Two New Species of Impatiens from China, and Taxonomic Insights into the Longifilamenta Group, Which Is Endemic to China
Abstract
:1. Introduction
2. Results
2.1. Taxonomic Treatment of the New Species
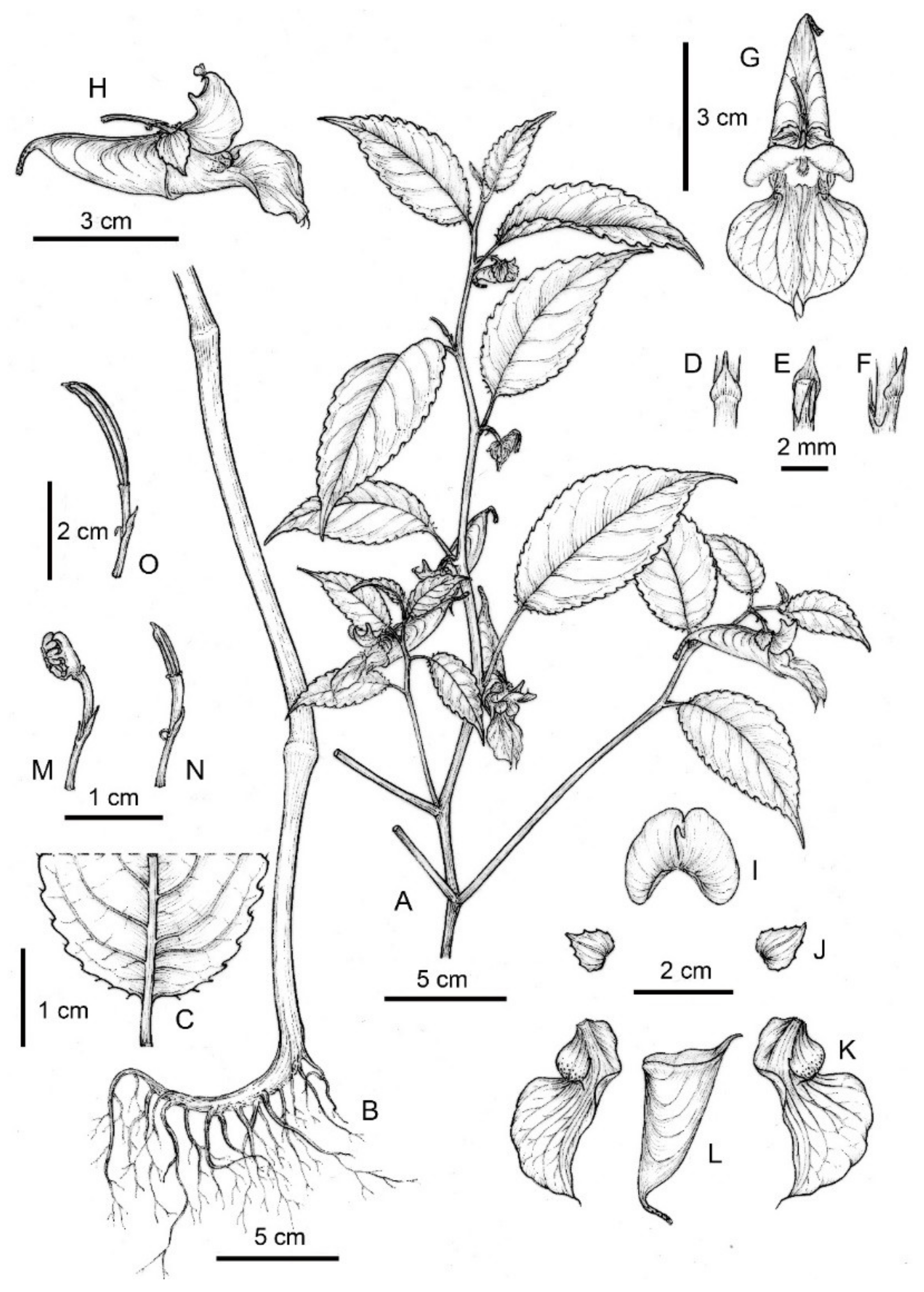
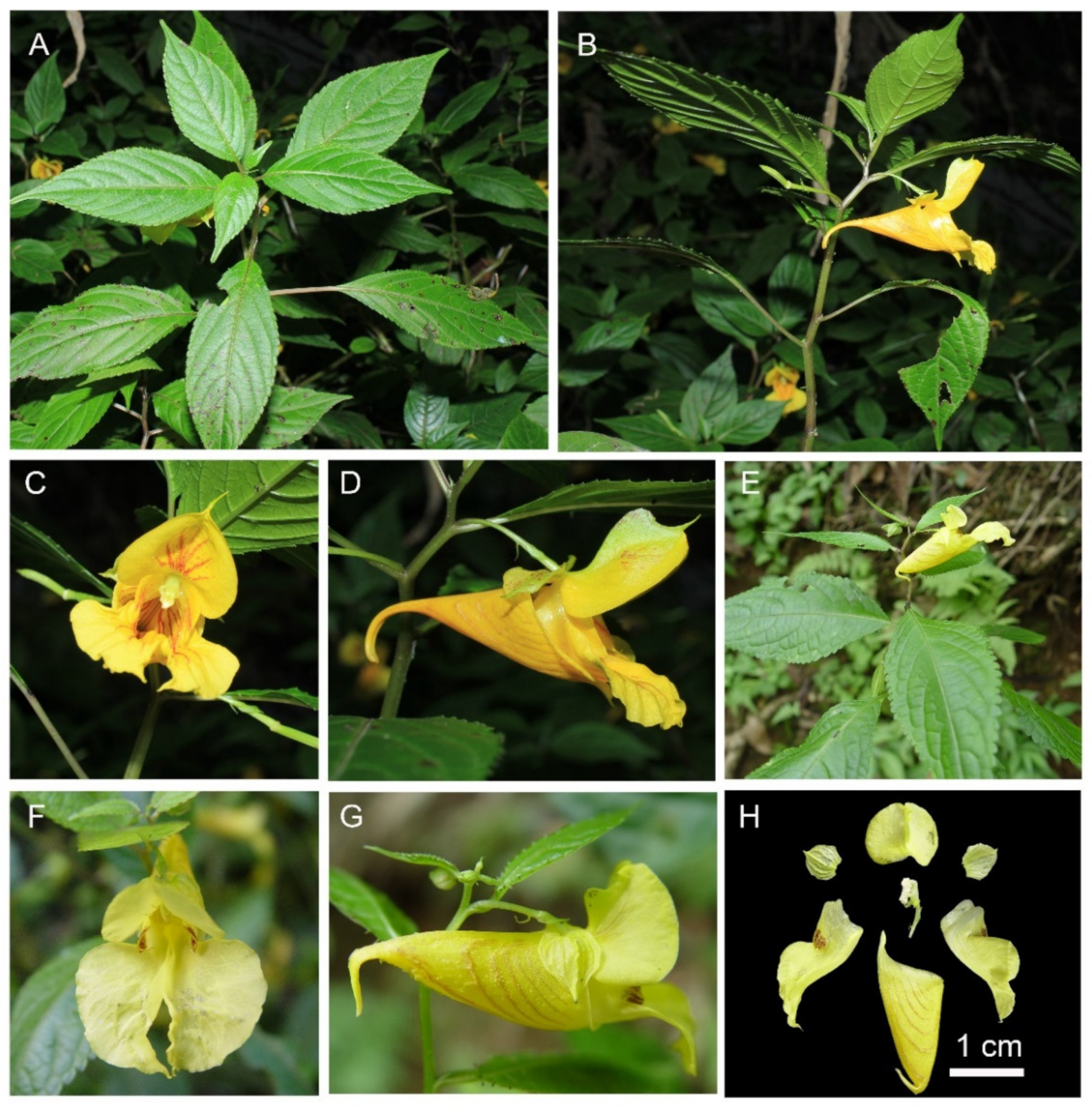
2.1.1. Taxonomic Description of Impatiens longshanensis
Diagnosis
Additional Material Examined
Description
Etymology
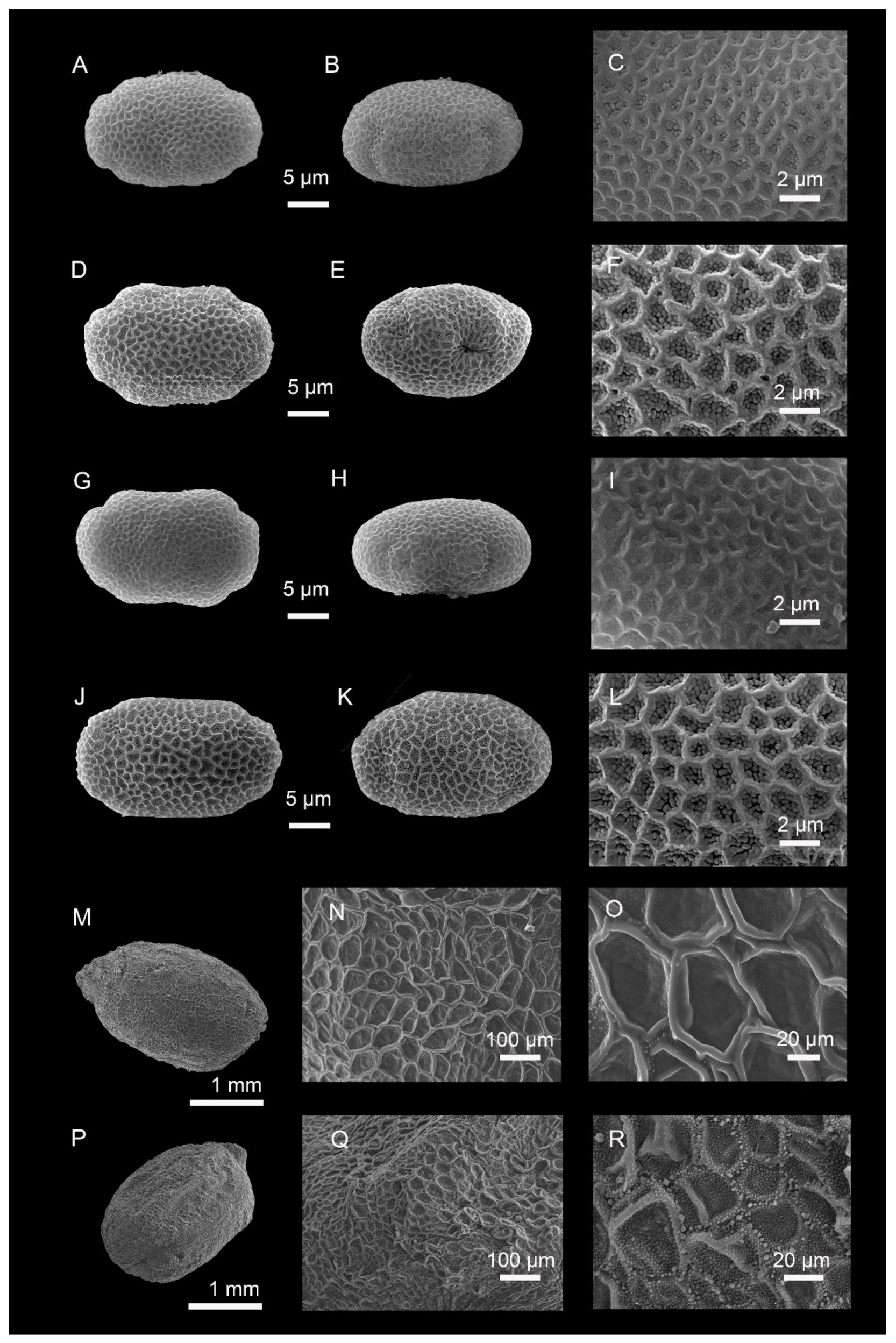
Micromorphological Observations
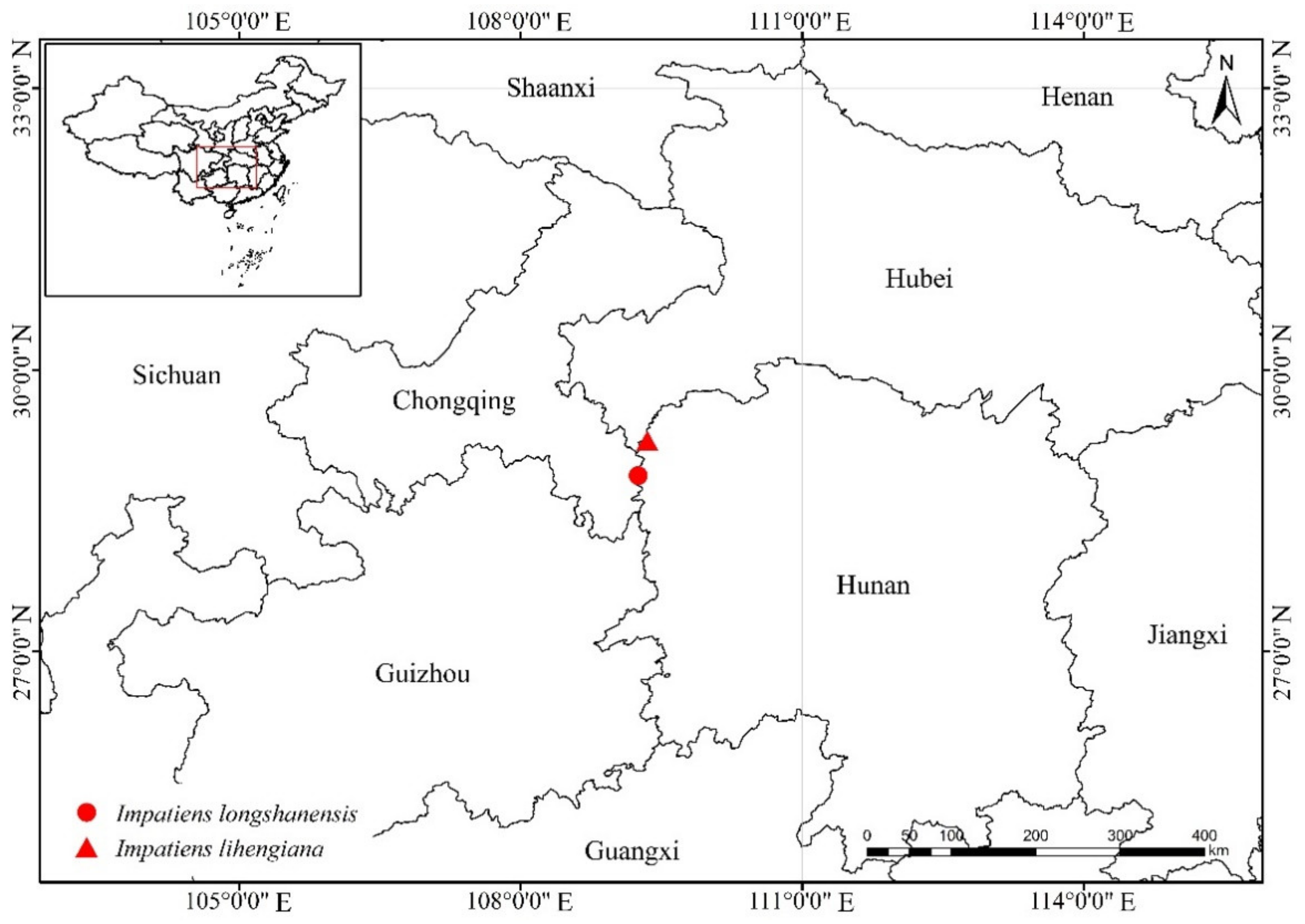
Habitat and Distribution
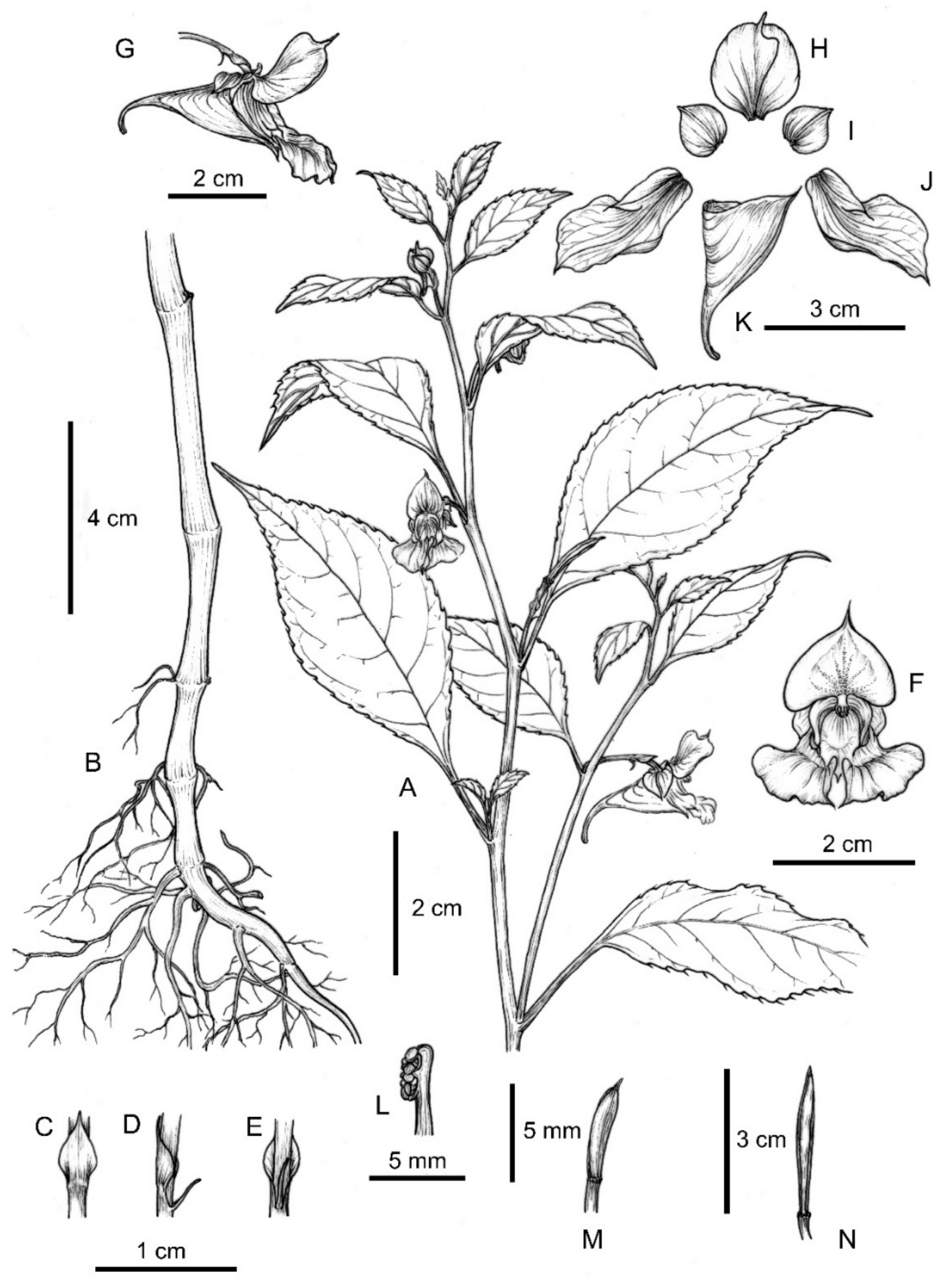
2.1.2. Taxonomic Description of Impatiens lihengiana
Diagnosis
Description
Etymology
Habitat and Distribution
Micromorphological Observations
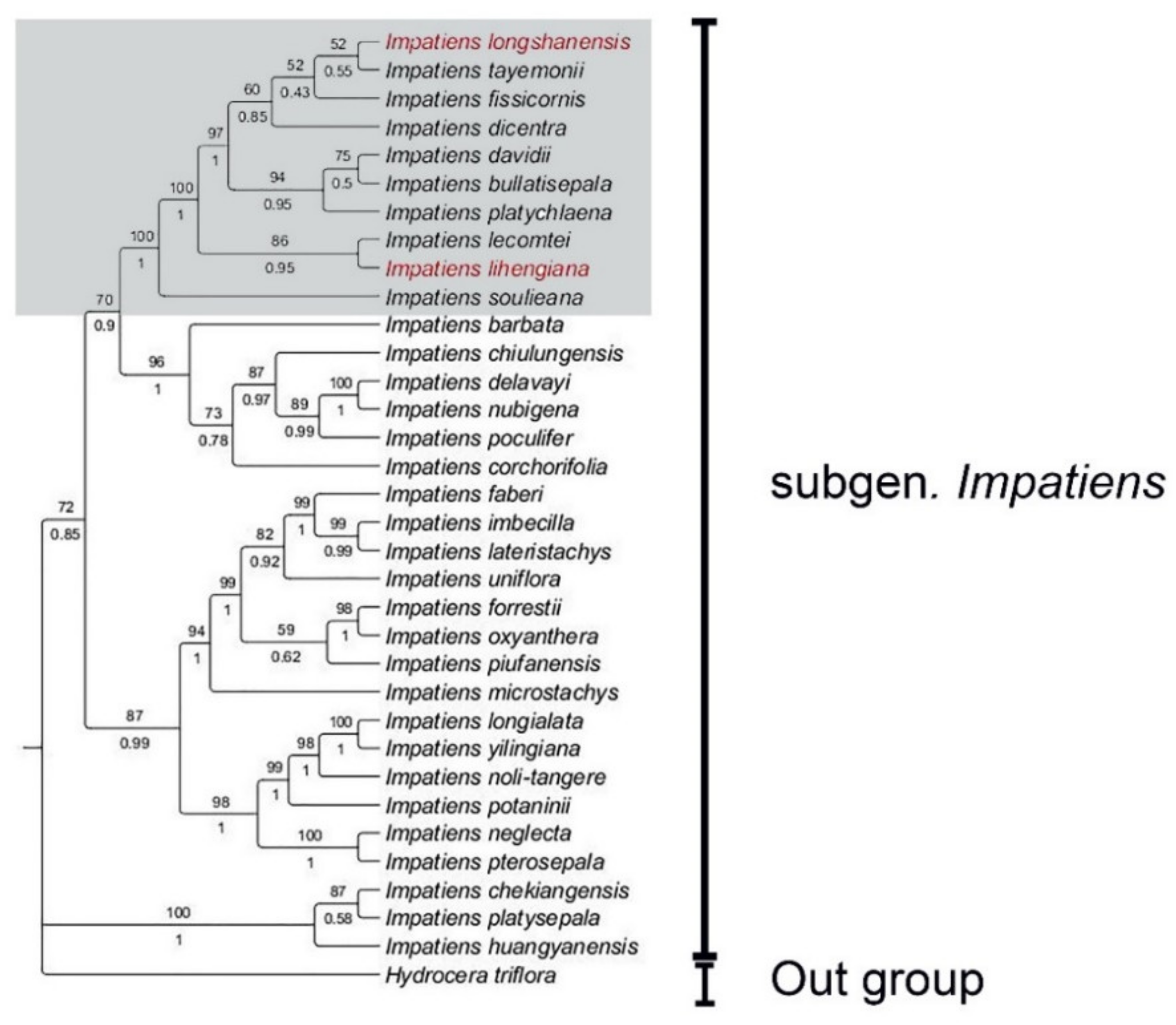
2.2. Molecular Phylogenetic Analysis
2.3. Taxonomic Insights
2.4. Analytical Key to the Taxa of Longifilamenta Group of Impatiens in China
- -
- 1a Basal lobes of lateral united petals with a filamentous long hair, distal lobes of lateral united petals apex retuse with aseta …………………………………………… 2
- -
- 1b Basal lobes of lateral united petals with a filamentous long hair, distal lobes of lateral united petals apex entire with a filamentous long hair or a seta ………………………………………………………………………………………… 3
- -
- 2a Flowers pale rose pink; lateral sepals abaxial midvein narrowly thickened; distal lobes of lateral united petals oblong or suboblong …………………… I. oblongipetala
- -
- 2b Flowers yellow; lateral sepals abaxial midvein narrowly carinate; distal lobes of lateral united petals dolabriform …………………………………………… I. soulieana
- -
- 3a Lower sepal funnelform or navicular ……………………………………………… 4
- -
- 3b Lower sepal saccate ………………………………………………………………… 13
- -
- 4a Lower sepal navicular, spur absent ………………………………… I. shennongensis
- -
- 4b Lower sepal funnelform, spur 2-lobed ……………………………………………… 5
- -
- 5a Lateral sepals margin 4-or 5-denticulate on one side ………………… I. weihsiensis
