Light Spectra and Root Stocks Affect Response of Greenhouse Tomatoes to Long Photoperiod of Supplemental Lighting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material and Experimental Design
2.2. Growth Measurements
2.3. Leaf Gas Exchange: Day and Night Measurements
2.4. Leaf Gas Exchange: Light Response Curves
2.5. Leaf Gas Exchange: CO2 Response Curves
2.6. Chlorophyll Fluorescence Imaging
2.7. Fruit Yield
2.8. Statistical Analysis
3. Results
3.1. Chlorophyll Fluorescence and Photosynthesis
3.2. Plant Parameters
3.3. Fruit Yield
4. Discussion
4.1. Effect of Photoperiod Extension on Leaf Physiology
4.2. Effect of Photoperiod Extension on Fruit Production
4.3. Interaction between Rootstocks and Photoperiod Length
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- McAvoy, R.J.; Janes, H.W.; Godfriaux, B.L.; Secks, M.; Duchai, D.; Wittman, W.K. The effect of total available photosynthetic photon flux on single truss tomato growth and production. J. Hortic. Sci. 1989, 64, 331–338. [Google Scholar] [CrossRef]
- IESO. Independent Electricity System Operator. Available online: www.ieso.ca/power-data (accessed on 27 January 2020).
- Hao, X.; Guo, X.; Lanoue, J.; Zhang, Y.; Cao, R.; Zheng, J.; Little, C.; Leonardos, D.; Kholsa, S.; Grodzinski, B.; et al. A review on smart application of supplemental lighting in greenhouse fruiting vegetable production. Acta Hortic. 2018, 1227, 499–506. [Google Scholar] [CrossRef]
- Demers, D.A.; Gosselin, A. Growing greenhouse tomato and sweet pepper under supplemental lighting: Optimal photoperiod, negative effects of long photoperiod and their causes. Acta Hortic. 2002, 580, 83–88. [Google Scholar] [CrossRef]
- Velez-Ramierez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Plants under continuous light. Trends Plant Sci. 2011, 16, 310–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haque, M.S.; De Sousa, A.; Soares, C.; Kjaer, K.H.; Fidalgo, F.; Rosenqvist, E.; Ottosen, C.-O. Temperature Variation under Continuous Light Restores Tomato Leaf Photosynthesis and Maintains the Diurnal Pattern in Stomatal Conductance. Front. Plant Sci. 2017, 8, 1602. [Google Scholar] [CrossRef] [Green Version]
