ind. Crops Prod. 2012, 37, 479–485. [Google Scholar] [CrossRef]
- Boukhatem, M.N.; Kameli, A.; Saidi, F. Essential oil of Algerian rose-scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control 2013, 34, 208–213. [Google Scholar] [CrossRef]
- Tahan, F.; Yaman, M. Can the Pelargonium sidoides root extract EPs® 7630 prevent asthma attacks during viral infections of the upper respiratory tract in children? Phytomedicine 2013, 20, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Moyo, M.; Van Staden, J. Medicinal properties and conservation of Pelargonium sidoides DC. J. Ethnopharmacol. 2014, 152, 243–255. [Google Scholar] [CrossRef]
- Colling, J.; Groenewald, J.H.; Makunga, N.P. Genetic alterations for increased coumarin production lead to metabolic changes in the medicinally important Pelargonium sidoides DC (Geraniaceae). Metab. Eng. 2010, 12, 561–572. [Google Scholar] [CrossRef] [PubMed]
- Mehrarad, F.; Ziarati, P.; Mousavi, Z. Removing heavy metals from pharmaceutical effluent by Pelargonium grandiflorum. Biomed. Pharmacol. J. 2016, 9, 151–161. [Google Scholar] [CrossRef]
- Lam, E.J.; Gálvez, M.E.; Cánovas, M.; Montofré, Í.L.; Keith, B.F. Assessment of the adaptive capacity of plant species in copper mine tailings in arid and semiarid environments. J. Soils Sediments 2018, 18, 2203–2216. [Google Scholar] [CrossRef]
- Patel, A.; Patra, D.D. Phytoextraction capacity of Pelargonium graveolens L’Hér. grown on soilamended with tannery sludge—Its effect on the antioxidant activityand oil yield. Ecol. Eng. 2015, 74, 20–27. [Google Scholar] [CrossRef]
- Chand, S.; Singh, G.; Rajkumari; Patra, D.D. Performance of rose scented geranium (Pelargonium graveolens) in heavy metal polluted soil vis-à-vis phytoaccumulation of metals. Int. J. Phytoremediat. 2016, 18, 754–760. [Google Scholar] [CrossRef]
- Pandey, J.; Chand, S.; Chaurasiya, S.; Kumari, R.; Patra, D.D.; Verma, R.K.; Singh, S. Effect of tannery sludge amendments on the activity of soil enzymes and phytoremediation potential of two economically important cultivars of geranium (Pelargonium graveolens). Soil Sediment Contam. 2019, 28, 395–410. [Google Scholar] [CrossRef]
- Dimitrova, M.; Mihaylova, D.; Popova, A.; Alexieva, J.; Tana Sapundzhieva, T.; Fidan, H. Phenolic profile, antibacterial and antioxidant activity of Pelargonium graveolens leaves’ extracts. Scientific Bulletin. Ser. F Biotechnol. 2015, 19, 130–135. [Google Scholar] [CrossRef]
- El Ouadi, Y.; Bendaif, H.; Mrabti, H.N.; Elmsellem, H.; Kadmi, Y.; Shariati, M.A.; Abdel-Rahman, I.; Hammouti, B.; Bouyanzer, A. Antioxidant activity of phenols and flavonoids contents of aqueous extract of Pelargonium graveolens orgin in the North-East Morocco. J. Microbiol. Biotechnol. Food Sci. 2017, 6, 1218–1220. [Google Scholar] [CrossRef]
- Ennaifer, M.; Bouzaiene, T.; Chouaibi, M.; Hamdi, M. Pelargonium graveolens Aqueous Decoction: A New Water-Soluble Polysaccharide and Antioxidant-Rich Extract. Biomed Res. Int. 2018, 2018, 11. [Google Scholar] [CrossRef]
