Influence of Hydrocarbon-Oxidizing Bacteria on the Growth, Biochemical Characteristics, and Hormonal Status of Barley Plants and the Content of Petroleum Hydrocarbons in the Soil
Abstract
1. Introduction
2. Results
2.1. The Growth of Plants under the Influence of Oil and Bacteria
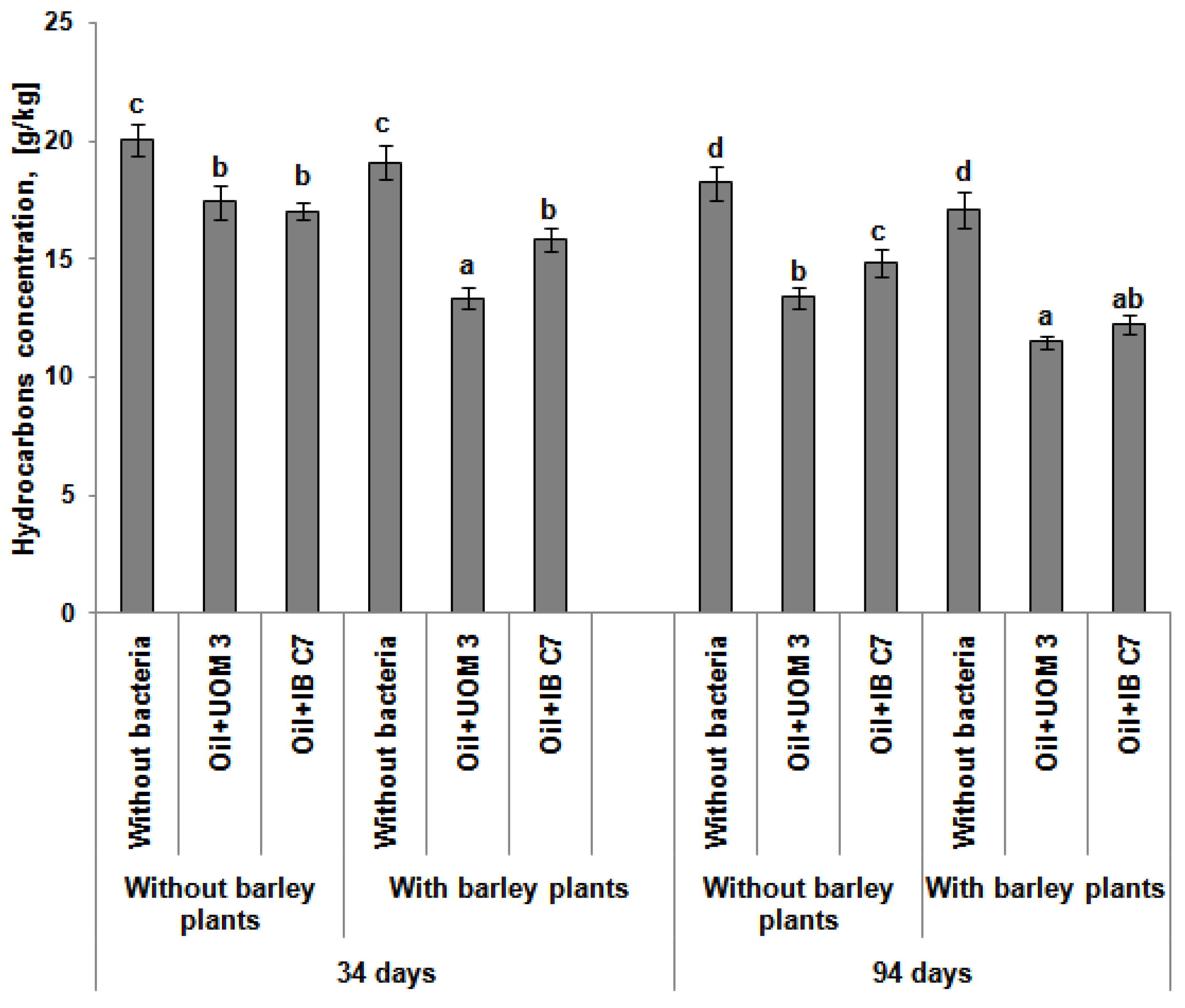
2.2. The Number of Microorganisms and the Content of Hydrocarbons in the Soil
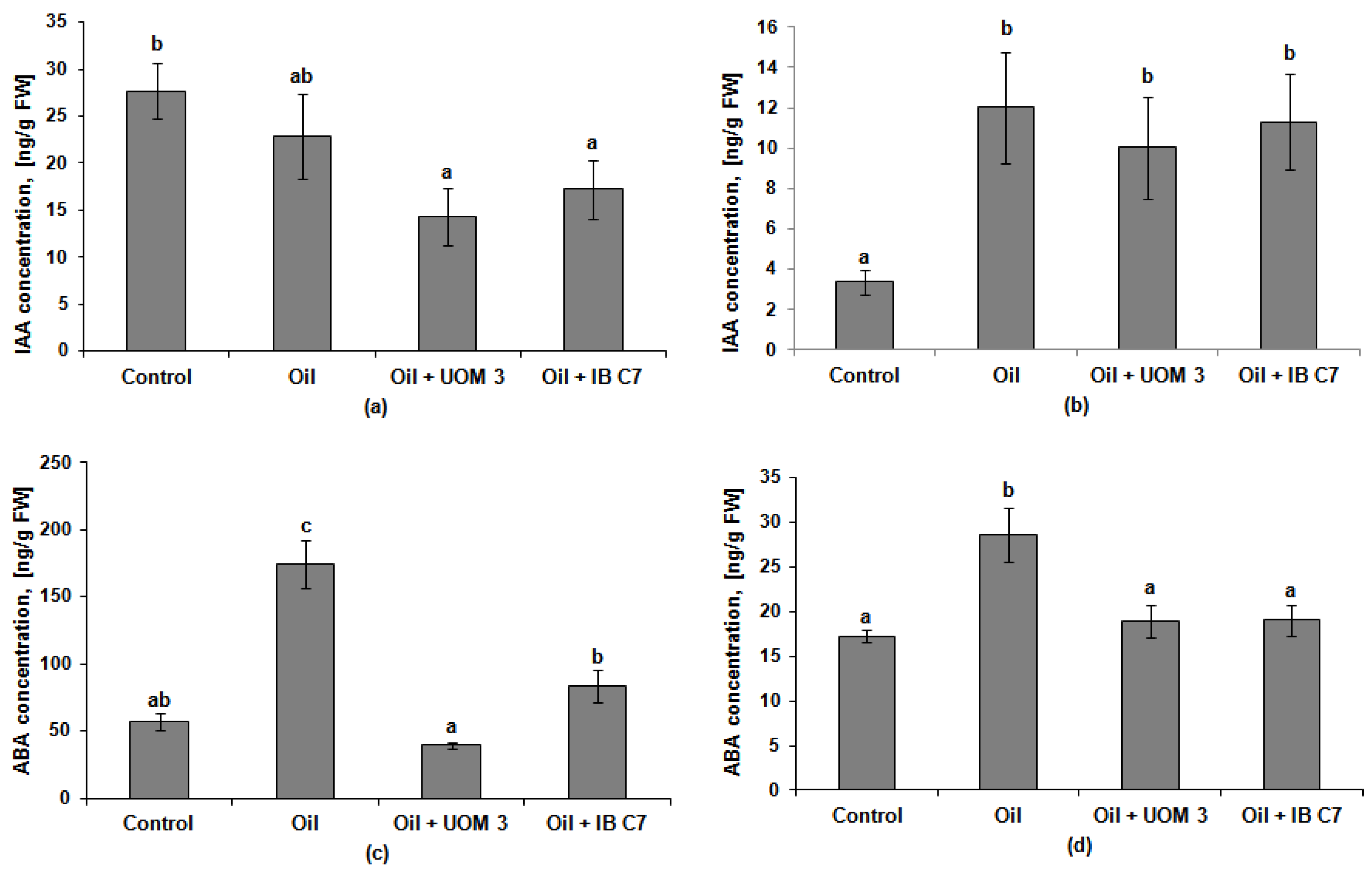
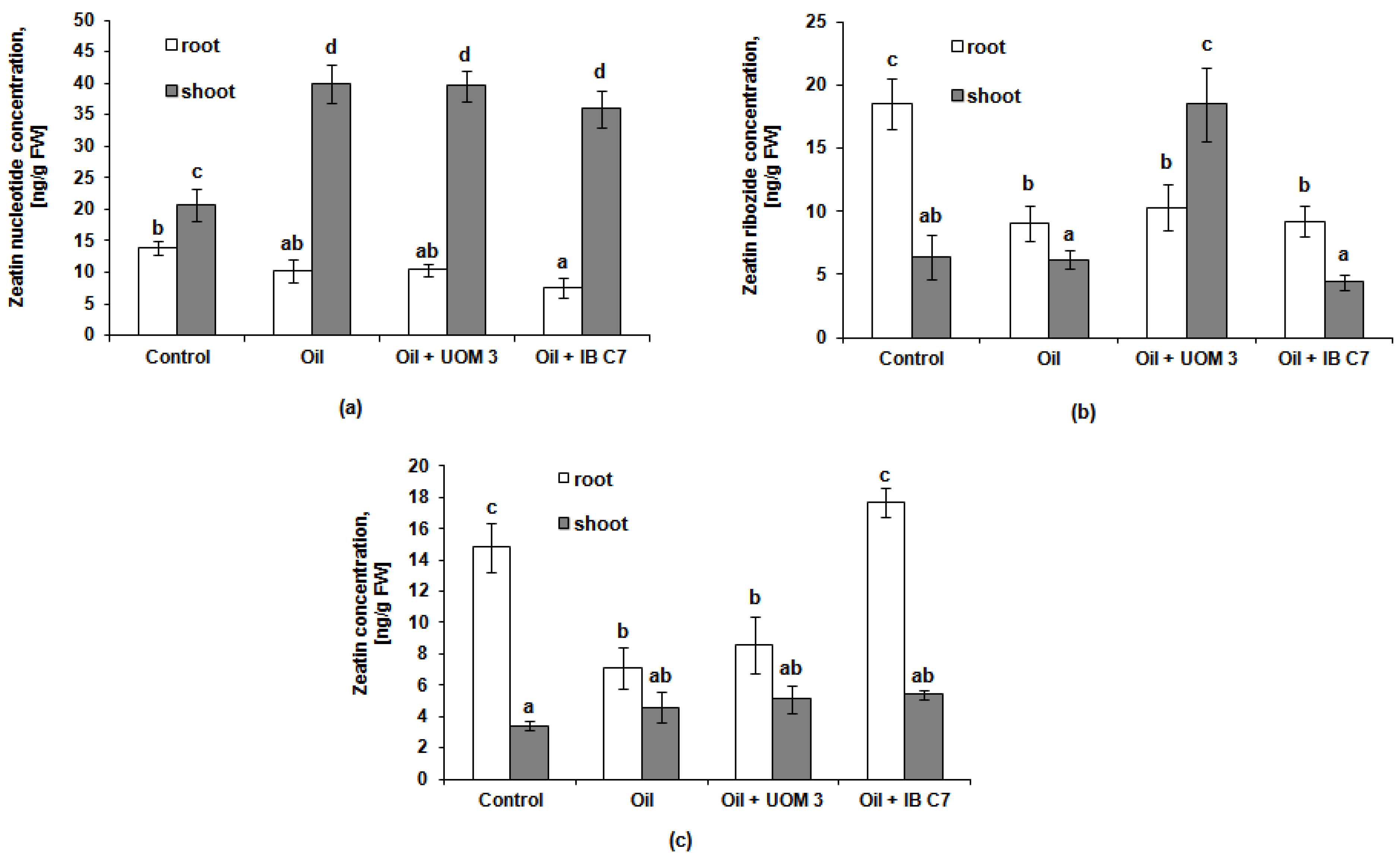
2.3. The Effect of Oil and Bacteria on the Content of Hormones in Plants
2.4. The Effect of Oil and Bacteria on the Synthesis of Plant Pigments and Proline
3. Discussion
4. Materials and Methods
4.1. Plant Growth Conditions and Treatments
- Clean soil + barley plants without bacterial treatment (control);
- Oil-contaminated soil;
- Oil-contaminated soil + barley plants without bacterial treatment;
- Oil-contaminated soil + Enterobacter sp. UOM 3;
- Oil-contaminated soil + Enterobacter sp. UOM 3 + barley plants;
- Oil-contaminated soil + P. hunanensis IB C7;
- Oil-contaminated soil + P. hunanensis IB C7 + barley plants.
4.2. Analysis of the Content of Pigments and Proline
4.3. Cultivation of the Microorganisms and Analysis of their Number
4.4. Analysis of the Content of Hydrocarbons in the Soil
4.5. Hormone Measurement
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wolińska, A.; Kuźniar, A.; Szafranek-Nakonieczna, A.; Jastrzębska, N.; Roguska, E.; Stępniewska, Z. Biological activity of autochthonic bacterial community in oil-contaminated soil. Water Air Soil Pollut. 2016, 227, 130. [Google Scholar] [CrossRef]
- Polyak, Y.M.; Bakina, L.G.; Chugunova, M.V.; Mayachkina, N.V.; Gerasimov, A.O.; Bure, V.M. Effect of remediation strategies on biological activity of oil-contaminated soil—A field study. Int. Biodeterior. Biodegrad. 2018, 126, 57–68. [Google Scholar] [CrossRef]
- Bungau, S.; Behl, T.; Aleya, L.; Bourgeade, P.; Aloui-Sossé, B.; Purza, A.L.; Abid, A.; Samuel, A.D. Expatiating the impact of anthropogenic aspects and climatic factors on long term soil monitoring and management. Environ. Sci. Pollut. Res. 2021, 28, 30528–30550. [Google Scholar] [CrossRef] [PubMed]
- Koshlaf, E.; Ball, A.S. Soil bioremediation approaches for petroleum hydrocarbon polluted environments. AIMS Microbiol. 2017, 3, 25–49. [Google Scholar] [CrossRef] [PubMed]
- Rohrbacher, F.; St-Arnaud, M. Root exudation: The ecological driver of hydrocarbon rhizoremediation. Agronomy 2016, 6, 19. [Google Scholar] [CrossRef]
- Chetverikov, S.; Vysotskaya, L.; Kuzina, E.; Arkhipova, T.; Bakaeva, M.; Rafikova, G.; Korshunova, T.; Chetverikova, D.; Hkudaygulov, G.; Kudoyarova, G. Effects of association of barley plants with hydrocarbon-degrading bacteria on the content of soluble organic compounds in clean and oil-contaminated sand. Plants 2021, 10, 975. [Google Scholar] [CrossRef] [PubMed]
