Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma
Abstract
:1. Introduction
2. Results
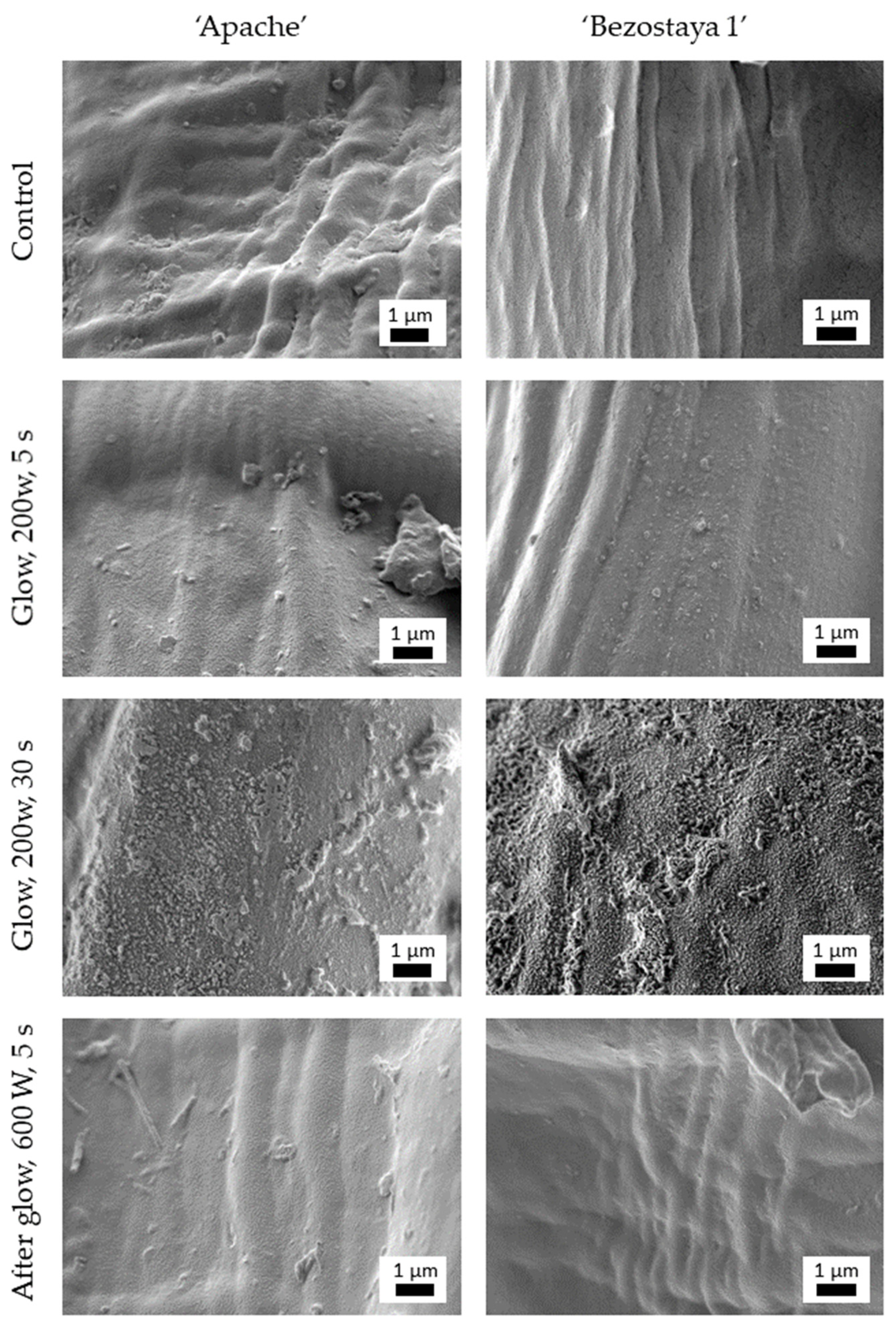
2.1. Glow and Afterglow Plasma Treatment Caused Morphological Changes on the Seed Surface
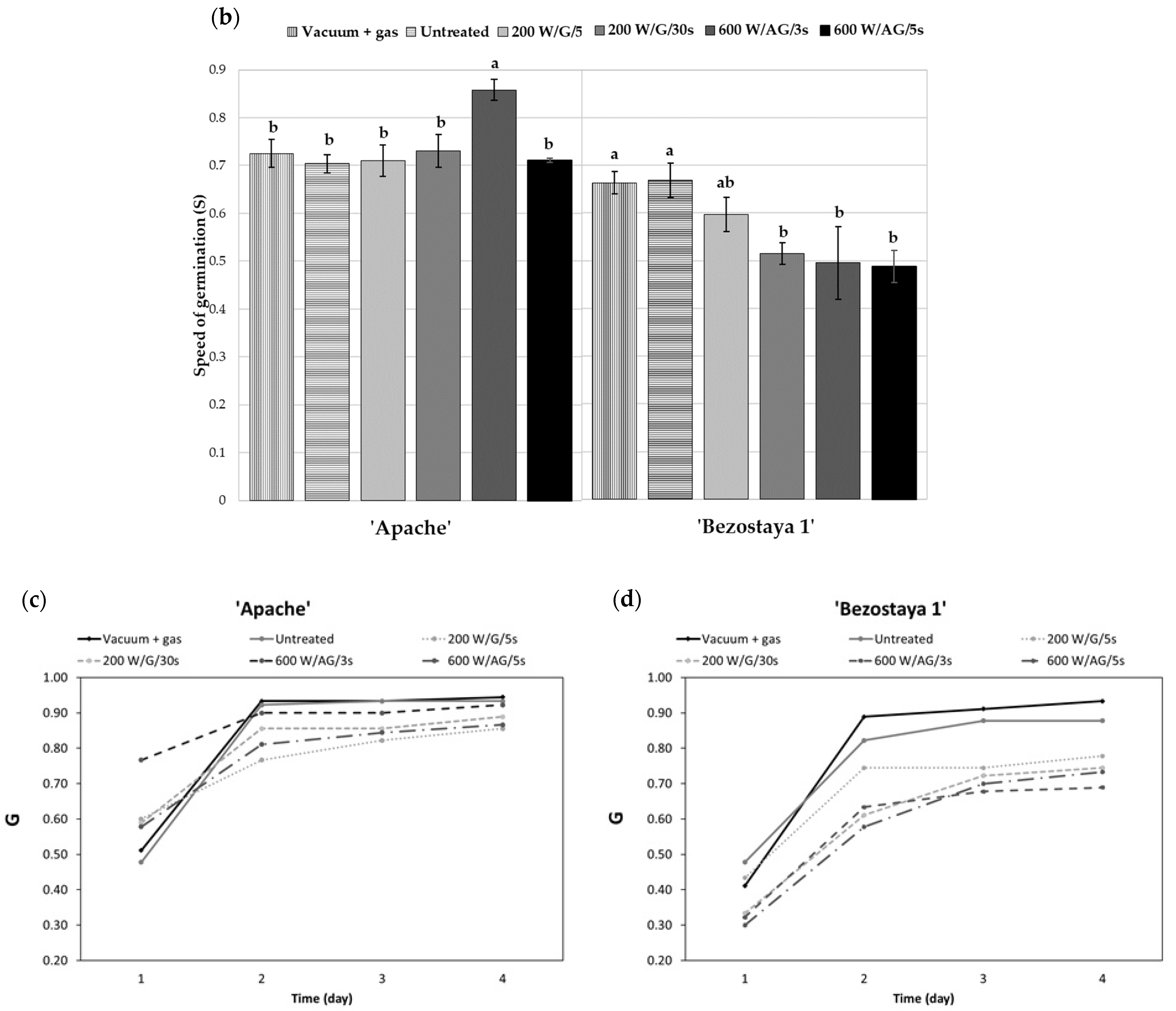
2.2. Germination Process
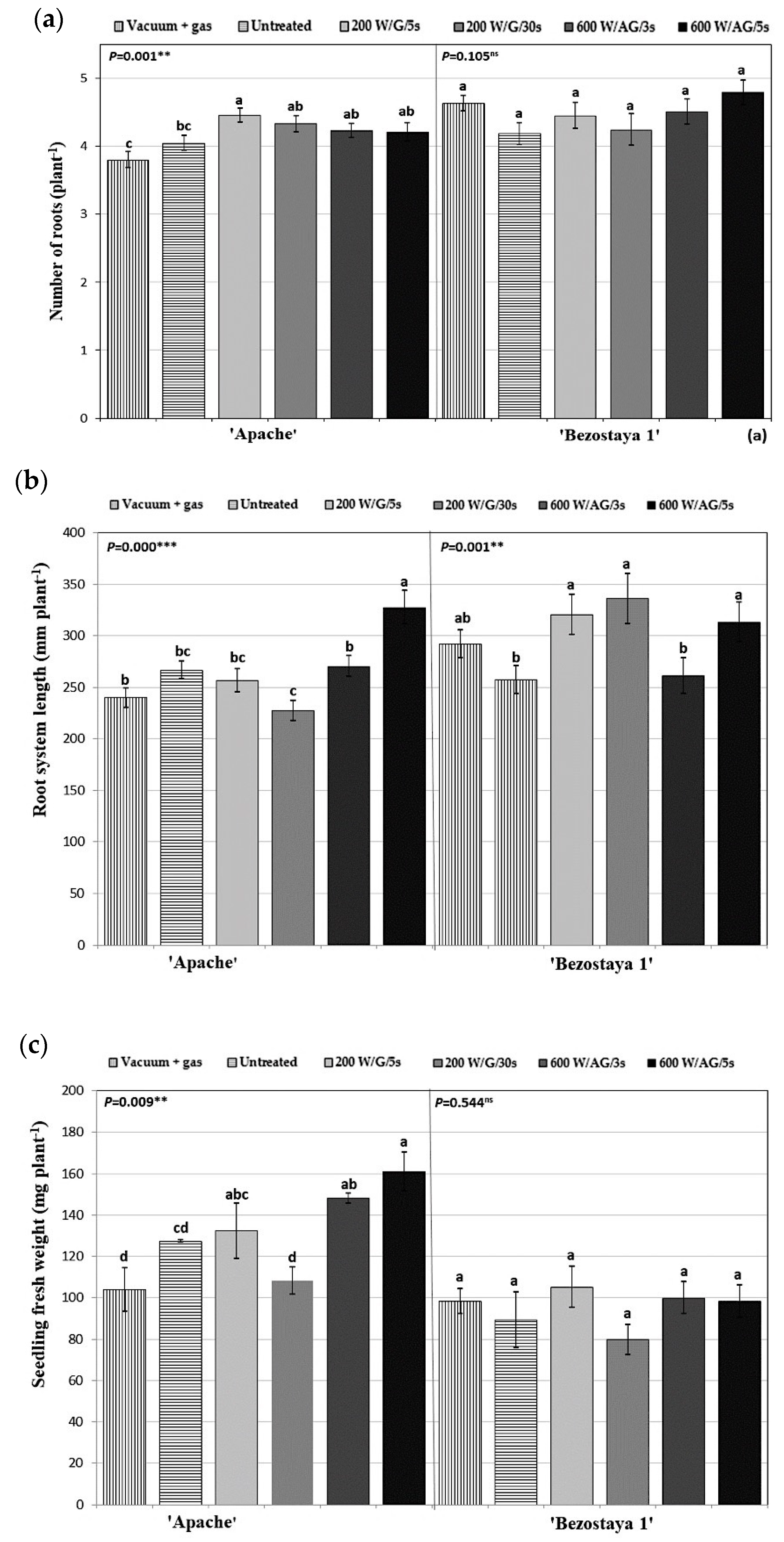
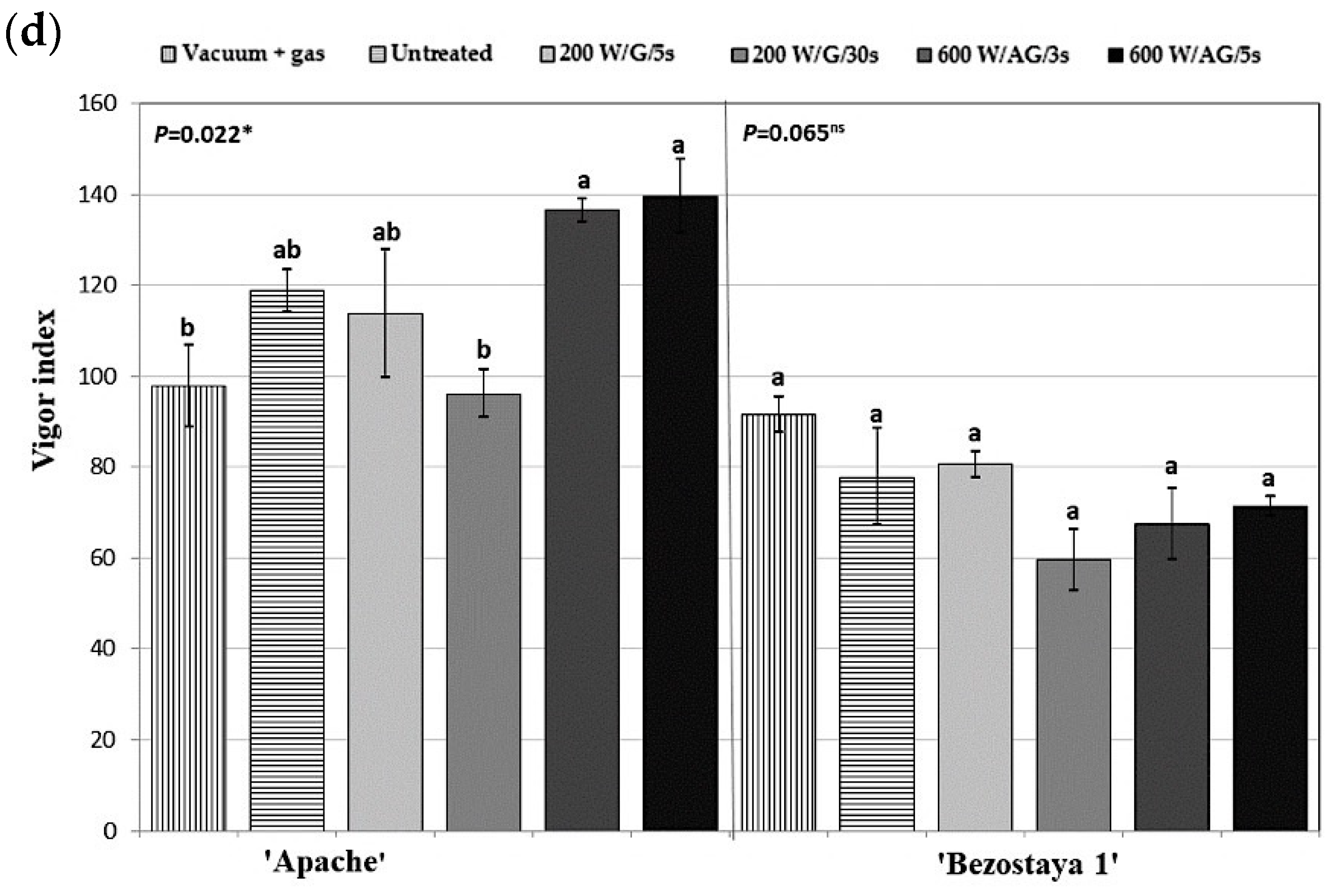
2.3. Morphological Characteristics of Seedlings
3. Discussion
4. Materials and Methods
4.1. Seed Material
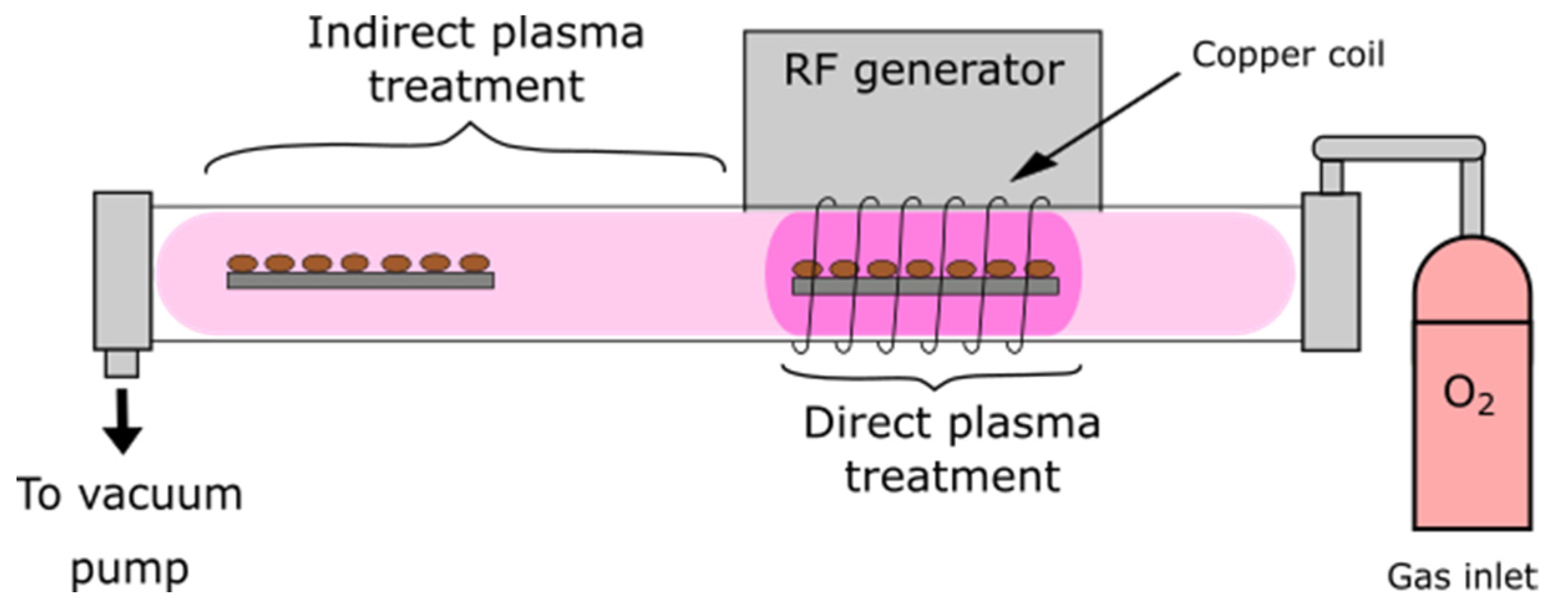
4.2. Characteristics of Plasma and Seed Treatment
4.3. SEM Imaging and Sample Preparation
4.4. Seed Germination, Growth Conditions, and Measurements (Parameters Assessed)
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Selcuk, M.; Oksuz, L.; Basaran, P. Decontamination of grains and legumes infected with Aspergillus spp. and Penicillum spp. by cold plasma treatment. Bioresour. Technol. 2008, 99, 5104–5109. [Google Scholar] [CrossRef]