- El Aanachi, S.; Gali, L.; Nacer, S.N.; Bensouici, C.; Dari, K.; Aassila, H. Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol. 2020, 29, 101819. [Google Scholar] [CrossRef]
- Savvas, D.; Ntatsi, G.; Passam, H.C. The European journal of plant science and biotechnology plant nutrition and physiological disorders in greenhouse grown tomato, pepper and eggplant. Eur. J. Plant Sci. Biotechnol. 2008, 2, 45–61. [Google Scholar]
- Signore, A.; Serio, F.; Santamaria, P. A targeted management of the nutrient solution in a soilless tomato crop according to plant needs. Front. Plant Sci. 2016, 7, 391. [Google Scholar] [CrossRef]
- Loreto, F.; Velikova, V. Isoprene produced by leaves protects the photosynthetic apparatus against ozone damage, quenches ozone products, and reduces lipid peroxidation of cellular membranes. Plant Physiol. 2001, 127, 1781–1787. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.L.; Mourato, M.P. Effect of excess copper on tomato plants: Growth parameters, enzyme activities, chlorophyll, and mineral content. J. Plant Nutr. 2006, 29, 2179–2198. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Papakyriakou, E.; Petropoulos, S.A.; Tzortzakis, N. The combined and single effect of salinity and copper stress on growth and quality of Mentha spicata plants. J. Hazard. Mater. 2019, 368, 584–593. [Google Scholar] [CrossRef]
- Reichman, S.M.; Menzies, N.W.; Asher, C.J.; Mulligan, D.R. Responses of four Australian tree species to toxic concentrations of copper in solution culture. J. Plant Nutr. 2006, 29, 1127–1141. [Google Scholar] [CrossRef]
- Öquist, G.; Chow, W.S.; Anderson, J.M. Photoinhibition of photosynthesis represents a mechanism for the long-term regulation of photosystem II. Planta 1992, 186, 450–460. [Google Scholar] [CrossRef]
- Yruela, I. Copper in plants. Braz. J. Plant Physiol. 2005, 17, 145–156. [Google Scholar] [CrossRef]
- Guidi, L.; Lo Piccolo, E.; Landi, M. Chlorophyll fluorescence, photoinhibition and abiotic stress: Does it make any difference the fact to be a C3 or C4 species? Front. Plant Sci. 2019, 10, 174. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wang, L.; Dixon, M.A. Response to copper toxicity for three ornamental crops in solution culture. HortScience 2004, 39, 1116–1120. [Google Scholar] [CrossRef]
- Osmond, C.B.; Grace, S.C. Perspectives of photoinhibition and photorespiration in the field: Quintessential inefficiencies of the light and dark reaction of photosynthesis? J. Exp. Bot. 1995, 46, 1415–1422. [Google Scholar] [CrossRef]
- Branco-Neves, S.; Soares, C.; de Sousa, A.; Martins, V.; Azenha, M.; Gerós, H.; Fidalgo, F. An efficient antioxidant system and heavy metal exclusion from leaves make Solanum cheesmaniae more tolerant to Cu than its cultivated counterpart. Food Energy Secur. 2017, 6, 123–133. [Google Scholar] [CrossRef]