- -
- 5b Lateral sepals margin entire ………………………………………………………… 6
- -
- 6a Lateral united petals clawed ………………………………………………………… 7
- -
- 6b Lateral united petals not clawed …………………………………………………… 8
- -
- 7a Lateral sepals broadly ovate-cordate, abaxial midvein cristate ……… I. toxophora
- -
- 7b Lateral sepals ovate-orbicular, abaxial midvein without cristate …… I. tayemonii
- -
- 8a Lateral sepals abaxial midvein carinate narrowly cristate or with a spinelike appendage …………………………………………………………………………………… 9
- -
- 8b Lateral sepals abaxial midvein without carinate ………………………………… 12
- -
- 9a Flowers pink ………………………………………………………………… I. lecomtei
- -
- 9b Flowers yellow ……………………………………………………………………… 10
- -
- 10a lateral sepals ovate, abaxial midvein a spinelike appendage; dorsal petal reniform ………………………………………………………………………… I. cornutisepala
- -
- 10b lateral sepals orbicular, abaxial midvein carinate or narrowly cristate; dorsal petal orbicular ……………………………………………………………………………11
- -
- 11a Bracts subulate; Lateral sepals 10–20 mm in diam, abaxial midvein acutely carinate ……………………………………………………………………………… I. brevipes
- -
- 11b Bracts ovate; Lateral sepals ca. 8 mm in diam, abaxial midvein narrowly cristate ……………………………………………………………………………… I. mussotii
- -
- 12a Flowers yellow; lateral sepals suborbicular, 1-veined ……………… I. lihengiana
- -
- 12b Flowers pale purple or purple-red; Lateral sepals ovate-orbicular,7-veined …………………………………………………………………… I. gongchengensis
- -
- 13a Lateral sepals margin dentate …………………………………………………… 14
- -
- 13b Lateral sepals margin entire ……………………………………………………… 18
- -
- 14a Lateral sepals inequilateral, coarsely dentate on one side ……………… I. dicentra
- -
- 14b Lateral sepals equilateral, dentate on both side ………………………………… 15
- -
- 15a Lateral sepals margin irregularly fimbriate-lacerate …………………………… 16
- -
- 15b Lateral sepals margin coarsely dentate …………………………………………… 17
- -