- Niemira, B.A.; Boyd, G.; Sites, J. Cold Plasma Rapid Decontamination of Food Contact Surfaces Contaminated with Salmonella Biofilms. J. Food Sci. 2014, 79, M917–M922. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.H.; Chang, H.C.; Chen, Y.-K.; Hung, C.L.; Lin, S.Y.; Chen, Y.S. An improved process for high nutrition of germinated brown rice production: Low-pressure plasma. Food Chem. 2016, 191, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Ramírez, A.; López-Santos, C.; Cantos, M.; García, J.L.; Molina, R.; Cotrino, J.; Espinós, J.P.; González-Elipe, A.R. Surface chemistry and germination improvement of Quinoa seeds subjected to plasma activation. Sci. Rep. 2017, 7, 5924. [Google Scholar] [CrossRef] [Green Version]
- Ling, L.; Jiangang, L.; Minchong, S.; Chunlei, Z.; Yuanhua, D. Cold plasma treatment enhances oilseed rape seed germination under drought stress. Sci. Rep. 2015, 5, 13033. [Google Scholar] [CrossRef] [PubMed]
- Mitra, A.; Li, Y.-F.; Klämpfl, T.G.; Shimizu, T.; Jeon, J.; Morfill, G.E.; Zimmermann, J.L. Inactivation of Surface-Borne Microorganisms and Increased Germination of Seed Specimen by Cold Atmospheric Plasma. Food Bioprocess. Technol. 2013, 7, 645–653. [Google Scholar] [CrossRef] [Green Version]
- Jiang, J.; He, X.; Li, L.; Li, J.; Shao, H.; Xu, Q.; Ye, R.; Dong, Y. Effect of Cold Plasma Treatment on Seed Germination and Growth of Wheat. Plasma Sci. Technol. 2014, 16, 54–58. [Google Scholar] [CrossRef] [Green Version]
- Meng, Y.; Qu, G.; Wang, T.; Sun, Q.; Liang, D.; Hu, S. Enhancement of Germination and Seedling Growth of Wheat Seed Using Dielectric Barrier Discharge Plasma with Various Gas Sources. Plasma Chem. Plasma Process. 2017, 37, 1105–1119. [Google Scholar] [CrossRef]
- Slade, A.J.; Fuerstenberg, S.I.; Loeffler, D.; Steine, M.N.; Facciotti, D. A reverse genetic, nontransgenic approach to wheat crop improvement by TILLING. Nat. Biotechnol. 2005, 23, 75–81. [Google Scholar] [CrossRef]