- Lange, B.; van der Ent, A.; Baker, A.J.M.; Echevarria, G.; Mahy, G.; Malaisse, F.; Meerts, P.; Pourret, O.; Verbruggen, N.; Faucon, M.P. Copper and cobalt accumulation in plants: A critical assessment of the current state of knowledge. New Phytol. 2017, 213, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Tschinkel, P.F.S.; Melo, E.S.P.; Pereira, H.S.; Silva, K.R.N.; Arakaki, D.G.; Lima, N.V.; Fernandes, M.R.; Leite, L.C.S.; Melo, E.S.P.; Melnikov, P.; et al. The hazardous level of heavy metals in different medicinal plants and their decoctions in water: A public health problem in Brazil. BioMed Res. Int. 2020, 2020. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Review of the existing maximum residue levels for copper compounds according to Article 12 of Regulation (EC) No 396/2005. EFSA J. 2018, 16. [Google Scholar] [CrossRef]
- Ghassabzadeh, H.; Mohadespour, A.; Torab-Mostaedi, M.; Zaheri, P.; Maragheh, M.G.; Taheri, H. Adsorption of Ag, Cu and Hg from aqueous solutions using expanded perlite. J. Hazard. Mater. 2010, 177, 950–955. [Google Scholar] [CrossRef]
- Matijevic, L.; Romic, D.; Romic, M. Soil organic matter and salinity affect copper bioavailability in root zone and uptake by Vicia faba L. plants. Environ. Geochem. Health 2014, 36, 883–896. [Google Scholar] [CrossRef]
- Eskandari, S.; Mozaffari, V. Interactive effect of soil salinity and copper application on growth and chemical composition of pistachio seedlings (cv. Badami). Commun. Soil Sci. Plant Anal. 2014, 45, 688–702. [Google Scholar] [CrossRef]
- Li, L.; Long, M.; Islam, F.; Farooq, M.A.; Wang, J.; Mwamba, T.M.; Shou, J.; Zhou, W. Synergistic effects of chromium and copper on photosynthetic inhibition, subcellular distribution, and related gene expression in Brassica napus cultivars. Environ. Sci. Pollut. Res. 2019, 26, 11827–11845. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Antoniou, O.; Tzionis, A.; Prasad, M.; Tzortzakis, N. Alternative soilless media using olive-mill and paper waste for growing ornamental plants. Environ. Sci. Pollut. Res. 2018, 25, 35915–35927. [Google Scholar] [CrossRef] [PubMed]
- Tzortzakis, N.G.; Economakis, C.D. Shredded maize stems as an alternative substrate medium: Effect on growth, flowering and yield of tomato in soilless culture. J. Veg. Sci. 2005, 11, 57–70. [Google Scholar] [CrossRef]
- Chrysargyris, A.; Antoniou, O.; Athinodorou, F.; Vassiliou, R.; Papadaki, A.; Tzortzakis, N. Deployment of olive-stone waste as a substitute growing medium component for Brassica seedling production in nurseries. Environ. Sci. Pollut. Res. 2019, 26. [Google Scholar] [CrossRef] [PubMed]
- Chrysargyris, A.; Laoutari, S.; Litskas, V.D.; Stavrinides, M.C.; Tzortzakis, N. Effects of water stress on lavender and sage biomass production, essential oil composition and biocidal properties against Tetranychus urticae (Koch). Sci. Hortic. 2016, 213, 96–103. [Google Scholar] [CrossRef]
