Colleters, Extrafloral Nectaries, and Resin Glands Protect Buds and Young Leaves of Ouratea castaneifolia (DC.) Engl. (Ochnaceae)
Abstract
1. Introduction
2. Results
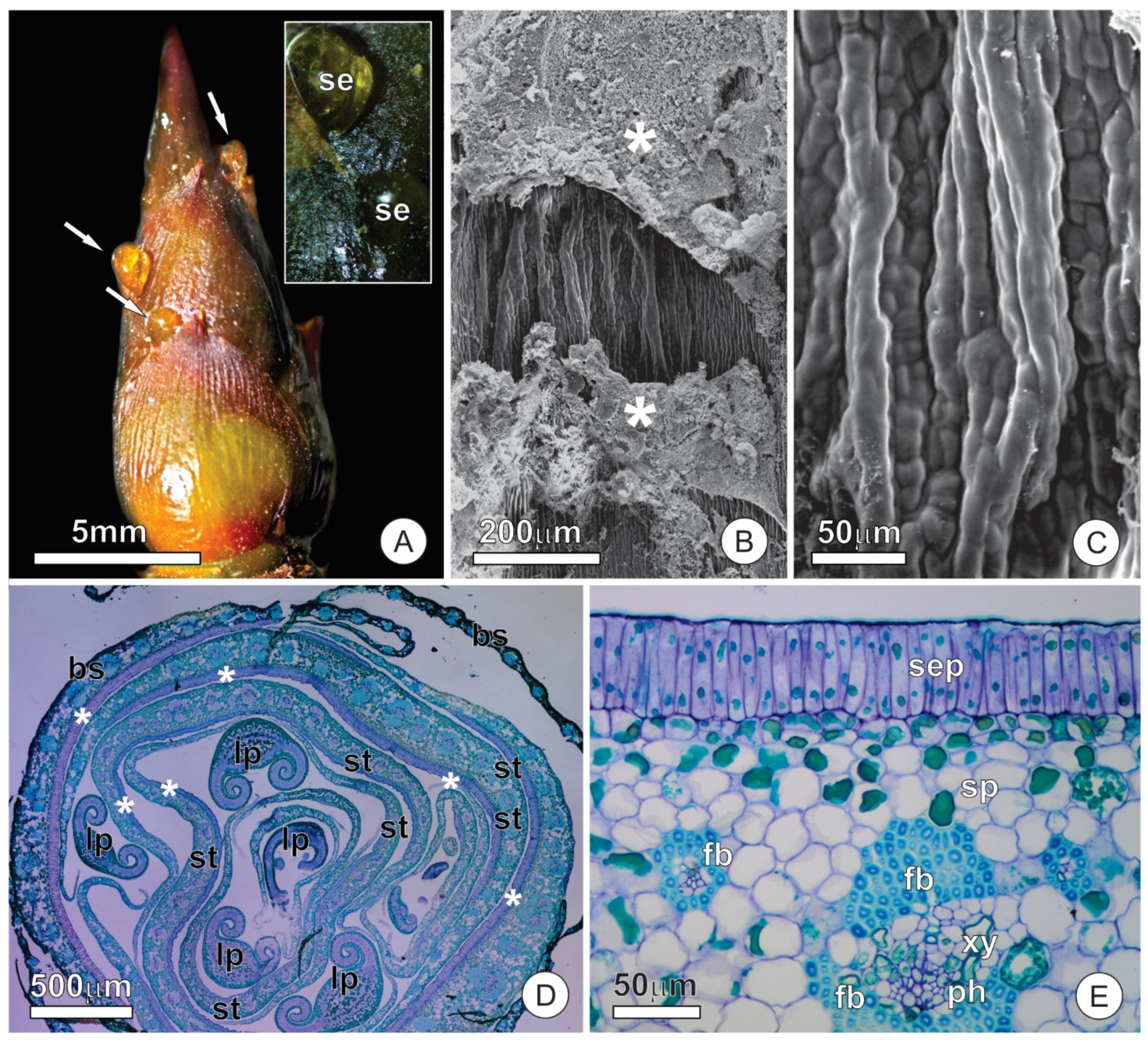
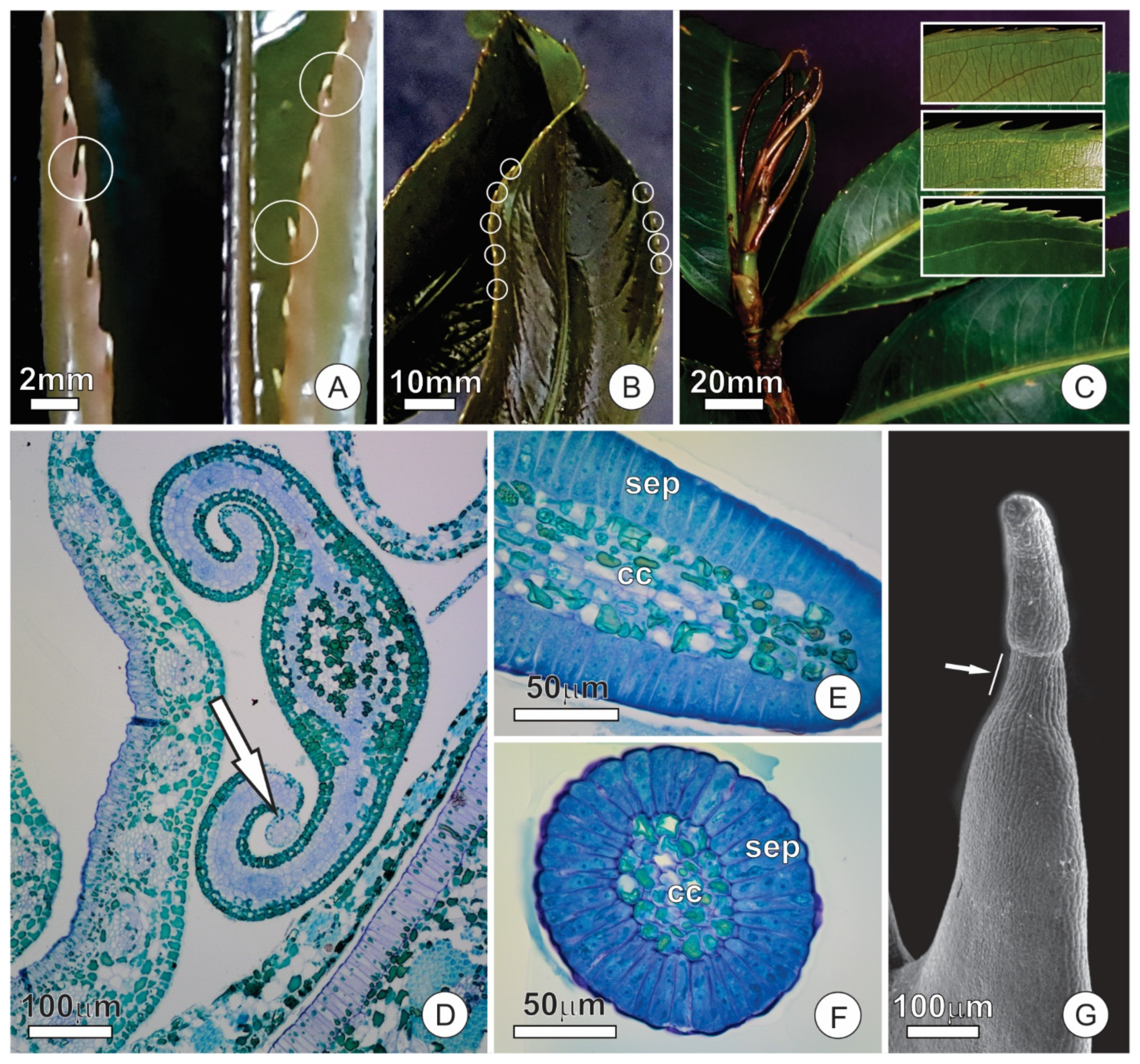
2.1. Bud Dynamics and Structural Aspects
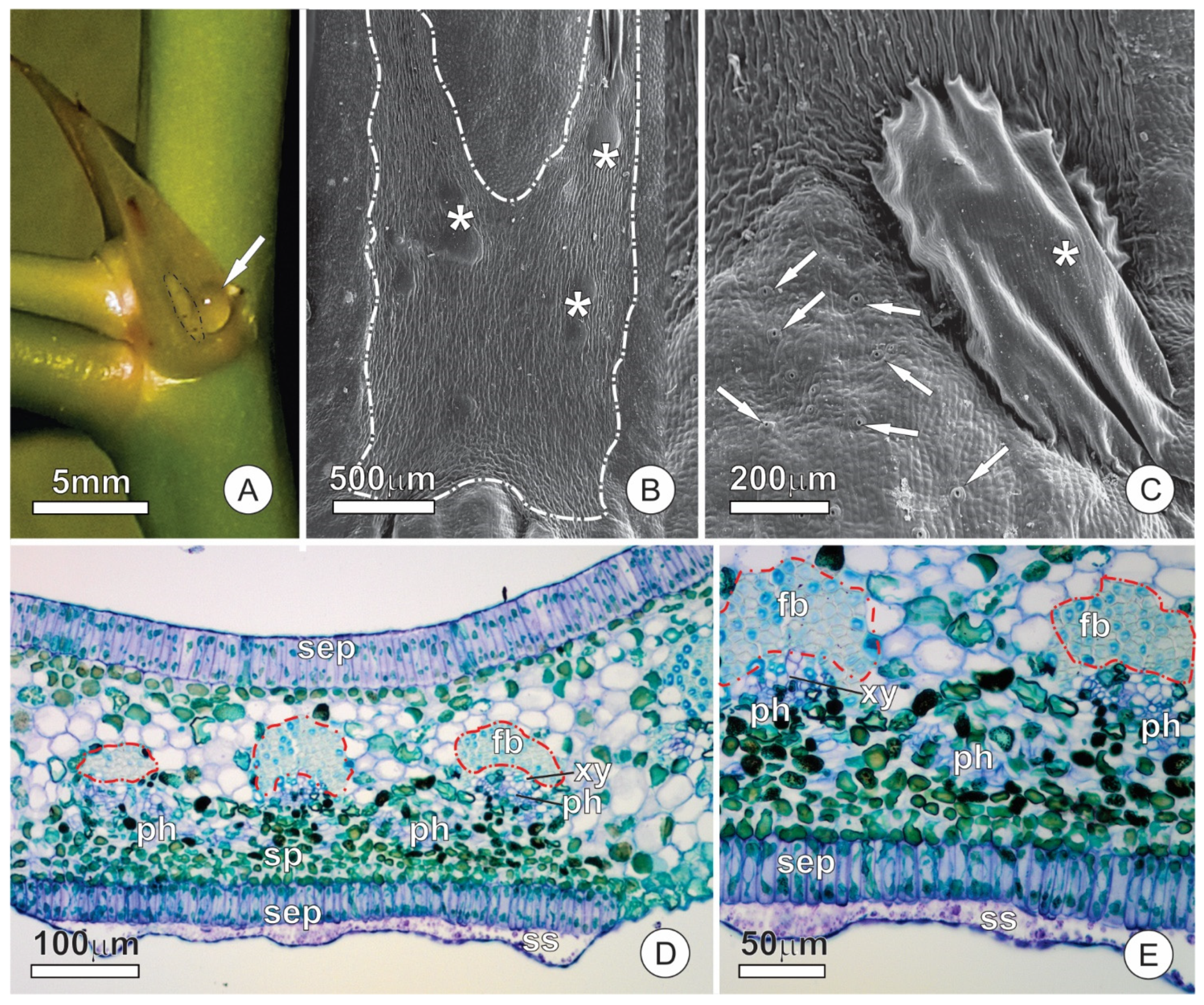
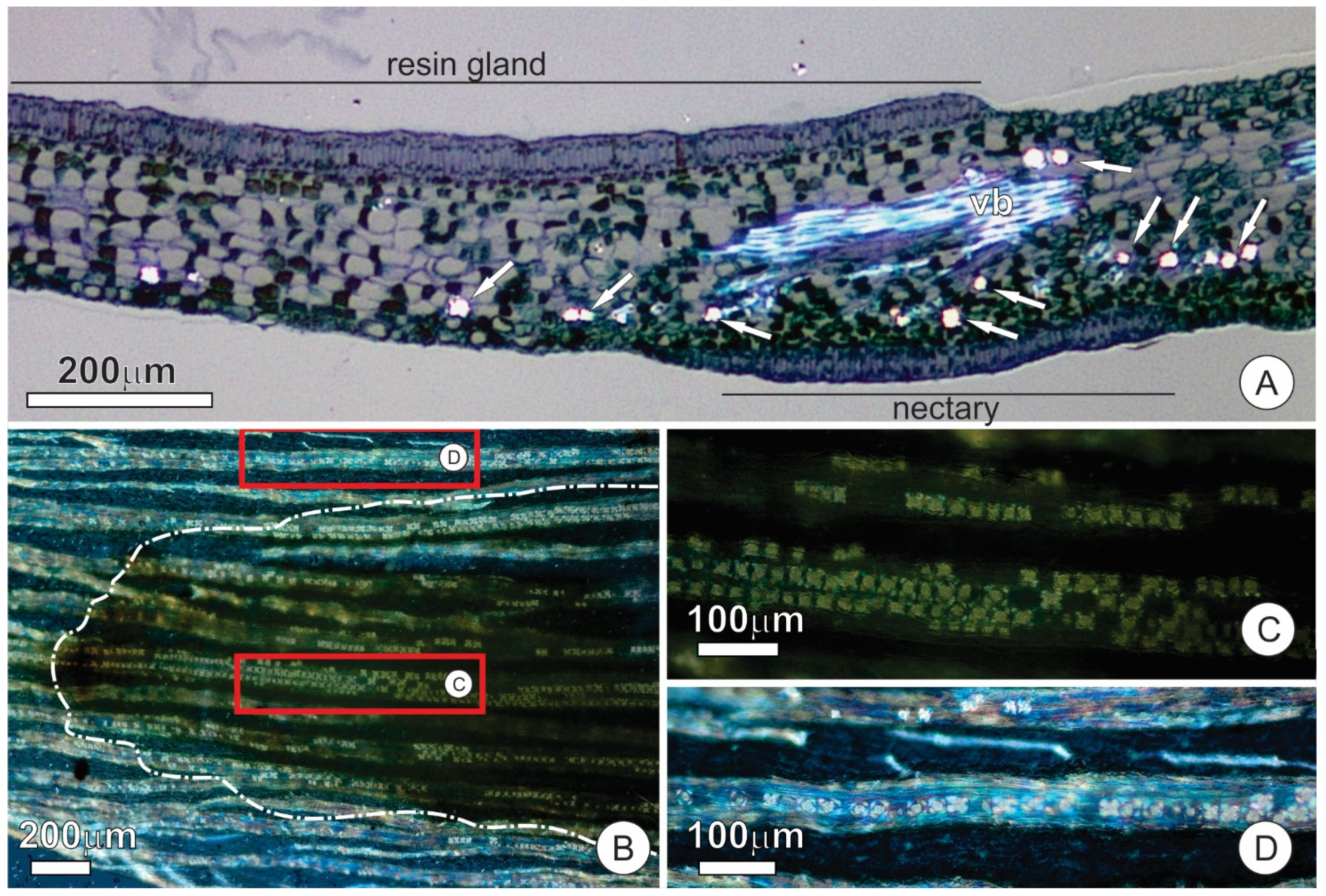
2.2. Resin Glands
2.3. Colleters
2.4. Extrafloral Nectaries