- Muratova, A.; Dubrovskaya, E.; Golubev, S.; Grinev, V.; Chernyshova, M.; Turkovskaya, O. The coupling of the plant and microbial catabolisms of phenanthrene in the rhizosphere of Medicago sativa. J. Plant Physiol. 2015, 188, 1–8. [Google Scholar] [CrossRef]
- Gkorezis, P.; Daghio, M.; Franzetti, A.; Van Hamme, J.D.; Sillen, W.; Vangronsveld, J. The interaction between plants and bacteria in the remediation of petroleum hydrocarbons: An environmental perspective. Front. Microbiol. 2016, 7, 1836. [Google Scholar] [CrossRef]
- Korshunova, T.Yu.; Chetverikov, S.P.; Bakaeva, M.D.; Kuzina, E.V.; Rafikova, G.F.; Chetverikova, D.V.; Loginov, O.N. Microorganisms in the elimination of oil pollution consequences (review). Appl. Biochem. Microbiol. 2019, 55, 344–354. [Google Scholar] [CrossRef]
- Viesser, J.A.; Sugai-Guerios, M.H.; Malucelli, L.C.; Pincerati, M.R.; Karp, S.G.; Maranho, L.T. Petroleum-tolerant rhizospheric bacteria: Isolation, characterization and bioremediation potential. Sci. Rep. 2020, 10, 2060. [Google Scholar] [CrossRef]
- Kim, J.I.; Baek, D.; Park, H.C.; Chun, H.J.; Oh, D.H.; Lee, M.K.; Cha, J.Y.; Kim, W.Y.; Kim, M.C.; Chung, W.S.; et al. Overexpression of Arabidopsis YUCCA6 in potato results in high-auxin developmental phenotypes and enhanced resistance to water deficit. Mol. Plant 2013, 6, 337–349. [Google Scholar] [CrossRef]
- Habib, S.H.; Kausar, H.; Halimi, M.S. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res. Int. 2016, 1–4, 1–10. [Google Scholar] [CrossRef]
- Arkhipova, T.; Martynenko, E.; Sharipova, G.; Kuzmina, L.; Ivanov, I.; Garipova, M.; Kudoyarova, G. Effects of plant growth promoting rhizobacteria on the content of abscisic acid and salt resistance of wheat plants. Plants 2020, 9, 1429. [Google Scholar] [CrossRef]
- Bakaeva, M.; Kuzina, E.; Vysotskaya, L.; Kudoyarova, G.; Arkhipova, T.; Rafikova, G.; Chetverikov, S.; Korshunova, T.; Chetverikova, D.; Loginov, O. Capacity of Pseudomonas strains to degrade hydrocarbons, produce auxins and maintain plant growth under normal conditions and in the presence of petroleum contaminants. Plants 2020, 9, 379. [Google Scholar] [CrossRef] [PubMed]
- Vysotskaya, L.B.; Kudoyarova, G.R.; Arkhipova, T.N.; Kuzina, E.V.; Rafikova, G.F.; Akhtyamova, Z.A.; Ivanov, R.S.; Chetverikov, S.P.; Chetverikova, D.V.; Bakaeva, M.D.; et al. The influence of the association of barley plants with petroleum degrading bacteria on the hormone content, growth and photosynthesis of barley plants grown in the oil-contaminated soil. Acta Physiol. Plant. 2021, 43, 67. [Google Scholar] [CrossRef]
- Li, Q.; Lu, Y.; Shi, Y.; Wang, T.; Ni, K.; Xu, L.; Liu, S.; Wang, L.; Xiong, Q.; Giesy, J.P. Combined effects of cadmium and fluoranthene on germination, growth and photosynthesis of soybean seedlings. J. Environ. Sci. 2013, 25, 1936–1946. [Google Scholar] [CrossRef]