- 16a Flowers yellow, to 4.5 cm deep; lateral sepals margin and abaxial midvein irregularly fimbriate-lacerate; dorsal petal orbicular …………………………… I. lacinulifera
- -
- 16b Flowers pale purple, 2–3 cm deep; lateral sepals margin irregularly lacerate; dorsal petal broadly reniform …………………………………………………… I. platyceras
- -
- 17a Lateral united petals clawed; lower sepal with a hooked spur; dorsal petal suborbicular ……………………………………………………………………… I. fissicornis
- -
- 17b Lateral united petals not clawed; lower sepal with a incurved spur; dorsal petal reniform …………………………………………………………………… I. longshanensis
- -
- 18a Lateral united petals clawed ……………………………………………………… 19
- -
- 18b Lateral united petals not clawed …………………………………………………… 22
- -
- 19a Dorsal petal orbicular or suborbicular …………………………………………… 20
- -
- 19b Dorsal petal broadly ovate ………………………………………………………… 21
- -
- 20a Lateral sepals yellow, broadly ovate, 9-veined ………………………… I. davidii
- -
- 20b Lateral sepals pale green, orbicular, abaxial midvein with a small sac at base ………………………………………………………………………………… I. vittata
- -
- 21a Lateral sepals abaxially plicated; basal lobes of lateral united petals oblong ………………………………………………………………………… I. plicatisepala
- -
- 21b Lateral sepals lateral veins reticulate and sunk on abaxial surface with bullate projections among veins; basal lobes of lateral united petals ovate to elliptic …………………………………………………………………………… I. bullatisepala
- -
- 22a Lateral sepals abaxial midvein not thickened, many veined ……… I. platychlaena
- -
- 22b Lateral sepals abaxial midvein fine or slightly thickened, carinate …………… 23
- -
- 23a Lateral sepals apex aristate-acuminate ……………………………… I. waldheimiana
- -
- 23b Lateral sepals apex without aristate-acuminate ………………………………… 24
- -
- 24a Flowers ca. 4 cm deep; lateral sepals abaxial midvein fine, turgid; basal lobes of lateral united petals oblate ……………………………………………………… I. robusta
- -