2.5. Histochemistry and Sugar Analysis
3. Discussion
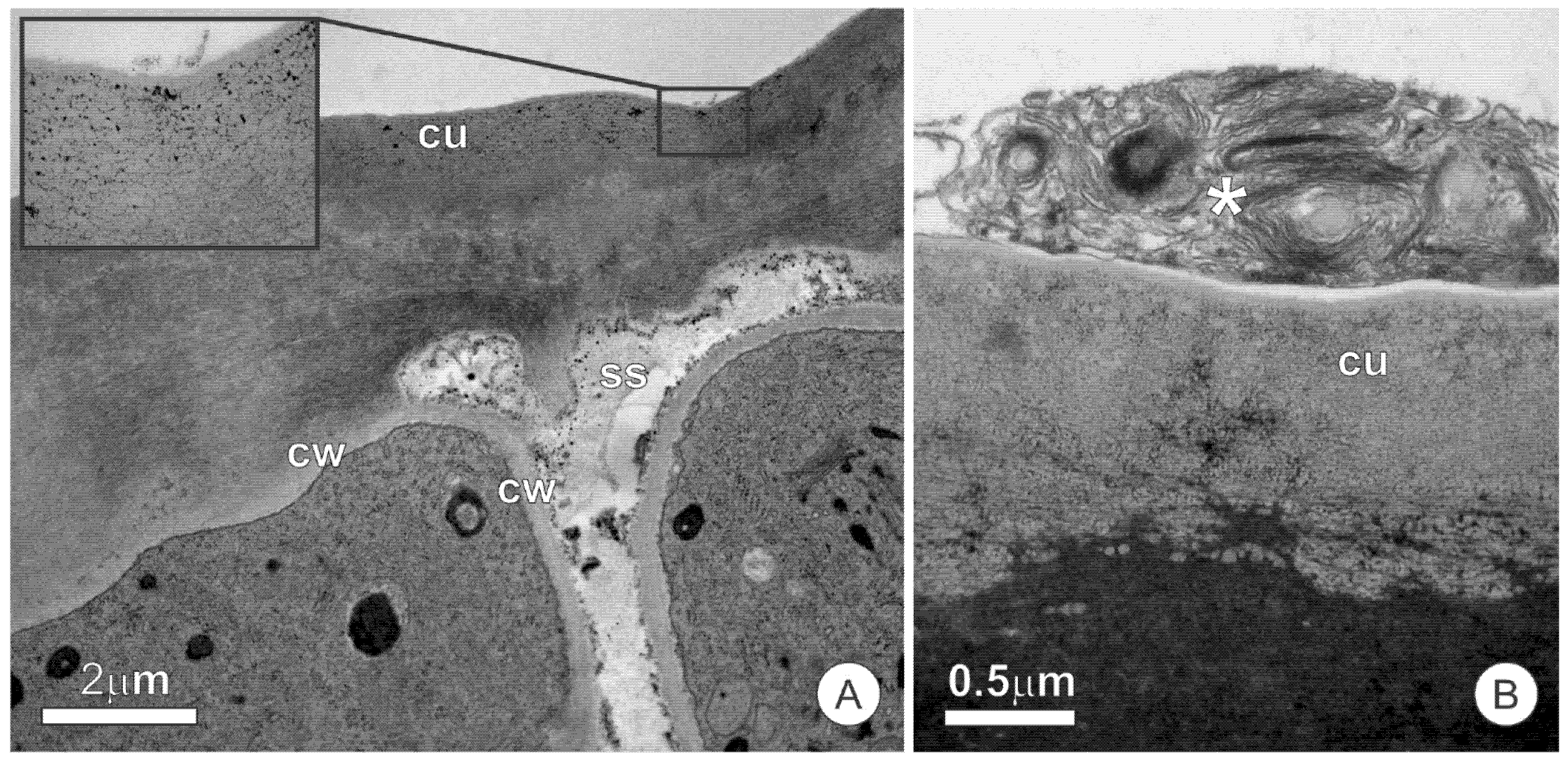
3.1. Anatomy
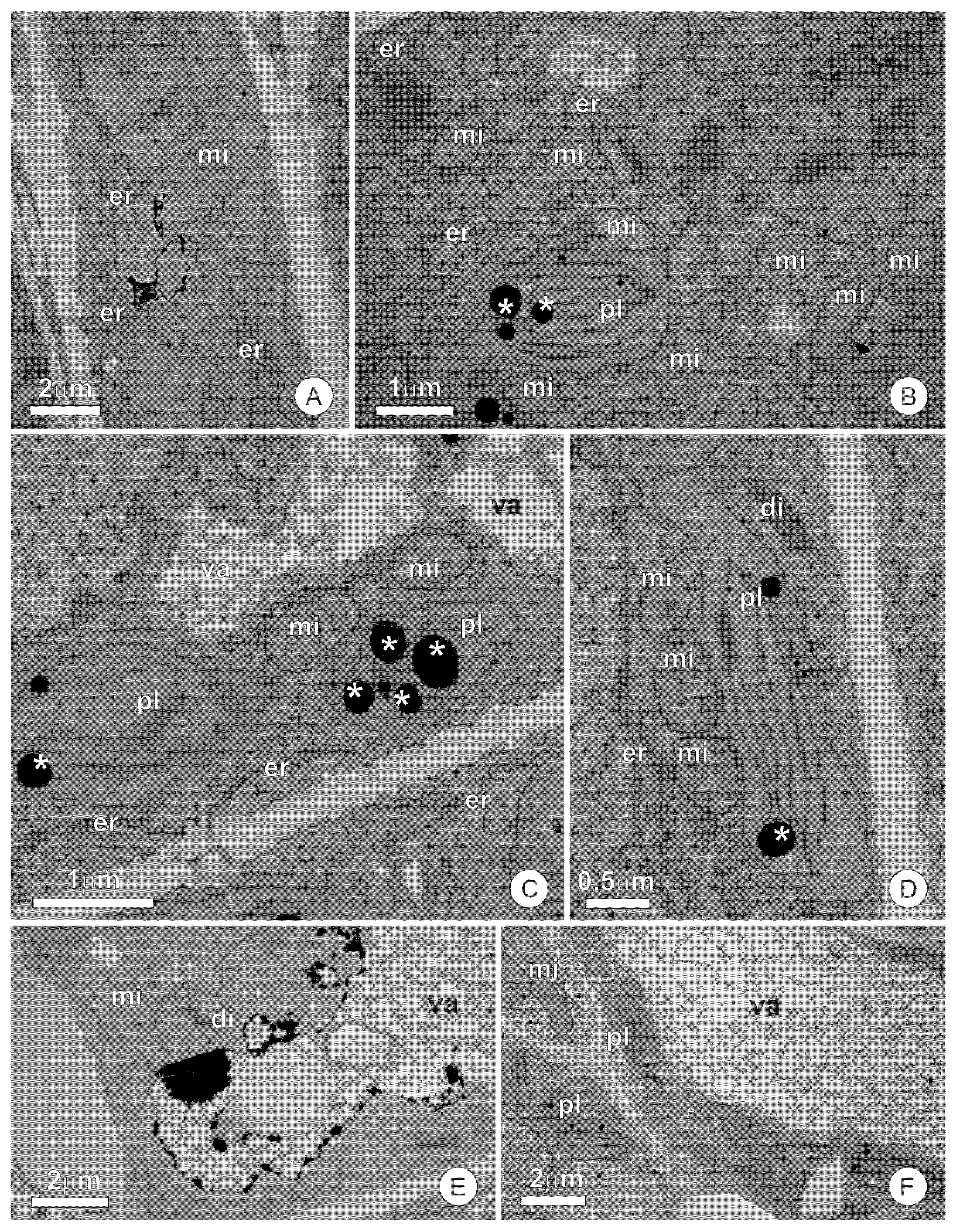
3.2. Ultrastructure and Secretion Mechanism
3.3. Functional Aspects
4. Materials and Methods
4.1. Plant Material
4.2. Light Microscopy
4.3. Electron Microscopy
4.4. Histochemistry
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schneider, J.V.; Bissiengou, P.; Amaral, M.C.E.; Tahir, A.; Fay, M.F.; Thines