- Han, G.; Cui, B.X.; Zhang, X.X.; Li, K.R. The effects of petroleum-contaminated soil on photosynthesis of Amorpha fruticosa seedlings. Int. J. Environ. Sci. Technol. 2016, 13, 2383–2392. [Google Scholar] [CrossRef]
- Ali, W.A.-A. Biodegradation and phytotoxicity of crude oil hydrocarbons in an agricultural soil. Chil. J. Agric. Res. 2019, 79, 266–277. [Google Scholar]
- Basumatary, B.; Bordoloi, S.; Sarma, H.P. Crude oil-contaminated soil phytoremediation by using Cyperus brevifolius (Rottb.) Hassk. Water Air Soil Pollut. 2012, 223, 3373–3383. [Google Scholar] [CrossRef]
- Skrypnik, L.; Maslennikov, P.; Novikova, A.; Kozhikin, M. Effect of crude oil on growth, oxidative stress and response of antioxidative system of two rye (Secale cereale L.) varieties. Plants 2021, 10, 157. [Google Scholar] [CrossRef] [PubMed]
- Macoustra, G.K.; King, C.K.; Wasley, J.; Robinson, S.A.; Jolley, D.F. Impact of hydrocarbons from a diesel fuel on the germination and early growth of subantarctic plants. Environ. Sci. Process. Impacts 2015, 17, 1238–1248. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panchenko, L.; Muratova, A.; Turkovskaya, O. Comparison of the phytoremediation potentials of Medicago falcata L. and Medicago sativa L. in aged oil-sludge-contaminated soil. Environ. Sci. Pollut. Res. 2017, 24, 3117–3130. [Google Scholar] [CrossRef]
- Devatha, C.P.; Vishnu Vishal, A.; Purna Chandra Rao, J. Investigation of physical and chemical characteristics on soil due to crude oil contamination and its remediation. Appl. Water Sci. 2019, 9, 89. [Google Scholar] [CrossRef]
- Gilyazov, M.; Osipova, R.; Ravzutdinov, A.; Kuzhamberdieva, S. Yield and chemical composition of spring wheat harvest on oil-contaminated grey forest soil. In International scientific and practical conference AgroSMART—Smart solutions for agriculture. KnE Life Sci. 2019, 338–346. [Google Scholar] [CrossRef]
- Agnello, A.C.; Bagard, M.; van Hullebusch, E.D.; Espositob, G.; Huguenot, D. Comparative bioremediation of heavy metals and petroleum hydrocarbons co-contaminated soil by natural attenuation, phytoremediation, bioaugmentation and bioaugmentation-assisted phytoremediation. Sci. Total Environ. 2016, 563–564, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Spaepen, S.; Vanderleyden, J. Auxin and plant-microbe interactions. Cold Spring Harb. Perspect. Biol. 2011, 3, a0014383. [Google Scholar] [CrossRef]
- Shi, T.-Q.; Peng, H.; Zeng, S.-Y.; Ji, R.-Y.; Shi, K.; Huang, H.; Ji, X.-J. Microbial production of plant hormones: Opportunities and challenges. Bioengineered 2017, 8, 124–128. [Google Scholar] [CrossRef] [PubMed]