- 24b Flowers 2.5–3 cm deep; lateral sepals abaxial midvein slightly thickened, carinate; basal lobes of lateral united petals ovate-lanceolate ………………………… I. conaensis
3. Discussion
4. Materials and Methods
4.1. Gross Traits
4.2. SEM Observations
4.3. DNA Sequencing and Phylogenetic Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blume, C. Balsaminaceae. In Prodromus Florae Peninsulae Indiae Orientalis; Parburry Allen & Co.: London, UK, 1834; Volume 1, p. 140. [Google Scholar]
- Linnaeus, C. Species Plantarum; Salvius: Stockholm, Sweden, 1753; Volume 2, p. 937. [Google Scholar]
- Grey-Wilson, C. Impatiens of Africa; Balkema: Rotterdam, The Netherlands, 1980. [Google Scholar]
- Mabberley, D.J. Mabberley’s Plant-Book, a Portable Dictionary of Plants, Their Classification and Uses, 4th ed.; Cambridge University Press: Cambridge, MA, USA, 2017; p. 1102. [Google Scholar]
- Chen, Y.L.; Akiyama, S.; Ohba, H. Balsaminaceae. In Flora of China; Wu, Z.-Y., Raven, P.H., Hong, D.Y., Eds.; Science Press: Beijing, China; Beijing & Missouri Botanical Garden Press: St. Louis, MO, USA, 2007; Volume 12, pp. 43–113. [Google Scholar]
- Yu, S.X.; Janssens, S.B.; Zhu, X.Y.; Lidén, M.; Gao, T.G.; Wang, W. Phylogeny of Impatiens (Balsaminaceae): Integrating molecular and morphological evidence into a new classification. Cladistics 2016, 32, 179–197. [Google Scholar] [CrossRef]
- Duric, M.J.; Subotic, A.R.; Prokic, L.T.; Trifunovic-Momcilov, M.M.; Cingel, A.D.; Dragicevic, M.B.; Simonovic, A.D.; Milosevic, S.M. Molecular characterization and expression of four aquaporin genes in Impatiens walleriana during drought stress and recovery. Plants 2021, 10, 154. [Google Scholar] [CrossRef]
- Cong, Y.Y.; Cai, X.Z.; Liu, K.M. Impatiens unguiculata (Balsaminaceae), a new species from Xizang, China. Ann. Bot. Fenn. 2013, 50, 165–168. [Google Scholar] [CrossRef]