, M.; Sosef, M.S.M.; Zizka, G.; Chatrou, L.W. Phylogenetics, ancestral state reconstruction, and a new infrafamilial classification of the pantropical Ochnaceae (Medusagynaceae, Ochanceae s.str., Quiinaceae) based on five DNA regions. Mol. Phylogenet. Evol. 2014, 78, 199–214. [Google Scholar] [CrossRef] [PubMed]
- Fiaschi, P.; Nicoletti de Fraga, C.; Yamamoto, K. Neotropical Ochnaceae s.l. (incl. Quiinaceae). In Neotropikey—Interactive Key and Information Resources for Flowering Plants of the Neotropics; Milliken, W., Klitgird, B., Baracat, A., Eds.; (2009 onwards); Available online: http://www.kew.org/science/tropamerica/neotropikey/families/Ochnaceae_s.l._(incl._Quiinaceae).htm (accessed on 12 March 2021).
- Yamamoto, K. Estudos Taxonômicos Sobre Ouratea parviflora (DC.) Baill. (Ochnaceae) e Espécies Afins Ocorrentes em Floresta Atlântica Nas Regiões Sudeste e Sul do Brasil. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brasil, 1995. [Google Scholar]
- Fraga, C.N.; Saavedra, M.M. A new cauliflorous white-flowered species of Ouratea (Ochnaceae) from the Brazilian Atlantic Forest. Phytotaxa 2014, 167, 119–126. [Google Scholar] [CrossRef]
- Weber, M.G.; Porturas, L.D.; Keeler, K.H. World List of Plants with Extrafloral Nectaries. Available online: www.extrafloralnectaries.org (accessed on 16 March 2021).