- Kudoyarova, G.; Arkhipova, T.; Korshunova, T.; Bakaeva, M.; Loginov, O.; Dodd, I.C. Phytohormone mediation of interactions between plants and non-symbiotic growth promoting bacteria under edaphic stresses. Front. Plant Sci. 2019, 10, 1368. [Google Scholar] [CrossRef] [PubMed]
- Chetverikov, S.P.; Bakaeva, M.D.; Korshunova, T.Y.; Kuzina, E.V.; Rafikova, G.F.; Chetverikova, D.V.; Vysotskaya, L.B.; Loginov, O.N. New strain Enterobacter sp. UOM 3 ‒ destructor of oil and producer of indole acetic acid. Nat. Tech. Sci. 2019, 7, 37–40. [Google Scholar] [CrossRef]
- Safronova, V.I.; Stepanok, V.V.; Engqvist, G.L.; Alekseyev, Y.V.; Belimov, A.A. Root-associated bacteria containing 1-aminocyclopropane-1-carboxylate deaminase improve growth and nutrient uptake by pea genotypes cultivated in cadmium supplemented soil. Biol. Fertil. Soils 2006, 42, 267–272. [Google Scholar] [CrossRef]
- Cheng, Z.; Park, E.; Glick, B.R. 1-Aminocyclopropane-1-carboxylate deaminase from Pseudomonas putida UW4 facilitates the growth of canola in the presence of salt. Can. J. Microbiol. 2007, 53, 912–918. [Google Scholar] [CrossRef]
- Greenberg, B.M.; Huang, X.-D.; Gurska, Y.; Gerhardt, K.E.; Lampi, M.A.; Khalid, A.; Isherwood, D.; Chang, P.; Wang, W.; Wang, H.; et al. Development and successful field tests of a multi-process phytoremediation system for decontamination of persistent petroleum and organic contaminants in soils. In Reclamation and Remediation: Policy and Practice; Tisch, B., Zimmerman, K., White, P., Beckett, P., Guenther, L., Macleod, A., Rowsome, S., Black, C., Eds.; Canadian Land Reclamation Association (CLRA): Calgary, AB, Canada, 2006; pp. 124–133. [Google Scholar]
- Huang, X.-D.; El-Alawi, Y.; Gurska, J.; Glick, B.R.; Greenberg, B.M. A multi-process phytoremediation system for decontamination of persistent total petroleum hydrocarbons (TPHs) from soils. Microchem. J. 2005, 81, 139–147. [Google Scholar] [CrossRef]
- Glick, B.R. Modifying a plant’s response to stress by decreasing ethylene production. In Phytoremediation and Rhizoremediation; Mackova, M., Dowling, D., Macek, T, Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 227–236. [Google Scholar]
- Benson, A.; Ram, G.; John, A.; Joe, M.M. Inoculation of 1-aminocyclopropane-1-carboxylate deaminase-producing bacteria along with biosurfactant application enhances the phytoremediation efficiency of Medicago sativa in hydrocarbon-contaminated soils. Bioremediation J. 2017, 21, 20–29. [Google Scholar] [CrossRef]
- Kang, S.M.; Shahzad, R.; Khan, M.A.; Hasnain, Z.; Lee, K.E.; Park, H.S.; Kim, L.R.; Lee, I.J. Ameliorative effect of indole-3-acetic acid- and siderophore-producing Leclercia adecarboxylata MO1 on cucumber plants under zinc stress. J. Plant Interact. 2021, 16, 30–41. [Google Scholar] [CrossRef]