- Kuang, R.P.; Duan, L.D.; Gu, J.Z.; Cai, X.Z.; Cong, Y.Y.; Liu, K.M. Impatiens liboensis sp. nov. (Balsaminaceae) from Guizhou, China. Nord. J. Bot. 2014, 32, 463–467. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, T.J.; Zhao, L.H. Impatiens menghuochengensis sp. nov. (Balsaminaceae) from Sichuan, China. Nord. J. Bot. 2014, 6, 839–843. [Google Scholar] [CrossRef]
- Cai, X.Z.; Hu, G.W.; Cong, Y.Y. Impatiens xanthinoides (Balsaminaceae), a new species from Yunnan, China. Phytotaxa 2015, 227, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y.H.; Liu, Y.N.; Jiang, H.; Zhu, X.X.; Zhang, W.; Yu, S.X. Impatiens pandurata (Balsaminaceae), a new species from Yunnan, China. Bot. Stud. 2015, 56, e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Z.C.; Pan, B.; Huang, F.Z.; Liu, Y. Impatiens gongchengensis (Balsaminaceae), a new species from Guangxi, Southern China. Taiwania 2020, 65, 1–4. [Google Scholar] [CrossRef]
- Peng, S.; Cong, Y.Y.; Tian, J.; Zhang, C.F.; Hu, G.W.; Wang, Q.F. Impatiens bullatisepala (Balsaminaceae), a new species from Guizhou, China. Phytotaxa 2021, 500, 217–224. [Google Scholar] [CrossRef]
- Song, Y.X.; Peng, S.; Cong, Y.Y.; Zheng, Y.M. Impatiens rapiformis, a new species of Impatiens with root tuber from Yunnan, China. Nord. J. Bot. 2021, 39. [Google Scholar] [CrossRef]
- Chen, Y.L. Balsaminaceae. In Flora Reipublicae Popularis Sinica; Science Press: Beijing, China, 2001; Volume 47, pp. 1–243. [Google Scholar]
- Yu, S.X. Balsaminaceae of China; Peking University Press: Beijing, China, 2012. [Google Scholar]
- Hooker, J.D. Les especes du genre “Impatiens” dans l’herbier du Museum de Paris. Nov. Arch. Mus. Nat. Hist. Paris. Ser. 1908, 10, 233–272. [Google Scholar]
- Zou, C.Y.; Liu, Y.; Li, J.; Yu, S.X. Impatiens plicatisepala (Balsaminaceae), a new species from Guangxi, China. Taiwania 2020, 65, 451–455. [Google Scholar] [CrossRef]
- Yu, S.X.; Zhou, X.R.; Chen, Y.L. Impatiens cornutisepala (Balsaminaceae), a New Species from Guangxi, China. Novon 2009, 19, 562–566. [Google Scholar] [CrossRef]