- Guo, Q.; Wang, Y.; Zhang, H.; Qu, G.; Wang, T.; Sun, Q.; Liang, D. Alleviation of adverse effects of drought stress on wheat seed germination using atmospheric dielectric barrier discharge plasma treatment. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Iranbakhsh, A.; Ardebili, N.O.; Ardebili, Z.O.; Shafaati, M.; Ghoranneviss, M. Non-thermal Plasma Induced Expression of Heat Shock Factor A4A and Improved Wheat (Triticum aestivum L.) Growth and Resistance Against Salt Stress. Plasma Chem. Plasma Process. 2017, 38, 29–44. [Google Scholar] [CrossRef]
- Sera, B.; Spatenka, P.; Sery, M.; Vrchotova, N.; Hruskova, I. Influence of Plasma Treatment on Wheat and Oat Germination and Early Growth. IEEE Trans. Plasma Sci. 2010, 38, 2963–2968. [Google Scholar] [CrossRef]
- Roy, N.C.; Hasan, M.M.; Talukder, M.R.; Hossain, M.D.; Chowdhury, A.N. Prospective Applications of Low Frequency Glow Discharge Plasmas on Enhanced Germination, Growth and Yield of Wheat. Plasma Chem. Plasma Process. 2017, 38, 13–28. [Google Scholar] [CrossRef]