- Vysotskaya, L.; Akhiyarova, G.; Feoktistova, A.; Akhtyamova, Z.; Korobova, A.; Ivanov, I.; Dodd, I.; Kuluev, B.; Kudoyarova, G. Effects of phosphate shortage on root growth and hormone content of barley depend on capacity of the roots to accumulate ABA. Plants 2020, 9, 1722. [Google Scholar] [CrossRef] [PubMed]
- Buer, C.; Kordbacheh, F.; Truong, T.T.; Hocart, C.H.; Djordjevic, M.A. Alteration of flavonoid accumulation patterns in transparent testa mutants disturbs auxin transport, gravity responses, and imparts long-term effects on root and shoot architecture. Planta 2013, 238, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Peer, W.A.; Cheng, Y.; Murphy, A.S. Evidence of oxidative attenuation of auxin signalling. J. Exp. Bot. 2013, 64, 2629–2639. [Google Scholar] [CrossRef]
- Sharma, P.; Jha, A.B.; Dubey, R.S.; Pessarakli, M. Reactive oxygen species, oxidative damage, and antioxidative defense mechanism in plants under stressful conditions. J. Bot. 2012. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Korobova, A.V.; Veselov, S.Y.; Dodd, I.C.; Kudoyarova, G.R. ABA mediation of shoot cytokinin oxidase activity: Assessing its impacts on cytokinin status and biomass allocation of nutrient deprived durum wheat. Funct. Plant Biol. 2009, 36, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Cui, K.; Xu, A.; Nie, L.; Huang, J.; Peng, S. Drought stress condition increases root to shoot ratio via alteration of carbohydrate partitioning and enzymatic activity in rice seedlings. Acta Physiol. Plant. 2015, 37, 9. [Google Scholar] [CrossRef]
- Werner, T.; Motyka, V.; Laucou, V.; Smets, R.; Van Onckelen, H.; Schmulling, T. Cytokinin-deficient transgenic Arabidopsis plants show multiple developmental alterations indicating opposite functions of cytokinins in the regulation of shoot and root meristem activity. Plant Cell 2003, 15, 2532–2550. [Google Scholar] [CrossRef]
- Huang, X.; Hou, L.; Meng, J.; You, H.; Li, Z.; Gong, Z.; Yang, S.; Shi, Y. The antagonistic action of abscisic acid and cytokinin signaling mediates drought stress response in Arabidopsis. Mol. Plant 2018, 11, 970–982. [Google Scholar] [CrossRef]
- He, Y.; Liu, Y.; Li, M.; Lamin-Samu, A.T.; Yang, D.; Yu, X.; Izhar, M.; Jan, I.; Ali, M.; Lu, G. The Arabidopsis SMALL AUXIN UP RNA32 protein regulates ABA-mediated responses to drought stress. Front. Plant Sci. 2021, 12, 625493. [Google Scholar] [CrossRef]
- Atta, A.M.; Mohamed, N.H.; Hegazy, A.K.; Moustafa, Y.M.; Mohamed, R.R.; Safwat, G.; Diab, A.A. Green technology for remediation of water polluted with petroleum crude oil: Using of Eichhornia crassipes (Mart.) Solms combined with magnetic nanoparticles capped with myrrh resources of Saudi Arabia. Nanomaterials 2020, 10, 262. [Google Scholar] [CrossRef] [PubMed]