- Cong, Y.Y.; Liu, K.M. Impatiens oblongipetala (Balsaminaceae), a New Species from Yunnan, China. Novon 2010, 20, 392–395. [Google Scholar] [CrossRef]
- Wang, Q.; Gadagkar, S.R.; Deng, H.P.; Yang, Z.M.; Yu, F.Q. Impatiens shennongensis (Balsaminaceae): A new species from Hubei, China. Phytotaxa 2016, 244, 96–100. [Google Scholar] [CrossRef]
- Wang, K.F.; Wang, X.Z. Outline of Palynology; Beijing Universit Press: Beijing, China, 1983. [Google Scholar]
- Lu, Y.Q. Pollen morphology of Impatiens L. (Balsaminaceae) and its taxonomic implications. Acta Phytotax. Sin. 1991, 29, 352–357. [Google Scholar]
- Lu, Y.Q.; Chen, Y.L. Seed morphology of Impatiens L. (Balsaminaceae) and its taxonomic significance. Acta Phytotax. Sin. 1991, 29, 252–257. [Google Scholar]
- Liu, C.J.; Lin, Q.; He, J.X. Methods and terminology of study on seed morphology from China. Acta Bot. Boreali Occident. Sin. 2004, 24, 178–188. [Google Scholar]
- Song, Y.; Yuan, Y.M.; Küpfer, P. Seedcoat micromorphology of Impatiens (Balsaminaceae) from China. Bot. J. Linn. Soc. 2005, 149, 195–208. [Google Scholar] [CrossRef]
- Yuan, Y.M.; Song, Y.; Geuten, K.; Rahelivololona, E.; Wohlhauser, S.; Fischer, E.; Küpfer, P. Phylogeny and biogeography of Balsaminaceae inferred from ITS sequence data. Taxon 2004, 53, 391–404. [Google Scholar] [CrossRef]
- Janssens, S.; Geuten, K.; Yuan, Y.M.; Song, Y.; Küpfer, P.; Smets, E. Phylogenetics of Impatiens and Hydrocera (Balsaminaceae) using chloroplast atpB-rbcL spacer sequences. Syst. Bot. 2006, 31, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Gao, F.; Jakovlić, I.; Zou, H.; Zhang, J.; Li, W.X.; Wang, G.T. PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. Mol. Ecol. Resour. 2020, 20, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software ver. 