- Lanoue, J.; Zheng, J.; Little, C.; Thibodeau, A.; Grodzinski, B.; Hao, X. Alternating Red and Blue Light-Emitting Diodes Allows for Injury-Free Tomato Production with Continuous Lighting. Front. Plant Sci. 2019, 10, 1114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez-Ramirez, A.; Van Ieperen, W.; Vreugdenhil, D.; Millenaar, F. Continuous light as a way to increase greenhouse tomato production: Expected challenges. Acta Hortic. 2012, 956, 51–57. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; van Ieperen, W.; Vreugdenhil, D.; van Poppel, P.M.J.A.; Heuvelink, E.; Millenaar, F.F. A single locus confers tolerance to continuous light and allows substantial yield increase in tomato. Nat. Commun. 2014, 5, 4549. [Google Scholar] [CrossRef] [Green Version]
- Kouřil, R.; Dekker, J.P.; Boekema, E.J. Supramolecular organization of photosystem II in green plants. Biochim. Et Biophys. Acta Bioenerg. 2012, 1817, 2–12. [Google Scholar] [CrossRef] [Green Version]
- Heuvelink, E.; Dorais, M. Crop growth and yield. In Tomatoes; Heuvelink, E., Ed.; CABI Publishing: Wallingford, UK, 2005; pp. 85–144. [Google Scholar]
- LaNoue, J.; Leonardos, E.D.; Grodzinski, B. Effects of Light Quality and Intensity on Diurnal Patterns and Rates of Photo-Assimilate Translocation and Transpiration in Tomato Leaves. Front. Plant Sci. 2018, 9, 756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrillo, E.; Herz, M.A.G.; Barta, A.; Kalyna, M.; Kornblihtt, A.R. Let there be light: Regulation of gene expression in plants. RNA Biol. 2014, 11, 1215–1220. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, R.; Yamano, T.; Murakami, K.; Fujiwara, K. Effects of spectral distribution and photosynthetic photon flux density for overnight LED light irradiation on tomato seedling growth and leaf injury. Sci. Hortic. 2016, 198, 363–369. [Google Scholar] [CrossRef]
- Velez-Ramirez, A.I.; Vreugdenhil, D.; Millenaar, F.F.; Van Ieperen, W. Phytochrome A Protects Tomato Plants From Injuries Induced by Continuous Light. Front. Plant Sci. 2019, 10, 19. [Google Scholar] [CrossRef]
- Hao, X.; Zhang, Y.; Guo, X.; Little, C.; Zheng, J. Dynamic temperature control strategy with a temperature drop improves responses of greenhouse tomatoes and sweet peppers to long photoperiods of supplemental lighting and saves energy. Acta Hortic. 2018, 1227, 291–298. [Google Scholar] [CrossRef]
- Hogewoning, S.; Trouwborst, G.; Maljaars, H.; Poorter, H.; Van Ieperen, W.; Harbinson, J. Blue light dose-responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. [Google Scholar] [CrossRef]
- Rahmatian, A.; Delshad, M.; Salehi, R. Effect of grafting on growth, yield and fruit quality of single and double stemmed tomato plants grown hydroponically. Hortic. Environ. Biotechnol. 2014, 55, 115–119. [Google Scholar] [CrossRef]