- Mostafa, A.A.; Hafez, R.M.; Hegazy, A.K.; Fattah, A.M.A.-E.; Mohamed, N.H.; Mustafa, Y.M.; Gobouri, A.A.; Azab, E. Variations of structural and functional traits of Azollapinnata R. Br. in response to crude oil pollution in arid regions. Sustainability 2021, 13, 2142. [Google Scholar] [CrossRef]
- Baruah, P.; Saikia, R.R.; Baruah, P.P.; Deka, S. Effect of crude oil contamination on the chlorophyll content and morpho-anatomy of Cyperus brevifolius (Rottb.) Hassk. Environ. Sci. Pollut. Res. 2014, 21, 12530–12538. [Google Scholar] [CrossRef] [PubMed]
- Tomar, R.S.; Jajoo, A. Fluoranthene, a polycyclic aromatic hydrocarbon, inhibits light as well as dark reactions of photosynthesis in wheat (Triticum aestivum). Ecotoxicol. Environ. Saf. 2014, 109, 110–115. [Google Scholar] [CrossRef]
- Kreslavski, V.D.; Brestic, M.; Zharmukhamedov, S.K.; Lyubimov, V.Y.; Lankin, A.V.; Jajoo, A.; Allakhverdiev, S.I. Mechanisms of inhibitory effects of polycyclic aromatic hydrocarbons in photosynthetic primary processes in pea leaves and thylakoid preparations. Plant Biol. 2017, 19, 683–688. [Google Scholar] [CrossRef]
- Al-Hawas, G.H.S.; Sukry, W.M.; Azzoz, M.M.; Al-Moaik, R.M.S. The effect of sublethal concentrations of crude oil on the metabolism of Jojoba (Simmodsia chinensis) seedlings. Int. Res. J. Plant Sci. 2012, 3, 54–62. [Google Scholar]
- Ng, J.L.P.; Hassan, S.; Truong, T.T.; Hocart, C.H.; Laffont, C.; Frugier, F.; Mathesius, U. Flavonoids and auxin transport inhibitors rescue symbiotic nodulation in the Medicago truncatula cytokinin perception mutant cre1. Plant Cell 2015, 27, 2210–2226. [Google Scholar] [CrossRef]
- Chen, J.; Wu, X.; Yao, X.; Zhu, Z.; Xu, S.; Zha, D. Exogenous 6-benzylaminopurine confers tolerance to low temperature by amelioration of oxidative damage in eggplant (Solanum melongena L.) seedlings. Braz. J. Bot. 2016, 39, 409–416. [Google Scholar] [CrossRef]
- Silva-Navas, J.; Moreno-Risueno, M.A.; Manzano, C.; Téllez-Robledo, B.; Navarro-Neila, S.; Carrasco, V.; Pollmann, S.; Gallego, F.J.; Del Pozo, J.C. Flavonols mediate root phototropism and growth through regulation of proliferation-to-differentiation transition. Plant Cell 2016, 28, 1372–1387. [Google Scholar] [CrossRef]
- Ghaleh, Z.R.; Sarmast, M.K.; Atashi, S. 6-Benzylaminopurine (6-BA) ameliorates drought stress response in tall fescue via the influencing of biochemicals and strigolactone-signaling genes. Plant Physiol. Biochem. 2020, 155, 877–887. [Google Scholar] [CrossRef]
- Nybakken, L.; Lie, N.H.; Julkunen-Titto, R.; Asplund, J.; Ohlson, M. Fertilization changes chemical defense in needles of mature Norway spruce (Picea abies). Front. Plant Sci. 2018, 9, 770. [Google Scholar] [CrossRef]
- Yuan, L.Y.; Yuan, Y.H.; Du, J.; Sun, J.; Guo, S.R. Effects of 24-epibrassinolide on nitrogen metabolism in cucumber seedlings under Ca(NO3)2 stress. Plant Physiol. Biochem. 2012, 61, 29–35. [Google Scholar] [CrossRef]