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalyaanamoorthy, S.; Minh, B.Q.; Wong, T.K.; VonHaeseler, A.; Jermiin, L.S. ModelFinder: Fast model selection for accurate phylogenetic estimates. Nat. Methods 2017, 14, 587–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Teslenko, M.; VanDerMark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Bot. 2012, 61, 539–542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, L.T.; Schmidt, H.A.; VonHaeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Minh, B.Q.; Nguyen, M.A.T.; von Haeseler, A. Ultrafast approximation for phylogenetic bootstrap. Mol. Biol. Evol. 2013, 30, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Bot. 2010, 59, 307–321. [Google Scholar] [CrossRef] [Green Version]


| Characters | Impatiens longshanensis | Impatiens dicentra |
|---|---|---|
| Plant height (cm) | 40–80 | 60–90 |
| Length of petiole (cm) | 1–3 | 2–5 |
| Lateral sepal shape | broadly ovate-orbicular, equilateral | broadly ovate-orbicular, inequilateral |
| Lateral sepal margin | coarsely dentate on both sides | coarsely dentate on one side, rarely entire |
| Lateral sepal dorsum | abaxial midvein inconspicuously thickened | abaxial midvein narrowly carinate |
| Lateral sepal color | green | yellow |
| Deep of lower sepal (cm) | 1.85–2.3 | 3–5 |
| Length of spur (cm) | ca.1 | vix 1 |
| Dorsal petal | reniform | orbicular |
| Length of lateral united petals (cm) | 2.2–2.9 | 2 |
| Basal lobes | oblong | lanceolate |
| Distal lobes | dolabriform | lanceolate |
| Characters | Impatiens lihengiana | Impatiens davidii | Impatiens platychlaena |
|---|---|---|---|
| Plant height (cm) | 60–70 | ca. 90 | 60–100 |
| Leaf shape | narrowly elliptic or narrowly ovate-elliptic | ovate-oblong, or ovate-lanceolate | ovate-oblong, ovate, or ovate-lanceolate |
| Length of petiole (cm) | 1.5–2.5 | 4–8 | 2–5 |
| Flower color | yellow | yellowish | bicolored: purple and yellow |
| Lateral sepal shape | suborbicular, purple spotted | broadly ovate | broadly orbicular, purple spotted |
| Lateral sepal dorsum | 1-veined | 9-veined | many veined |