- Khah, E.M.; Kakava, E.; Mavromatis, A.; Chachalis, D.; Goulas, C. Effect of grafting on growth and yield of tomato (Lycopersicon esculentum Mill.) in greenhouse and open-field. J. Appl. Hortic. 2006, 8, 3–7. [Google Scholar] [CrossRef]
- Kyriacou, M.C.; Rouphael, Y.; Colla, G.; Zrenner, R.; Schwarz, D. Vegetable grafting: The implications of a growing agronomic imperative for vegetatble fruit quality and nutritive value. Front. Plant Sci. 2017, 8, 741. [Google Scholar] [CrossRef] [PubMed]
- Ontario Ministry of Agriculture. Food and Rural Affairs. Growing Greenhouse Vegetables in Ontario; Queen’s Printer: Toronto, ON, Canada, 2010. [Google Scholar]
- Kusuma, P.; Pattison, P.M.; Bugbee, B. From physics to fixtures to food: Current and potential LED efficacy. Hortic. Res. 2020, 7, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaiser, E.; Ouzounis, T.; Giday, H.; Schipper, R.; Heuvelink, E.; Marcelis, L.F.M. Adding Blue to Red Supplemental Light Increases Biomass and Yield of Greenhouse-Grown Tomatoes, but Only to an Optimum. Front. Plant Sci. 2019, 9, 2002. [Google Scholar] [CrossRef] [Green Version]
- Kaiser, E.; Weerheim, K.; Schipper, R.; Dieleman, J.A. Partial replacement of red and blue by green light increases biomass and yield in tomato. Sci. Hortic. 2019, 249, 271–279. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. Am. Soc. Agric. Eng. 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Bloch, G.; Bar-Shai, N.; Cytter, Y.; Green, R. Time is honey: Circadian clocks of bees and flowers and how their interactions may influence ecological communities. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160256. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- LaNoue, J.; Leonardos, E.D.; Khosla, S.; Hao, X.; Grodzinski, B. Effect of elevated CO2 and spectral quality on whole plant gas exchange patterns in tomatoes. PLoS ONE 2018, 13, e0205861. [Google Scholar] [CrossRef] [PubMed]
- Farquhar, G.D.; Von Caemmerer, S.; Berry, J.A. A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 1980, 149, 78–90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McMurtrie, R.E.; Wang, Y. Mathematical models of the photosynthetic response of tree stands to rising CO2 concentrations and temperatures. Plant Cell Environ. 1993, 16, 1–13. [Google Scholar] [CrossRef]
- Bernacchi, C.J.; Singsaas, E.L.; Pimentel, C.; Portis, A.R., Jr.; Long, S.P. Improved temperature response functions for models of rubisco-limited photosynthesis. Plant Cell Environ. 2001, 24, 253–259. [Google Scholar] [CrossRef]