- Scogings, P.F. Foliar flavonol concentration in Sclerocarya birrea saplings responds to nutrient fertilisation according to growth-differentiation balance hypothesis. Phytochem. Lett. 2018, 23, 180–184. [Google Scholar] [CrossRef]
- Padilla, F.M.; Pena-Fleitas, M.T.; Gallardo, M.; Thompson, R.B. Evaluation of optical sensor measurements of canopy reflectance and of leaf flavonols and chlorophyll contents to assess crop nitrogen status of muskmelon. Eur. J. Agron. 2014, 58, 39–52. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Cerovic, Z.G.; Ghozlen, N.B.; Milhade, C.; Obert, M.; Debuisson, S.; Le Moigne, M. Nondestructive diagnostic test for nitrogen nutrition of grapevine (Vitis vinifera L.) based on dualex leaf-clip measurements in the field. J. Agric. Food Chem. 2015, 63, 3669–3680. [Google Scholar] [CrossRef]
- Gong, Z.; Chen, W.; Bao, G.; Sun, J.; Ding, X.; Fan, C. Physiological response of Secale cereale L. seedlings under freezing-thawing and alkaline salt stress. Environ. Sci. Pollut. Res. 2020, 27, 1499–1507. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; Barczyk, G.; Nadgórska-Socha, A. Antioxidant responses of Triticum aestivum plants to petroleum derived substances. Ecotoxicology 2018, 27, 1353–1367. [Google Scholar] [CrossRef]
- Rusin, M.; Gospodarek, J.; Nadgórska-Socha, A. Soil pollution by petroleum-derived substances and its bioremediation: The effect on Aphis fabae Scop. infestation and antioxidant response in Vicia faba L. Agronomy 2020, 10, 147. [Google Scholar] [CrossRef]
- York, L.M.; Carminati, A.; Mooney, S.J.; Ritz, K.; Bennett, M.J. The holistic rhizosphere: Integrating zones, processes, and semantics in the soil influenced by roots. J. Exp. Bot. 2016, 67, 3629–3643. [Google Scholar] [CrossRef] [PubMed]
- Zaborowska, M.; Kucharski, J.; Wyszkowska, J. Biological activity of soil contaminated with cobalt, tin, and molybdenum. Environ. Monit. Assess. 2016, 188, 398. [Google Scholar] [CrossRef]
- Tomkiel, M.; Baćmaga, M.; Borowik, A.; Wyszkowska, J.; Kucharski, J. The sensitivity of soil enzymes, microorganisms and spring wheat to soil contamination with carfentrazone-ethyl. J. Environ. Sci. Health. Part B 2018, 53, 97–107. [Google Scholar] [CrossRef]
- Xu, J.G.; Johnson, R.L. Nitrogen dynamics in soils with different hydrocarbon contents planted to barley and field pea. Can. J. Soil Sci. 1997, 77, 453–458. [Google Scholar] [CrossRef]
- Vysotskaya, L.B.; Arkhipova, T.N.; Kuzina, E.V.; Rafikova, G.F.; Akhtyamova, Z.A.; Ivanov, R.S.; Timergalina, L.N.; Kudoyarova, G.R. Comparison of responses of different plant species to oil pollution. Biomics 2019, 11, 86–100. [Google Scholar] [CrossRef]
- ImageJ Software. V. 1.48. National Institutes of Health. USA. Available online: http://imagej.nih.gov/ij/http://imagej.nih.gov/ij/ (accessed on 25 July 2021).