- Suzart, L.R.; Daniel, J.F.S.; Carvalho, M.G.; Kaplan, M.A.C. Biodiversidade flavonoídica e aspectos farmacológicos em espécies dos gêneros Ouratea e Luxemburgia (Ochnaceae). Quim. Nova 2007, 30, 984–987. [Google Scholar] [CrossRef]
- Fahn, A. Secretory Tissue in Plants; Academic Press: London, UK, 1979. [Google Scholar]
- Groom, P. On bud-protection in dicotyledons. Trans. Linn. Soc. Lond. 2nd Ser. Bot. 1892, 3, 255–266. [Google Scholar] [CrossRef][Green Version]
- Tresmondi, F.; Canaveze, Y.; Guimarães, E.; Machado, S.R. Colleters in Rubiaceae from forest and savanna: The link between secretion and environment. Sci. Nat. 2017, 104, 17. [Google Scholar] [CrossRef]
- Voigt, D.; Kim, J.; Jantschke, A.; Varenberg, M. Robust, universal, and persistent bud secretion adhesion in horse-chestnut trees. Sci. Rep. 2020, 10, 16925. [Google Scholar] [CrossRef]
- Campos, B.H.; Guimarães, E.; Canaveze, Y.; Machado, S.R. Epicormic bud protection traits vary along a latitudinal gradient in a neotropical savana. Sci. Nat. 2021, 108, 11. [Google Scholar] [CrossRef]
- Coley, P.D.; Kursar, T.A. Anti-herbivore defences of young tropical leaves: Physiological constrains and ecological tradeoffs. In Tropical Forest Plant Ecophysiology; Mulkey, S.S., Chazdon, L.R., Eds.; Springer: Boston, MA, USA, 1996; pp. 305–336. [Google Scholar] [CrossRef]
- Pausas, J.G.; Pratt, R.B.; Keeley, J.E.; Jacobsen, A.L.; Ramirez, A.R.; Vilagrosa, A.; Paula, S.; Kaneakua-Pia, I.N.; Davis, S.D. Towards understanding resprouting at the global scale. New Phytol. 2016, 209, 945–954. [Google Scholar] [CrossRef]
- Amaral, M.C.E.; Bittrich, V. Ochnaceae. In Flowering Plants. Eudicots. The Families and Genera of Vascular Plants; Kubitzki, K., Ed.; Springer: Berlin/Heidelberg, Germany, 2014; Volume 11, pp. 253–268. [Google Scholar] [CrossRef]
- Queiroz-Lima, A.; Amorim, A.M.; Cardoso, D.B. A new bristle-leaved species of Sauvagesia (Ochnaceae) endemic to the Espinhaço range, Brazil. Syst. Bot. 2017, 42, 346–350. [Google Scholar] [CrossRef]
- Reinales, S.; Parra-O, C. Phylogenetic position and evolution of glandular structures of the unusual and narrowly distributed genus Rhytidanthera (Ochnaceae). Bot. J. Linn. Soc. 2020, 193, 84–99. [Google Scholar] [CrossRef]
- Rios, A.B.M.; Menino, G.C.D.O.; Dalvi, V.C. Leaf teeth in eudicots: What can anatomy elucidate? Bot. J. Linn. Soc. 2020, 193, 504–522. [Google Scholar] [CrossRef]
- Lorenzi, H. Árvores Brasileiras: Manual de Identificação e Cultivo de Plantas Arbóreas do Brasil; Instituto Plantarum: Nova Odessa, Brazil, 2000. [Google Scholar]
- Barbosa-Campos, M.T.; Castro, S.A.B.; Kuster, V.C.; Santos, L.N.; Lemos-Filho, J.P.; Vale, F.H.A. How the long-life span leaves of Ouratea castaneifolia Engl. (Ochnaceae) differ in distinct light conditions. Braz. J. Bot. 2018, 41, 403–414. [Google Scholar] [CrossRef]
- Curtis, J.D.; Lersten, N.R. Morphology, seasonal variation, and function of resin glands on buds and leaves of Populus deltoides (Salicaceae). Am. J. Bot. 1974, 61, 835–845. [Google Scholar] [CrossRef]
- Thomas, V. Structural, functional and phylogenetic aspects of the colleter. Ann. Bot. 1991, 68, 287–305. [Google Scholar] [CrossRef]
- Simões, A.O.; CASTRO, M.D.M.; Kinoshita, L.S. Calycine colleters of seven species of Apocynaceae (Apocynoideae) from Brazil. Bot. J. Linn. Soc. 2006, 152, 387–398. [Google Scholar] [CrossRef]
- Machado, S.R.; Morellato, P.C.; Sajo, M.G.; Oliveira, P.S. Morphological patterns of extrafloral nectaries in woody plant species of the Brazilian cerrado. Plant Biol. 2008, 10, 660–673. [Google Scholar] [CrossRef]
- Possobom, C.C.F.; Guimarães, E.; Machado, S.R. Structure and secretion mechanisms of floral glands in Diplopterys pubipetala (Malpighiaceae), a neotropical species. Flora 2015, 211, 26–39. [Google Scholar] [CrossRef]
- Araújo, J.S.; Meira, R.M.S.A. Comparative anatomy of calyx and foliar glands of Banisteriopsis C. B. Rob. (Malpighiaceae). Acta. Bot. Brasil. 2016, 30, 112–123. [Google Scholar] [CrossRef]
- Ribeiro, J.C.; Ferreira, M.J.P.; Demarco, D. Colleters in Asclepiadoideae (Apocynaceae): Protection of meristems against desiccation and new functions assigned. Int. J. Plant Sci. 2017, 178, 465–477. [Google Scholar] [CrossRef]
- Harley, R.M.; Giulietti, A.M.; Leite, K.R.B. Two new species and a new record of Sauvagesia (Ochnaceae) in the chapada diamantina of Bahia, Brazil. Kew Bull. 2005, 60, 571–580. [Google Scholar]
- Cardoso, D.B.O.S. A new species of Sauvagesia (Ochnaceae) from the Espinhaço range of Minas Gerais, Brazil. Brittonia 2011, 63, 150–155. [Google Scholar] [CrossRef]