- Baker, N.R. Chlorophyll fluorescence: A probe of photosynthesis in vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Hillman, W.S. Injury of tomato plants by continuous light and unfavorable photoperiodic cycles. Am. J. Bot. 1956, 43, 89–96. [Google Scholar] [CrossRef]
- Matsuda, R.; Ozawa, N.; Fujiwara, K. Leaf photosynthesis, plant growth, and carbohydrate accumulation of tomato under different photoperiods and diurnal temperature differences. Sci. Hortic. 2014, 170, 150–158. [Google Scholar] [CrossRef]
- Haque, M.S.; Kjaer, K.H.; Rosenqvist, E.; Ottosen, C.-O. Continuous light increases growth, daily carbon gain, antioxidants, and alters carbohydrate metabolism in a cultivated and a wild tomato species. Front. Plant Sci. 2015, 6, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velez-Ramirez, A.I.; Dünner-Planella, G.; Vreugdenhil, D.; Millenaar, F.F.; Van Ieperen, W. On the induction of injury in tomato under continuous light: Circadian asynchrony as the main triggering factor. Funct. Plant Biol. 2017, 44, 597. [Google Scholar] [CrossRef] [PubMed]
- Azcón-Bieto, J.; Federico, R.; Giartosio, C.E. Inhibition of Photosynthesis by Carbohydrates in Wheat Leaves. Plant Physiol. 1983, 73, 681–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kristoffersen, T. Interactions of photoperiod and temperature in growth and development of young tomato plants (Lycopersicon esculentum Mill.). Physiol. Plant. 1963, 16, 1–98. [Google Scholar]
- Lanoue, J.; Zheng, J.; Little, C.; Grodzinski, B.; Hao, X. Continuous Light Does Not Compromise Growth and Yield in Mini-Cucumber Greenhouse Production with Supplemental LED Light. Plants 2021, 10, 378. [Google Scholar] [CrossRef]
- Liu, Y.; Li, T.; Qi, H.; Li, J.; Yin, X. Effects of grafting on carbohydrate accumulation and sugar-metbolic enzyme activities in muskmelon. Afr. J. Biotechnol. 2010, 9, 25–35. [Google Scholar]
- Barry, H.G.; Castle, W.S.; Davies, F.S. Rootstocks and plant water relations affect sugar accumulation of citrus fruit via osmotic adjustment. J. Am. Soc. Hort. Sci. 2014, 129, 881–889. [Google Scholar] [CrossRef]
- Velez-Ramierez, A.I.; van Ieperen, W.; Vreugdenhil, D.; Millenaar, F.F. Continuous-light tolerance in tomato is graft-transferable. Planta 2015, 241, 285–290. [Google Scholar] [CrossRef]
- Paponov, M.; Kechasov, D.; Lacek, J.; Verheul, M.J.; Paponov, I. Supplemental Light-Emitting Diode Inter-Lighting Increases Tomato Fruit Growth Through Enhanced Photosynthetic Light Use Efficiency and Modulated Root Activity. Front. Plant Sci. 2020, 10, 1656. [Google Scholar] [CrossRef] [PubMed]