- Wojdyło, A.; Oszmiański, J.; Czemerys, R. Antioxidant activity and phenolic compounds in 32 selected herbs. Food Chem. 2007, 105, 940–949. [Google Scholar] [CrossRef]
- Tzortzakis, N.G.; Tzanakaki, K.; Economakis, C.D. Effect of origanum oil and vinegar on the maintenance of postharvest quality of tomato. Food Nutr. Sci. 2011, 2, 974–982. [Google Scholar] [CrossRef][Green Version]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef]
- De Azevedo Neto, A.D.; Prisco, J.T.; Enéas-Filho, J.; De Abreu, C.E.B.; Gomes-Filho, E. Effect of salt stress on antioxidative enzymes and lipid peroxidation in leaves and roots of salt-tolerant and salt-sensitive maize genotypes. Environ. Exp. Bot. 2006, 56, 87–94. [Google Scholar] [CrossRef]
- Benimeli, C.S.; Medina, A.; Navarro, C.M.; Medina, R.B.; Amoroso, M.J.; Gómez, M.I. Bioaccumulation of copper by Zea mays: Impact on root, shoot and leaf growth. Water Air Soil Pollut. 2010, 210, 365–370. [Google Scholar] [CrossRef]
- Azooz, M.M.; Abou-Elhamd, M.F.; Al-Fredan, M.A. Biphasic effect of copper on growth, proline, lipid peroxidation and antioxidant enzyme activities of wheat (Triticum aestivum cv. Hasaawi) at early growing stage. Aust. J. Crop Sci. 2012, 6, 688–694. [Google Scholar]



| DAT | Cu2+ (μM) | Plant Height | Leaf Number | Total Upper Fresh Biomass | Total Upper Biomass Dry Matter Content |
|---|---|---|---|---|---|
| 35 days | 4 | 43.16 ± 2.19 Y | 32.50 ± 4.66 a | 57.14 ± 3.78 ab | 12.19 ± 0.48 |
| 25 | 44.33 ± 1.72 | 24.66 ± 1.17 b | 68.39 ± 2.75 a | 12.19 ± 0.28 | |
| 50 | 42.33 ± 2.67 | 25.50 ± 3.52 b | 70.02 ± 4.69 a | 12.16 ± 0.91 | |
| 100 | 42.83 ± 2.15 | 22.33 ± 2.84 b | 50.72 ± 6.10 b | 13.16 ± 0.91 | |
| 49 days | 4 | 53.00 ± 4.57 | 58.00 ± 6.29 | 160.04 ± 0.80 | 12.89 ± 0.07 b |
| 25 | 50.00 ± 5.19 | 41.83 ± 6.87 | 152.92 ± 21.32 | 12.68 ± 0.15 b | |
| 50 | 54.83 ± 9.22 | 42.33 ± 8.36 | 156.89 ± 10.77 | 13.77 ± 0.08 a | |
| 100 | 56.40 ± 2.22 | 52.80 ± 4.66 | 133.55 ± 20.87 | 13.72 ± 0.19 a | |
| Significance | |||||
| Days (D) | ns | * | *** | ns | |
| Copper (Cu) | ns | ns | ns | ns | |
| D x Cu | ns | ns | ns | ns | |
| DAT | Cu2+ (μM) | Stomatal Resistance | Fv/Fm | Chl a | Chl b | Total Chl |
|---|---|---|---|---|---|---|
| 35 days | 4 | 0.90 ± 0.13 b Y | 0.83 ± 0.003 a | 23.47 ± 0.35 | 36.32 ± 0.77 | 59.77 ± 1.04 |
| 25 | 1.33 ± 0.08 ab | 0.83 ± 0.001 a | 23.80 ± 2.21 | 35.12 ± 2.57 | 58.90 ± 4.77 | |
| 50 | 1.34 ± 0.15 ab | 0.83 ± 0.050 ab | 23.97 ± 0.39 | 35.59 ± 0.71 | 59.54 ± 0.83 | |
| 100 | 1.61 ± 0.19 a | 0.82 ± 0.003 b | 23.49 ± 2.57 | 34.56 ± 2.15 | 58.03 ± 5.72 | |
| 49 days | 4 | 6.75 ± 0.50 b | 0.80 ± 0.006 a | 28.14 ± 1.10 a | 39.76 ± 0.96 a | 66.55 ± 1.39 a |
| 25 | 9.11 ± 1.20 ab | 0.78 ± 0.005 ab | 26.81 ± 0.43 ab | 36.62 ± 0.14 ab | 66.81 ± 2.60 a | |
| 50 | 9.05 ± 0.78 ab | 0.78 ± 0.007 ab | 24.79 ± 0.85 b | 38.68 ± 1.52 ab | 66.81 ± 2.60 ab | |
| 100 | 10.58 ± 1.38 a | 0.75 ± 0.019 b | 21.46 ± 0.91 c | 35.22 ± 1.30 b | 56.66 ± 2.17 b | |
| Significance | ||||||