- Parkin, J. The extra-floral nectaries of Hevea brasiliensis, Müll.-Arg. (the Para Rubber Tree), an example of bud-scales serving as nectaries. Ann. Bot. 1904, 18, 217–226. [Google Scholar] [CrossRef]
- Marinho, L.C.; Amorim, A.M.; Cardoso, D.B.O.S. Stirring up a wasp nest: Two new species of the taxonomically complex genus Ouratea (Ochnaceae). Syst. Bot. 2018, 43, 760–766. [Google Scholar] [CrossRef]
- Elias, T.S. Extrafloral nectaries: Their structure and distribution. In The Biology of Nectaries; Bentley, B., Elias, T., Eds.; Columbia University Press: New York, NY, USA, 1983; pp. 174–203. [Google Scholar]
- Frey-Wyssling, A. The phloem supply to the nectaries. Acta. Bot. Neerl. 1955, 4, 358–369. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Ultrastructure and post-floral secretion of the pericarpial nectaries of Erythrina speciosa (Fabaceae). Ann. Bot. 2009, 104, 937–944. [Google Scholar] [CrossRef]
- Fahn, A. Structure and function of secretory cells. Adv. Bot. Res. 2000, 31, 37–75. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Salino, A.; Paiva, E.A.S. Colleters in Thelypteridaceae: Unveiling mucilage secretion and its probable role in ferns. Flora 2017, 228, 65–70. [Google Scholar] [CrossRef]
- Mohan, J.S.S.; Inamdar, J.A. Ultrastructure and secretion of extrafloral nectaries of Plumeria rubra L. Ann. Bot. 1986, 57, 389–401. [Google Scholar] [CrossRef]
- Fernandes, V.F.; Thadeo, M.; Dalvi, V.C.; Meira, R.M.S.A. Secretory structures in Casearia sylvestris Sw. (Salicaceae): Diversity, mechanisms of secretion, and exudate complexity. Int. J. Plant Sci. 2018, 178, 288–301. [Google Scholar] [CrossRef]
- Machado, S.R.; Paleari, L.M.; Paiva, E.A.S.; Rodrigues, T.M. Colleters on the inflorescence axis of Croton glandulosus (Euphorbiaceae): Structural and functional characterization. Int. J. Plant Sci. 2015, 176, 86–93. [Google Scholar] [CrossRef]
- Ballego-Campos, I.; Paiva, E.A.S. Mucilage secretion in the inflorescences of Aechmea blanchetiana: Evidence of new functions of scales in bromeliaceae. Flora 2018, 246–247, 1–9. [Google Scholar] [CrossRef]
- Fahn, A.; Benayoun, J. Ultrastructure of resin ducts in Pinus halepensis development, possible sites of resin synthesis, and mode of its elimination from the protoplast. Ann. Bot. 1976, 40, 857–863. [Google Scholar] [CrossRef]
- Thomson, W.W.; Platt-Aloia, K.A.; Endress, A.G. Ultrastructure of oil gland development in the leaf of Citrus sinensis L. Bot. Gaz. 1976, 137, 330–340. [Google Scholar] [CrossRef]
- McGarvey, D.J.; Croteau, R. Terpenoid metabolism. Plant Cell 1995, 7, 1015–1026. [Google Scholar] [CrossRef]
- Paiva, E.A.S. How do secretory products cross the plant cell wall to be released? A new hypothesis involving cyclic mechanical actions of the protoplast. Ann. Bot. 2016, 117, 533–554. [Google Scholar] [CrossRef]
- Sadala-Castilho, R.; Machado, S.R.; Sa-Haiad, B.; Lima, H.A. Oil-resin glands in Velloziaceae flowers: Structure, ontogenesis and secretion. Plant Syst. Evol. 2016, 302, 585–599. [Google Scholar] [CrossRef]
- Paiva, E.A.S. How does the nectar of stomata-free nectaries cross the cuticle? Acta. Bot. Bras. 2017, 31, 525–530. [Google Scholar] [CrossRef]
- Mayer, J.L.S.; Cardoso-Gustavson, P.; Appezzato-da-Gloria, B. Colleters in monocots: New record for orchidaceae. Flora 2011, 206, 185–190. [Google Scholar] [CrossRef]
- Paiva, E.A.S.; Martins, L.C. Calycinal trichomes in Ipomoea cairica (Convolvulaceae): Ontogenesis, structure and functional aspects. Aust. J. Bot. 2011, 59, 91–98. [Google Scholar] [CrossRef]
- Almeida, A.L.; Paiva, E.A.S. Colleters in Mabea fistulifera Mart. (Euphorbiaceae): Anatomy and biology of the secretory process. Flora 2019, 258, 151439. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Colleters in Cariniana estrellensis (Lecythidaceae): Structure, secretion and evidences for young leaf protection. J. Torrey Bot. Soc. 2012, 139, 1–8. [Google Scholar] [CrossRef]
- Dell, B. Distribution and function of resins and glandular hairs in Western Australian plants. J. R Soc. West. Aust. 1977, 59, 119–123. [Google Scholar]
- Oliveira, P.S.; Leitao-Filho, H.F. Extrafloral Nectaries: Their taxonomic distribution and abundance in the woody flora of cerrado vegetation in Southeast Brazil. Biotropica 1987, 19, 140–148. [Google Scholar] [CrossRef]
- Oliveira, P.S.; Pie, M.R. Interaction between ants and plants bearing extrafloral nectaries in cerrado vegetation. An. Soc. Entomol. Bras. 1988, 27, 161–176. [Google Scholar] [CrossRef]
- Paiva, E.A.S. Are calcium oxalate crystals a dynamic calcium store in plants? New Phytol. 2019, 223, 1707–1711. [Google Scholar] [CrossRef]