- Albacete, A.; Ghanem, M.E.; Martínez-Andújar, C.; Acosta, M.; Sánchez-Bravo, J.; Martínez, V.; Lutts, S.; Dodd, I.C.; Pérez-Alfocea, F. Hormonal changes in relation to biomass partitioning and shoot growth impairment in salinized tomato (Solanum lycopersicum L.). Plants J. Exp. Bot. 2008, 59, 4119–4131. [Google Scholar] [CrossRef] [PubMed]
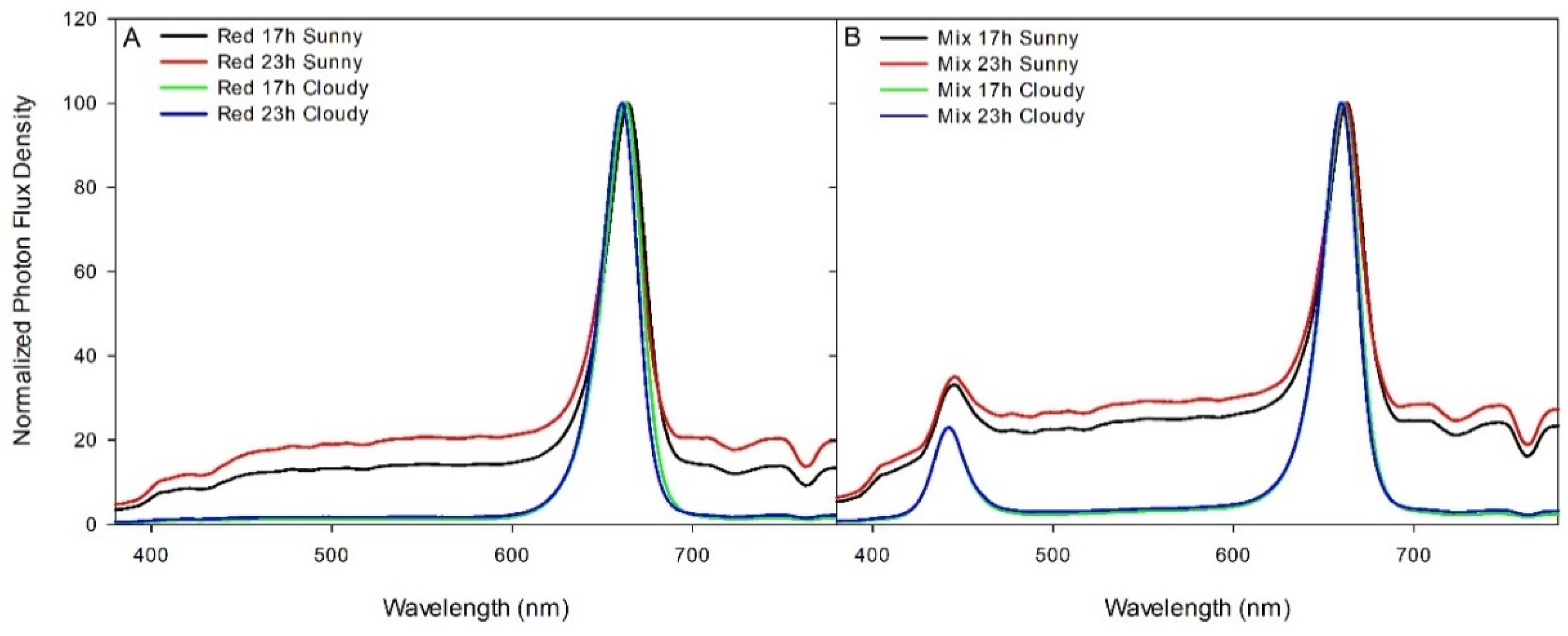







| Lighting Treatment | Supplemental Light Intensity (µmol m−2 s−1) | R:Fr—Sunny Day | R:Fr—Cloudy Day | PSS—Sunny Day | PSS—Cloudy Day | Supplemental DLI (mol m−2 d−1) |
|---|---|---|---|---|---|---|
| Red 17 h | 176 ± 7 | 3.78 | 25.56 | 0.82 | 0.88 | 10.79 ± 0.41 |
| Red 23 h | 127 ± 4 | 2.81 | 17.77 | 0.80 | 0.87 | 10.54 ± 0.36 |
| Mix 17 h | 169 ± 4 | 2.55 | 15.29 | 0.79 | 0.86 | 10.32 ± 0.27 |
| Mix 23 h | 134 ± 5 | 2.30 | 12.01 | 0.78 | 0.86 | 11.09 ± 0.42 |
| Lighting Treatment | Respiration (µmol CO2 m−2 s−1) | LCP (µmol m−2 s−1) | QY (µmol CO2 m−2 s−1/ µmol m−2 s−1) | Pnmax (µmol CO2 m−2 s−1) |
|---|---|---|---|---|
| 20 DIT | ||||
| Red 17 h | −1.61 ± 0.24 A | 26.23 ± 3.71 A | 0.062 ± 0.006 A | 28.34 ± 2.13 A |
| Red 23 h | −1.31 ± 0.13 A | 20.79 ± 2.33 A | 0.064 ± 0.005 A | 25.97 ± 2.67 A |
| Mix 17 h | −1.52 ± 0.38 A | 23.07 ± 5.64 A | 0.065 ± 0.005 A | 29.59 ± 2.16 A |
| Mix 23 h | −1.47 ± 0.22 A | 25.12 ± 3.08 A | 0.058 ± 0.004 A | 29.68 ± 1.23 A |
| 54 DIT | ||||
| Red 17 h | −1.65 ± 0.05 B,C,D | 32.48 ± 2.59 B | 0.047 ± 0.003 B | 25.80 ± 0.23 A |
| Red 23 h | −1.45 ± 0.04 A,B,C | 86.14 ± 7.37 A | 0.017 ± 0.002 C | 8.88 ± 1.88 B |
| Mix 17 h | −1.38 ± 0.24 A,B | 21.45 ± 5.13 B | 0.062 ± 0.002 A | 23.87 ± 2.76 A |
| Mix 23 h | −1.98 ± 0.18 C,D | 131.60 ± 10.21 A | 0.015 ± 0.0003 C | 8.24 ± 1.10 B |
| 134 DIT | ||||
| Red 17 h | −1.71 ± 0.08 A | 29.58 ± 0.93 A | 0.055 ± 0.002 A | 24.19 ± 1.24 B |
| Red 23 h | −1.45 ± 0.24 A | 25.44 ± 4.78 A | 0.055 ± 0.002 A | 25.93 ± 1.03 AB |
| Mix 17 h | −2.31 ± 0.49 A | 39.00 ± 9.12 A | 0.058 ± 0.002 A | 28.15 ± 2.36 AB |
| Mix 23 h | −1.83 ± 0.24 A | 31.08 ± 3.30 A | 0.058 ± 0.002 A | 35.00 ± 1.36 A |
| Lighting Treatment | Vcmax (µmol CO2 m−2 s−1) | Jmax (µmol e− m−2 s−1) | Pnmax (µmol CO2 m−2 s−1) |
|---|---|---|---|
| 20 DIT | |||
| Red 17 h | 26.98 ± 1.24 A | 100.16 ± 8.26 A | 19.71 ± 0.82 A |
| Red 23 h | 24.48 ± 0.66 A | 85.61 ± 4.22 A | 18.26 ± 0.53 A |
| Mix 17 h | 27.19 ± 0.45 A | 103.18 ± 3.04 A | 20.13 ± 0.31 A |
| Mix 23 h | 26.23 ± 0.29 A | 95.80 ± 1.64 A | 19.35 ± 0.20 A |
| 54 DIT | |||