- Dobrin, D.; Magureanu, M.; Mandache, N.B.; Ionita, M.-D. The effect of non-thermal plasma treatment on wheat germination and early growth. Innov. Food Sci. Emerg. Technol. 2015, 29, 255–260. [Google Scholar] [CrossRef]
- Volin, J.C.; Denes, F.S.; Young, R.A.; Park, S.M.T. Modification of Seed Germination Performance through Cold Plasma Chemistry Technology. Crop. Sci. 2000, 40, 1706–1718. [Google Scholar] [CrossRef]
- Yodpitak, S.; Mahatheeranont, S.; Boonyawan, D.; Sookwong, P.; Roytrakul, S.; Norkaew, O. Cold plasma treatment to improve germination and enhance the bioactive phytochemical content of germinated brown rice. Food Chem. 2019, 289, 328–339. [Google Scholar] [CrossRef]
- Bafoil, M.; Le Ru, A.; Merbahi, N.; Eichwald, O.; Dunand, C.; Yousfi, M. New insights of low-temperature plasma effects on germination of three genotypes of Arabidopsis thaliana seeds under osmotic and saline stresses. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bormashenko, E.; Shapira, Y.; Grynyov, R.; Whyman, G.; Bormashenko, Y.; Drori, E. Interaction of cold radiofrequency plasma with seeds of beans (Phaseolus vulgaris). J. Exp. Bot. 2015, 66, 4013–4021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bermúdez-Aguirre, D. Advances in Cold Plasma Applications for Food Safety and Preservation, 1st ed.; Bermudez-Aguirre, D., Ed.; Academic Press: Cambridge, MA, USA, 2019; ISBN 9780128149218. [Google Scholar]
- Han, L.; Patil, S.; Boehm, D.; Milosavljević, V.; Cullen, P.J.; Bourke, P. Mechanisms of Inactivation by High-Voltage Atmospheric Cold Plasma Differ for Escherichia coli and Staphylococcus aureus. Appl. Environ. Microbiol. 2016, 82, 450–458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fridman, G.; Brooks, A.D.; Balasubramanian, M.; Fridman, A.; Gutsol, A.; Vasilets, V.; Ayan, H.; Friedman, G. Comparison of Direct and Indirect Effects of Non-Thermal Atmospheric-Pressure Plasma on Bacteria. Plasma Process. Polym. 2007, 4, 370–375. [Google Scholar] [CrossRef]
- Anjum, T.; Bajwa, R. Importance of Germination Indices in Interpretation of Allelochemical Effects. Int. J. Agric. Biol. 2005, 7, 417–419. [Google Scholar]
- Manschadi, A.M.; Hammer, G.L.; Christopher, J.T.; DeVoil, P. Genotypic variation in seedling root architectural traits and implications for drought adaptation in wheat (Triticum aestivum L.). Plant Soil 2008, 303, 115–129. [Google Scholar] [CrossRef]
- Finch-Savage, W.E.; Bassel, G.W. Seed vigour and crop establishment: Extending performance beyond adaptation. J. Exp. Bot. 2016, 67, 567–591. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zemljič, A. Agricultural, I. of S. Izbor in Opis Sort Ozimnih Žit Za Setev v Letu 2015/16. Available online: http://www.kgzs-ms.si/wp-content/uploads/2015/09/OPIS-SORT-OZ-ŽIT-2015.pdf (accessed on 23 February 2021).