| Lateral sepal color | yellow-green | yellow | brown to purple-red when dry |
| Lower sepal | funnelform, base gradually narrowed into an incurved spur, 10–12 mm long | saccate, abruptly narrowed into a hooked spur, ca. 8 mm long | deeply saccate, abruptly narrowed into an incurved spur, ca. 6 mm long |
| Dorsal petal apex | long rostellate | emarginate, shortly rostellate | retuse |
| Lateral united petals | not clawed, 2.5–2.8 cm | clawed,1.5–2 cm | not clawed, 2.5–3 cm |
| Basal lobes | ovate-lanceolate, apex with a filamentous long hair | oblong, apex acuminate or caudate | orbicular, apex with a filamentous long hair |
| Distal lobes | dolabriform, apex obtuse, constricted into a filamentous hair | dolabriform, apex obtuse | dolabriform, longer, with a long filamentous hair |
| Capsule | linear | linear-cylindric | linear |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, Y.-X.; Xiao, Y.; Peng, S.; Cong, Y.-Y.; Hu, G.-W. Two New Species of Impatiens from China, and Taxonomic Insights into the Longifilamenta Group, Which Is Endemic to China. Plants 2021, 10, 1697. https://doi.org/10.3390/plants10081697
Song Y-X, Xiao Y, Peng S, Cong Y-Y, Hu G-W. Two New Species of Impatiens from China, and Taxonomic Insights into the Longifilamenta Group, Which Is Endemic to China. Plants. 2021; 10(8):1697. https://doi.org/10.3390/plants10081697
Chicago/Turabian StyleSong, Yong-Xiu, Yan Xiao, Shuai Peng, Yi-Yan Cong, and Guang-Wan Hu. 2021. "Two New Species of Impatiens from China, and Taxonomic Insights into the Longifilamenta Group, Which Is Endemic to China" Plants 10, no. 8: 1697. https://doi.org/10.3390/plants10081697
APA StyleSong, Y.-X., Xiao, Y., Peng, S., Cong, Y.-Y., & Hu, G.-W. (2021). Two New Species of Impatiens from China, and Taxonomic Insights into the Longifilamenta Group, Which Is Endemic to China. Plants, 10(8), 1697. https://doi.org/10.3390/plants10081697