| Red 17 h | 17.71 ± 0.46 B | 55.61 ± 1.67 B | 13.07 ± 0.31 B |
| Red 23 h | 10.56 ± 2.11 C | 30.84 ± 6.66 C | 7.80 ± 1.52 C |
| Mix 17 h | 26.05 ± 0.20 A | 96.30 ± 1.58 A | 19.08 ± 0.14 A |
| Mix 23 h | 6.54 ± 0.14 C | 18.26 ± 0.39 C | 4.97 ± 0.12 C |
| 134 DIT | |||
| Red 17 h | 23.17 ± 1.38 A | 80.98 ± 7.66 A | 17.00 ± 1.03 A |
| Red 23 h | 22.33 ± 0.51 A | 79.51 ± 2.48 A | 17.02 ± 0.39 A |
| Mix 17 h | 21.76 ± 0.10 A | 78.50 ± 0.64 A | 16.72 ± 0.11 A |
| Mix 23 h | 22.77 ± 0.90 A | 77.00 ± 3.36 A | 16.69 ± 0.53 A |
| Leaf Length (cm) | Leaf Width (cm) | ||||
|---|---|---|---|---|---|
| Cultivar | TE | TK | TE | TK | |
| Lighting Treatment | Leaf Rank | ||||
| 31 DIT | |||||
| Red 17 h | 5th | 46.33 ± 0.80 A | 47.83 ± 1.25 A | 36.00 ± 1.51 A | 42.50 ± 2.17 A |
| Red 23 h | 5th | 46.17 ± 1.14 A | 43.83 ± 1.89 A | 39.83 ± 0.98 A | 35.67 ± 3.69 A |
| Mix 17 h | 5th | 45.83 ± 0.95 A | 47.67 ± 1.69 A | 45.83 ± 0.95 A | 38.67 ± 1.74 A |
| Mix 23 h | 5th | 42.67 ± 1.43 A | 44.50 ± 1.18 A | 42.67 ± 1.43 A | 38.67 ± 2.08 A |
| 60 DIT | |||||
| Red 17 h | 5th | 38.67 ± 1.09 A | 42.33 ± 1.56 A | 36.67 ± 3.16 A | 40.50 ± 1.34 A |
| 10th | 46.50 ± 1.33 A | 49.67 ± 1.61 A | 61.50 ± 3.28 A | 66.33 ± 4.24 A | |
| Red 23 h | 5th | 41.67 ± 0.84 A | 41.67 ± 1.05 AB | 40.50 ± 1.12 A | 41.67 ± 2.12 A |
| 10th | 50.17 ± 0.83 A | 48.50 ± 1.45 A | 67.00 ± 2.80 A | 61.50 ± 3.91 A | |
| Mix 17 h | 5th | 42.17 ± 1.14 A | 42.67 ± 0.95 AB | 42.17 ± 2.84 A | 41.17 ± 0.95 A |
| 10th | 50.33 ± 1.26 A | 51.50 ± 1.11 A | 67.00 ± 3.09 A | 67.50 ± 4.03 A | |
| Mix 23 h | 5th | 39.67 ± 0.99 A | 38.00 ± 0.73 B | 39.67 ± 2.85 A | 36.67 ± 1.99 A |
| 10th | 47.33 ± 2.16 A | 50.00 ± 1.73 A | 62.33 ± 3.82 A | 62.50 ± 3.54 A | |
| 139 DIT | |||||
| Red 17 h | 5th | 39.17 ± 1.19 A | 38.83 ± 0.91 A | 35.00 ± 2.41 A | 33.67 ± 1.87 A |
| 10th | 45.17 ± 0.83 A | 46.83 ± 1.19 A | 56.67 ± 1.86 A | 55.50 ± 2.49 A | |
| 15th | 47.17 ± 2.09 A | 45.17 ± 1.01 A | 56.50 ± 3.66 A | 57.50 ± 3.02 A | |
| Red 23 h | 5th | 37.50 ± 0.92 A | 40.33 ± 1.02 A | 35.17 ± 3.17 A | 38.00 ± 2.28 A |
| 10th | 44.17 ± 2.47 A | 45.00 ± 1.75 A | 56.00 ± 2.77 A | 54.83 ± 2.41 A | |
| 15th | 46.17 ± 1.22 A | 45.83 ± 1.38 A | 56.83 ± 2.56 A | 54.00 ± 1.29 A | |
| Mix 17 h | 5th | 37.67 ± 1.31 A | 39.33 ± 1.20 A | 34.50 ± 1.34 A | 35.17 ± 2.47 A |
| 10th | 42.17 ± 2.26 A | 43.33 ± 1.73 A | 53.83 ± 2.76 A | 49.67 ± 3.02 A | |
| 15th | 44.33 ± 1.41 A | 49.17 ± 1.38 A | 50.83 ± 4.07 A | 55.50 ± 2.32 A | |
| Mix 23 h | 5th | 40.33 ± 1.54 A | 39.50 ± 0.89 A | 38.17 ± 1.42 A | 40.00 ± 1.73 A |