| Days (D) | *** | *** | ns | ns | ns | |
| Copper (Cu) | * | ns | * | ns | ns | |
| D x Cu | ns | ns | ns | ns | ns | |
| DAT | Cu2+ (μM) | Accumulation Rate-AR (mg kg−1 DW day−1) | Bioaccumulation Coefficient (BAC) | Translocation Factor (TF) | |||||
|---|---|---|---|---|---|---|---|---|---|
| Leaves | Stems | Petioles | Roots | Leaves | Stems | Petioles | |||
| 35 days | 4 | 94.94 ± 18.78 b Y | 140.66 ± 13.50 a | 124.48 ± 4.71 a | 104.66 ± 1.17 a | 575.99 ± 90.69 a | 0.25 ± 0.03 a | 0.22 ± 0.03 a | 0.19 ± 0.03 a |
| 25 | 145.97 ± 9.78 ab | 24.03 ± 0.66 b | 21.66 ± 0.64 b | 19.34 ± 0.45 b | 193.77 ± 15.76 b | 0.12 ± 0.01 b | 0.11 ± 0.01 b | 0.10 ± 0.01 b | |
| 50 | 165.14 ± 30.56 a | 12.40 ± 0.14 b | 11.49 ± 0.41 c | 11.89 ± 0.24 c | 84.51 ± 14.58 b | 0.15 ± 0.03 b | 0.14 ± 0.02 b | 0.11 ± 0.03 b | |
| 100 | 124.05 ± 12.80 ab | 6.27 ± 0.14 b | 5.89 ± 0.11 c | 6.67 ± 0.13 d | 73.67 ± 6.63 b | 0.08 ± 0.01 b | 0.08 ± 0.00 b | 0.09 ± 0.01 b | |
| 49 days | 4 | 549.13 ± 23.12 | 133.81 ± 6.98 a | 104.82 ± 2.14 a | 124.29 ± 6.70 a | 212.23 ± 3.35 a | 0.63 ± 0.02 a | 0.49 ± 0.00 a | 0.58 ± 0.03 a |
| 25 | 676.26 ± 170.78 | 23.60 ± 0.71 b | 18.66 ± 2.62 b | 19.89 ± 0.66 b | 137.88 ± 4.70 b | 0.17 ± 0.01 b | 0.13 ± 0.02 b | 0.14 ± 0.00 b | |
| 50 | 965.19 ± 42.52 | 11.31 ± 0.72 c | 9.96 ± 0.77 c | 8.58 ± 0.62 c | 124.42 ± 21.48 b | 0.09 ± 0.02 c | 0.08 ± 0.01 c | 0.07 ± 0.01 c | |
| 100 | 955.28 ± 260.27 | 6.01 ± 0.35 c | 6.49 ± 0.45 c | 4.37 ± 0.06 c | 67.25 ± 0.92 c | 0.09 ± 0.00 c | 0.09 ± 0.01 c | 0.06 ± 0.00 c | |
| Significance | |||||||||
| Days (D) | *** | ns | *** | * | *** | *** | *** | *** | |
| Copper (Cu) | ns | *** | *** | *** | *** | *** | *** | *** | |
| D x Cu | ns | ns | *** | *** | *** | *** | *** | *** | |
| DAT | Cu2+ (μM) | Tolerance Indices-TI (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Biomass | Plant height | Leaf No | Leaf FW | Stem FW | Petiole FW | Root FW | Leaf DW | Stem DW | Petiole DW | Root DW | ||
| 35 days | 4 | 100.00 ± 0.00 ab Y | 100.00 ± 0.00 | 100.00 ± 0.00 a | 100.00 ± 0.00 ab | 100.00 ± 0.00 ab | 100.00 ± 0.00 b | 100.00 ± 0.00 | 100.00 ± 0.00 ab | 100.00 ± 0.00 | 100.00 ± 0.00 b | 100.00 ± 0.00 |
| 25 | 111.30 ± 1.93 a | 102.70 ± 3.98 | 75.89 ± 3.61 b | 114.85 ± 0.12 a | 117.05 ± 11.33 a | 139.71 ± 4.48 a | 113.49 ± 4.23 | 108.33 ± 1.82 a | 110.93 ± 10.65 | 127.97 ± 1.03 a | 98.97 ± 6.01 | |
| 50 | 113.84 ± 7.53 a | 98.07 ± 6.20 | 78.46 ± 10.85 b | 117.98 ± 8.13 a | 120.51 ± 9.77 a | 138.56 ± 6.94 a | 93.54 ± 9.51 | 111.93 ± 8.57 a | 109.79 ± 7.88 | 134.52 ± 2.06 a | 117.89 ± 10.14 | |
| 100 | 88.31 ± 8.01 b | 99.22 ± 4.98 | 68.71 ± 8.76 b | 88.34 ± 10.83 b | 81.90 ± 8.49 b | 100.82 ± 23.47 b | 91.53 ± 15.81 | 84.16 ± 10.72 b | 87.92 ± 3.94 | 111.31 ± 7.21 b | 93.33 ± 12.07 | |
| 49 days | 4 | 100.00 ± 0.00 b | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 | 100.00 ± 0.00 b |