- Denčič, S.; Kobiljski, B.; Jestrocić, Z.; Orbović, B.; Pavlović, M. Efekat ruskih sorti Bezostaya 1 i Mirmovska 808 na selekciju pšenice u Institutu za ratarstvo i povrtarstvo u Novom Sadu. Sel. Semen. 2009, 15, 39–45. [Google Scholar]
- Zahoranová, A.; Henselová, M.; Hudecová, D.; Kaliňáková, B.; Kováčik, D.; Medvecká, V.; Černák, M. Effect of Cold Atmospheric Pressure Plasma on the Wheat Seedlings Vigor and on the Inactivation of Microorganisms on the Seeds Surface. Plasma Chem. Plasma Process. 2015, 36, 397–414. [Google Scholar] [CrossRef]
- Keser, M.; Gummadov, N.; Akin, B.; Belen, S.; Mert, Z.; Taner, S.; Topal, A.; Yazar, S.; Morgounov, A.; Sharma, R.C.; et al. Genetic gains in wheat in Turkey: Winter wheat for dryland conditions. Crop. J. 2017, 5, 533–540. [Google Scholar] [CrossRef]
- Postic, F.; Beauchêne, K.; Gouache, D.; Doussan, C. Scanner-Based Minirhizotrons Help to Highlight Relations between Deep Roots and Yield in Various Wheat Cultivars under Combined Water and Nitrogen Deficit Conditions. Agronomy 2019, 9, 297. [Google Scholar] [CrossRef] [Green Version]
- Salem, K.F.M.; Salem, K.; El-Zanaty, A.; Esmail, R. Assessing Wheat (Triticum aestivum L.) Genetic Diversity Using Morphological Characters and Microsatallite Markers Maize Breeding and Biotechnological View project Genetic engineering View project Assessing Wheat (Triticum aestivum L.) Genetic Diversity Using Morphological Characters and Microsatallite Markers. World J. Agric. Sci. 2008, 4, 538–544. [Google Scholar]
- Junior, C.A.; Vitoriano, J.D.O.; Da Silva, D.L.S.; Farias, M.D.L.; Dantas, N.B.D.L. Water uptake mechanism and germination of Erythrina velutina seeds treated with atmospheric plasma. Sci. Rep. 2016, 6, srep33722. [Google Scholar] [CrossRef]
- Filatova, I.; Azharonok, V.; Shik, A.; Antoniuk, A.; Terletskaya, N. Fungicidal effects of plasma and Radio-Wave pre-treatments on seeds of grain crops and legumes. In Plasma for Bio-Decontamination, Medicine and Food Security; Springer: Dordrecht, The Netherlands, 2012; pp. 469–479. [Google Scholar]
- Belderok, B.; Mesdag, J.; Donner, D.A. Bread-Making Quality of Wheat: A Century of Breeding in Europe, 1st ed.; Belderok, B., Mesdag, J., Eds.; Springer Science and Business Media LLC: Berlin, Germany, 2000. [Google Scholar]

| Source of Variation | Degree of Freedom | Rn | Ri | Rt | Pl | Fw | R/S | SV |
|---|---|---|---|---|---|---|---|---|
| Variety (V) | 1 | 13.18 *** | 2.47 ns | 16.20 *** | 1.30 ns | 44.25 *** | 0.25 ns | 98.36 *** |
| Treatment (T) | 5 | 2.21 ns | 6.41 *** | 4.73 *** | 4.35 *** | 4.59 ** | 0.87 ns | 3.40 * |
| V × T | 5 | 3.24 ** | 19.92 *** | 5.78 *** | 2.18 ns | 2.32 ns | 0.09 ns | 5.11 ** |
| Residuals | 22 | 1.42035 | 1086.79 | 13,707.6 | 1030.5 | 249.69 | 0.02 | 163.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starič, P.; Grobelnik Mlakar, S.; Junkar, I. Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma. Plants 2021, 10, 1728. https://doi.org/10.3390/plants10081728
Starič P, Grobelnik Mlakar S, Junkar I. Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma. Plants. 2021; 10(8):1728. https://doi.org/10.3390/plants10081728
Chicago/Turabian StyleStarič, Pia, Silva Grobelnik Mlakar, and Ita Junkar. 2021. "Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma" Plants 10, no. 8: 1728. https://doi.org/10.3390/plants10081728
APA StyleStarič, P., Grobelnik Mlakar, S., & Junkar, I. (2021). Response of Two Different Wheat Varieties to Glow and Afterglow Oxygen Plasma. Plants, 10(8), 1728. https://doi.org/10.3390/plants10081728