| 10th | 46.67 ± 1.76 A | 42.17 ± 1.19 A | 59.17 ± 2.47 A | 53.83 ± 2.50 A | |
| 15th | 43.33 ± 2.30 A | 45.50 ± 1.71 A | 58.17 ± 2.89 A | 57.83 ± 2.23 A | |
| Chlorophyll a + b (µg cm−2) | Carotenoids (µg cm−2) | ||||
|---|---|---|---|---|---|
| Cultivar | TE | TK | TE | TK | |
| Lighting Treatment | Leaf Rank | ||||
| 31 DIT | |||||
| Red 17 h | 5th | 39.98 ± 0.90 A | 38.05 ± 2.79 A | 7.93 ± 0.15 A | 7.59 ± 0.47 A |
| Red 23 h | 5th | 38.19 ± 1.68 A | 36.62 ± 1.21 A | 7.63 ± 0.28 A | 7.37 ± 0.20 A |
| Mix 17 h | 5th | 38.61 ± 1.72 A | 38.80 ± 1.74 A | 7.70 ± 0.29 A | 7.73 ± 0.29 A |
| Mix 23 h | 5th | 40.11 ± 1.81 A | 40.44 ± 1.13 A | 7.94 ± 0.39 A | 8.00 ± 0.19 A |
| 60 DIT | |||||
| Red 17 h | 5th | 26.77 ± 1.36 B | 31.47 ± 1.28 A | 5.68 ± 0.24 B | 6.50 ± 0.22 A |
| 10th | 34.30 ± 1.42 B | 36.61 ± 1.02 AB | 6.98 ± 0.24 B | 7.37 ± 0.29 A | |
| Red 23 h | 5th | 29.31 ± 2.31 B | 29.91 ± 1.91 AB | 6.21 ± 0.51 B | 6.22 ± 0.34 AB |
| 10th | 34.49 ± 2.17 B | 33.75 ± 0.96 AB | 7.00 ± 0.37 B | 6.89 ± 0.21 A | |
| Mix 17 h | 5th | 39.47 ± 3.49 A | 38.21 ± 2.16 A | 8.45 ± 0.77 A | 7.63 ± 0.36 A |
| 10th | 41.46 ± 2.02 A | 38.62 ± 1.36 A | 8.17 ± 0.33 A | 8.26 ± 0.39 A | |
| Mix 23 h | 5th | 28.47 ± 2.73 B | 22.75 ± 2.42 B | 5.98 ± 0.60 B | 4.95 ± 0.44 B |
| 10th | 34.63 ± 1.07 AB | 32.36 ± 1.34 B | 7.04 ± 0.18 B | 6.88 ± 0.38 A | |
| 139 DIT | |||||
| Red 17 h | 5th | 40.94 ± 1.89 A | 41.94 ± 1.25 B | 8.08 ± 0.31 A | 8.25 ± 0.20 B |
| 10th | 43.86 ± 1.54 A | 44.16 ± 1.25 A | 8.56 ± 0.25 A | 8.61 ± 0.20 A | |
| 15th | 34.10 ± 2.20 A | 37.82 ± 2.02 A | 6.94 ± 0.37 A | 7.57 ± 0.34 A | |
| Red 23 h | 5th | 42.59 ± 0.54 A | 42.78 ± 0.86 B | 8.36 ± 0.09 A | 8.39 ± 0.14 B |
| 10th | 44.80 ± 1.76 A | 45.43 ± 0.94 A | 8.71 ± 0.29 A | 8.82 ± 0.15 A | |
| 15th | 37.11 ± 1.16 A | 32.57 ± 1.55 A | 7.45 ± 0.19 A | 6.68 ± 0.26 A | |
| Mix 17 h | 5th | 43.26 ± 1.17 A | 46.36 ± 1.13 A | 8.46 ± 0.19 A | 8.97 ± 0.18 A |
| 10th | 45.19 ± 2.26 A | 44.43 ± 1.09 A | 8.77 ± 0.36 A | 8.65 ± 0.18 A | |
| 15th | 35.89 ± 3.57 A | 38.33 ± 1.94 A | 7.22 ± 0.61 A | 7.65 ± 0.32 A | |
| Mix 23 h | 5th | 45.65 ± 2.23 A | 44.30 ± 1.31 AB | 8.85 ± 0.36 A | 8.63 ± 0.21 AB |
| 10th | 42.53 ± 1.06 A | 45.38 ± 0.87 A | 8.35 ± 0.17 A | 8.81 ± 0.14 A | |
| 15th | 34.38 ± 2.25 A | 31.98 ± 1.89 A | 6.98 ± 0.38 A | 6.58 ± 0.33 A | |
| Fruit per Stem | Fruit Weight per Stem (kg stem−1) | |||
|---|---|---|---|---|
| Cultivar | TE | TK | TE | TK |
| February | ||||
| Lighting Treatment | ||||