- Paiva, E.A.S.; Ballego-Campos, I.; Gibernau, M. True nectar or stigmatic secretion? Structural evidence elucidates an old controversy regarding nectaries in Anthurium. Am. J. Bot. 2021, 108, 37–50. [Google Scholar] [CrossRef]
- Schnell, R.; Cusset, G.; Quenum, M. Contribution a l’etude des glandes extra-florales chez quelques groupes de plantes tropicales. Rev. Générale Bot. 1963, 70, 269–342. [Google Scholar]
- Metcalfe, C.R.; Chalk, L. Anatomy of the Dicotyledons, 2nd ed.; Claredon Press: Oxford, UK, 1979. [Google Scholar]
- Konarska, A. Characteristics of flower nectaries of Hedera helix L. (Araliaceae). Acta. Sci. Pol. Hortoru. Cultus 2014, 13, 109–122. [Google Scholar]
- Gish, M.; Mescher, M.C.; Moraes, C.M. Mechanical defenses of plant extrafloral nectaries against herbivory. Commun. Integr. Biol. Biol. 2016, 9, 3. [Google Scholar] [CrossRef]
- Gonçalves-Souza, P.; Gonçalves, E.G.; Paiva, E.A.S. Extrafloral nectaries in Philodendron (Araceae): Distribution and structure. Bot. J. Linn. Soc. 2016, 180, 229–240. [Google Scholar] [CrossRef]
- Pereira, P.S.; Gonçalves, L.A.; Silva, M.J.; Rezende, M.H. Extrafloral nectaries of four varieties of Chamaecrista ramosa (Vogel) H.S. Irwin & Barneby (Fabaceae): Anatomy, chemical nature, mechanisms of nectar secretion, and elimination. Protoplasma 2018, 255, 1635–1647. [Google Scholar] [CrossRef]
- Mesquita-Neto, J.N.; Paiva, E.A.S.; Galetto, L.; Schlindwein, C. Nectar secretion of floral buds of Tococa guianensis mediates interactions with generalist ants that reduce florivory. Front. Plant Sci. 2020, 11, 627. [Google Scholar] [CrossRef] [PubMed]
- Karnovsky, M.J. A formaldehyde glutaraldehyde fixative of high osmolality for use in electron microscopy. J. Cell Biol. 1965, 27, 137A–138A. [Google Scholar]
- O’Brien, T.; Feder, N.; McCully, M.E. Polychromatic staining of plant cell walls by toluidine blue O. Protoplasma 1964, 59, 368–373. [Google Scholar] [CrossRef]
- Spurr, A.R. A low-viscosity epoxy resin embedding medium for electron microscopy. J. Ultrastruct. Res. 1969, 26, 31–43. [Google Scholar] [CrossRef]
- Roland, J.C. General preparation and staining of thin sections. In Electron Microscopy of Plant Cells; Hall, J., Ed.; Elsevier: New York, NY, USA, 1978; pp. 1–62. [Google Scholar]
- Brundrett, M.C.; Kendrick, B.; Peterson, C.A. Efficient lipid staining in plant material with Sudan Red 7B or Fluoral Yellow 088 in polyethylene glycol-glycerol. Biotech. Histochem. 1991, 66, 111–116. [Google Scholar] [CrossRef]
- Johansen, D.A. Plant Microtechnique; McGraw-Hill Book Co.: New York, NY, USA, 1940. [Google Scholar]
- David, R.; Carde, J.P. Coloration différentielle des inclusions lipidiques et terpéniques des pseudophylles du Pin maritime au moyen du réactif Nadi. C R Acad. Sci. Paris 1964, 258, 1338–1340. [Google Scholar]
- Vidal, B.C. Dichroism in collagen bundles stained with Xylidine-Ponceau 2R. Ann. D Histochim. 1970, 15, 289–296. [Google Scholar]


| Test | Target Substance | Resin Gland | Colleter | Nectary | |||
|---|---|---|---|---|---|---|---|
| Protoplast | Secretion | Protoplast | Secretion | Protoplast | Secretion | ||
| NADI reagent | Terpenoids (essential oils) | + | + | − | − | − | − |
| Ruthenium Red | Mucilage | − | − | + | + | − | − |
| Sudan Red B | Lipids | − | − | + | + | − | − |
| Xylidine Ponceau | Proteins | − | − | + | + | + | − |
| Glucose strip-test | Sugars (glucose) | N/A | N/A | N/A | N/A | N/A | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paiva, E.A.S.; Couy-Melo, G.A.; Ballego-Campos, I. Colleters, Extrafloral Nectaries, and Resin Glands Protect Buds and Young Leaves of Ouratea castaneifolia (DC.) Engl. (Ochnaceae). Plants 2021, 10, 1680. https://doi.org/10.3390/plants10081680
Paiva EAS, Couy-Melo GA, Ballego-Campos I. Colleters, Extrafloral Nectaries, and Resin Glands Protect Buds and Young Leaves of Ouratea castaneifolia (DC.) Engl. (Ochnaceae). Plants. 2021; 10(8):1680. https://doi.org/10.3390/plants10081680
Chicago/Turabian StylePaiva, Elder A. S., Gabriel A. Couy-Melo, and Igor Ballego-Campos. 2021. "Colleters, Extrafloral Nectaries, and Resin Glands Protect Buds and Young Leaves of Ouratea castaneifolia (DC.) Engl. (Ochnaceae)" Plants 10, no. 8: 1680. https://doi.org/10.3390/plants10081680
APA StylePaiva, E. A. S., Couy-Melo, G. A., & Ballego-Campos, I. (2021). Colleters, Extrafloral Nectaries, and Resin Glands Protect Buds and Young Leaves of Ouratea castaneifolia (DC.) Engl. (Ochnaceae). Plants, 10(8), 1680. https://doi.org/10.3390/plants10081680