| 25 | 219.37 ± 77.36 a | 94.33 ± 9.80 | 72.12 ± 11.84 | 92.36 ± 14.09 | 100.74 ± 12.76 | 91.83 ± 12.71 | 94.11 ± 3.02 | 89.62 ± 14.77 | 103.03 ± 13.16 | 91.55 ± 14.43 | 92.56 ± 1.19 b | |
| 50 | 104.63 ± 6.51 b | 103.45 ± 17.39 | 72.98 ± 14.42 | 96.47 ± 5.56 | 102.19 ± 8.24 | 92.66 ± 6.20 | 86.56 ± 10.47 | 98.95 ± 5.91 | 115.32 ± 7.77 | 101.20 ± 5.87 | 106.55 ± 13.72 ab | |
| 100 | 89.36 ± 15.10 b | 106.41 ± 4.20 | 91.03 ± 8.03 | 85.44 ± 14.69 | 83.03 ± 12.44 | 79.57 ± 10.38 | 98.23 ± 1.84 | 87.38 ± 15.64 | 93.41 ± 15.21 | 87.41 ± 12.84 | 124.10 ± 1.03 a | |
| Significance | ||||||||||||
| Days (D) | ns | ns | ns | ns | ns | *** | ns | ns | ns | *** | ns | |
| Copper (Cu) | * | ns | ns | ns | * | * | ns | ns | ns | ns | ns | |
| D x Cu | ns | ns | ns | ns | ns | * | ns | ns | ns | ns | ns | |
| Leaf Cu | Root Cu | Phenols | Flavonoids | FRAP | DPPH | ABTS | H2O2 | MDA | |
|---|---|---|---|---|---|---|---|---|---|
| 35 DAT | |||||||||
| Leaf Cu | 1 | ||||||||
| Root Cu | 0.8406 | 1 | |||||||
| Phenols | −0.3210 | −0.0500 | 1 | ||||||
| Flavonoids | 0.4770 | −0.0714 | −0.4142 | 1 | |||||
| FRAP | 0.4453 | −0.0852 | −0.2426 | 0.9835 | 1 | ||||
| DPPH | 0.5612 | 0.0339 | −0.3562 | 0.9920 | 0.9866 | 1 | |||
| ABTS | 0.5018 | 0.0037 | −0.1406 | 0.9551 | 0.9901 | 0.9750 | 1 | ||
| H2O2 | −0.3121 | −0.0544 | 0.9996 | −0.3893 | −0.2161 | −0.3310 | −0.1139 | 1 | |
| MDA | 0.5334 | 0.1429 | −0.9396 | 0.6778 | 0.5357 | 0.6432 | 0.4571 | −0.9308 | 1 |
| 49 DAT | |||||||||
| Leaf Cu | 1 | ||||||||
| Root Cu | 0.7078 | 1 | |||||||
| Phenols | 0.8042 | 0.8743 | 1 | ||||||
| Flavonoids | 0.7933 | 0.9364 | 0.9890 | 1 | |||||
| FRAP | 0.4427 | 0.9454 | 0.7185 | 0.8086 | 1 | ||||
| DPPH | 0.8973 | 0.3775 | 0.4626 | 0.4440 | 0.0855 | 1 | |||
| ABTS | 0.7591 | 0.8380 | 0.9954 | 0.9751 | 0.6881 | 0.4034 | 1 | ||
| H2O2 | 0.4695 | 0.3948 | 0.7745 | 0.6815 | 0.2355 | 0.1705 | 0.8267 | 1 | |
| MDA | −0.1303 | −0.6349 | −0.1807 | −0.3241 | −0.7818 | 0.0074 | −0.1173 | 0.4215 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chrysargyris, A.; Maggini, R.; Incrocci, L.; Pardossi, A.; Tzortzakis, N. Copper Tolerance and Accumulation on Pelargonium graveolens L’Hér. Grown in Hydroponic Culture. Plants 2021, 10, 1663. https://doi.org/10.3390/plants10081663
Chrysargyris A, Maggini R, Incrocci L, Pardossi A, Tzortzakis N. Copper Tolerance and Accumulation on Pelargonium graveolens L’Hér. Grown in Hydroponic Culture. Plants. 2021; 10(8):1663. https://doi.org/10.3390/plants10081663
Chicago/Turabian StyleChrysargyris, Antonios, Rita Maggini, Luca Incrocci, Alberto Pardossi, and Nikolaos Tzortzakis. 2021. "Copper Tolerance and Accumulation on Pelargonium graveolens L’Hér. Grown in Hydroponic Culture" Plants 10, no. 8: 1663. https://doi.org/10.3390/plants10081663
APA StyleChrysargyris, A., Maggini, R., Incrocci, L., Pardossi, A., & Tzortzakis, N. (2021). Copper Tolerance and Accumulation on Pelargonium graveolens L’Hér. Grown in Hydroponic Culture. Plants, 10(8), 1663. https://doi.org/10.3390/plants10081663