| Red 17 h | 9 ± 1 A | 8 ± 2 A | 0.68 ± 0.06 AB | 0.63 ± 0.07 A |
| Red 23 h | 7 ± 2 A | 10 ± 1 A | 0.52 ± 0.10 B | 0.67 ± 0.05 A |
| Mix 17 h | 11 ± 2 A | 8 ± 2 A | 0.88 ± 0.12 A | 0.65 ± 0.15 A |
| Mix 23 h | 7 ± 1 A | 8 ± 1 A | 0.50 ± 0.04 B | 0.52 ± 0.02 A |
| March | ||||
| Red 17 h | 21 ± 2 A | 22 ± 2 A | 1.66 ± 0.20 AB | 1.90 ± 0.14 A |
| Red 23 h | 16 ± 2 A | 17 ± 1 BC | 1.13 ± 0.16 AB | 1.07 ± 0.08 B |
| Mix 17 h | 20 ± 2 A | 21 ± 2 AB | 1.70 ± 0.10 A | 1.72 ± 0.12 A |
| Mix 23 h | 17 ± 2 A | 12 ± 1 C | 0.97 ± 0.11 B | 0.70 ± 0.03 C |
| April | ||||
| Red 17 h | 20 ± 1 A | 22 ± 1 A | 1.97 ± 0.26 A | 2.23 ± 0.13 A |
| Red 23 h | 22 ± 2 A | 21 ± 1 A | 2.06 ± 0.14 A | 1.77 ± 0.07 B |
| Mix 17 h | 19 ± 1 A | 22 ± 1 A | 1.93 ± 0.15 A | 2.05 ± 0.10 AB |
| Mix 23 h | 16 ± 1 B | 12 ± 1 B | 1.42 ± 0.06 A | 0.95 ± 0.05 C |
| May | ||||
| Red 17 h | 26 ± 1 A | 24 ± 1 A | 3.47 ± 0.09 A | 3.32 ± 0.17 A |
| Red 23 h | 24 ± 1 A | 25 ± 2 A | 3.50 ± 0.10 A | 3.22 ± 0.10 A |
| Mix 17 h | 25 ± 1 A | 27 ± 1 A | 3.47 ± 0.10 A | 3.45 ± 0.13 A |
| Mix 23 h | 25 ± 1 A | 26 ± 1 A | 3.66 ± 0.14 A | 3.37 ± 0.21 A |
| Cumulative | ||||
| Red 17 h | 74 ± 1 A | 75 ± 3 A | 7.29 ± 0.17 AB | 8.08 ± 0.36 A |
| Red 23 h | 72 ± 1 A | 72 ± 1 A | 7.51 ± 0.08 A | 6.75 ± 0.11 B |
| Mix 17 h | 73 ± 1 A | 77 ± 2 A | 7.73 ± 0.15 A | 7.87 ± 0.20 A |
| Mix 23 h | 64 ± 1 B | 57 ± 2 B | 6.54 ± 0.16 B | 5.54 ± 0.20 C |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanoue, J.; Thibodeau, A.; Little, C.; Zheng, J.; Grodzinski, B.; Hao, X. Light Spectra and Root Stocks Affect Response of Greenhouse Tomatoes to Long Photoperiod of Supplemental Lighting. Plants 2021, 10, 1674. https://doi.org/10.3390/plants10081674
Lanoue J, Thibodeau A, Little C, Zheng J, Grodzinski B, Hao X. Light Spectra and Root Stocks Affect Response of Greenhouse Tomatoes to Long Photoperiod of Supplemental Lighting. Plants. 2021; 10(8):1674. https://doi.org/10.3390/plants10081674
Chicago/Turabian StyleLanoue, Jason, Alyssa Thibodeau, Celeste Little, Jingming Zheng, Bernard Grodzinski, and Xiuming Hao. 2021. "Light Spectra and Root Stocks Affect Response of Greenhouse Tomatoes to Long Photoperiod of Supplemental Lighting" Plants 10, no. 8: 1674. https://doi.org/10.3390/plants10081674
APA StyleLanoue, J., Thibodeau, A., Little, C., Zheng, J., Grodzinski, B., & Hao, X. (2021). Light Spectra and Root Stocks Affect Response of Greenhouse Tomatoes to Long Photoperiod of Supplemental Lighting. Plants, 10(8), 1674. https://doi.org/10.3390/plants10081674