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Dzerzhinskaya, I.S. Culture Media for the Isolation and Cultivation of Microorganisms; Astrakhan State Technical University: Astrakhan, Russia, 2008; 348p. [Google Scholar]
- Raymond, R.L. Microbial oxidation of n-paraffinic hydrocarbons. Dev. Ind. Microbiol. 1961, 2, 23–32. [Google Scholar]
- Veselov, S.Y.; Kudoyarova, G.R.; Egutkin, N.L.; Guili-Zade, V.Z.; Mustafina, A.R.; Kof, E.M. Modified solvent partitioning scheme providing increased specificity and rapidity of immunoassay for indole-3-acetic acid. Plant Physiol. 1992, 86, 93–96. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Vysotskaya, L.B.; Arkhipova, T.N.; Kuzmina, L.Y.; Galimsyanova, N.F.; Sidorova, L.V.; Gabbasova, I.M.; Melentiev, A.I.; Veselov, S.Y. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol. Plant. 2017, 39, 253. [Google Scholar] [CrossRef]
- Kudoyarova, G.R.; Melentiev, A.I.; Martynenko, E.V.; Timergalina, L.N.; Arkhipova, T.N.; Shendel, G.V.; Kuz’mina, L.Y.; Dodd, I.C.; Veselov, S.Y. Cytokinin producing bacteria stimulate amino acid deposition by wheat roots. Plant Physiol. Biochem. 2014, 83, 285–291. [Google Scholar] [CrossRef]


| Variants of Experiments | Fresh Mass | Root Mass/ Shoot Mass | |
|---|---|---|---|
| Root | Shoot | ||
| Control | 34.4 ± 2.0 b | 318.4 ± 7.5 c | 0.11 ± 0.012 a |
| Oil | 25.1 ± 1.1 a | 64.9 ± 1.0 a | 0.39 ± 0.080 b |
| Oil + UOM 3 | 30.1 ± 2.1 ab | 80.3 ± 4.3 ab | 0.37 ± 0.058 b |
| Oil + IB C7 | 41.9 ± 0.8 c | 85.9 ± 2.8 b | 0.49 ± 0.043 b |
| Variants of Experiments | Bushiness (pcs) | Shoot Height (cm) | The Number of Ears (pcs) | The Length of the Main Spike (cm) | The Number of Spikelets per Spike (pcs) | Dry Mass of the Shoot (g) |
|---|---|---|---|---|---|---|
| Control | 4.96 ± 0.28 b | 52.24 ± 1.34 c | 2.96 ± 0.26 c | 6.50 ± 0.23 d | 15.82 ± 0.65 c | 0.496 ± 0.022 c |
| Oil | 1.80 ± 0.05 a | 28.00 ± 0.45 a | 1.32 ± 0.05 a | 1.87 ± 0.08 a | 5.38 ± 0.20 a | 0.185 ± 0.009 a |
| Oil + UOM 3 | 1.93 ± 0.06 a | 29.44 ± 0.47 b | 1.43 ± 0.05 a | 2.57 ± 0.07 c | 6.06 ± 0.19 b | 0.213 ± 0.009 b |
| Oil + IB C7 | 1.91 ± 0.06 a | 28.82 ± 0.41 ab | 1.61 ± 0.05 b | 2.25 ± 0.07 b | 5.69 ± 0.20 ab | 0.213 ± 0.010 b |
| Variants of Experiments | Heterotrophic Microorganisms, ×107 | Hydrocarbon-Oxidizing Microorganisms, ×106 | Oligonitrophilic Microorganisms, ×105 | ||||
|---|---|---|---|---|---|---|---|
| 34 Days after Germination | 94 Days after Germination | 34 Days after Germination | 94 Days after Germination | 34 Days after Germination | 94 Days after Germination | ||
| Without plants | Without bacteria | (1.1 ± 0.2) a | (1.2 ± 0.2) a | (1.4 ± 0.3) a | (1.5 ± 0.3) a | (0.3 ± 0.1) a | (2.0 ± 0.1) a |
| UOM 3 | (1.6 ± 0.4) ab | (2.4 ± 0.6) bc | (7.2 ± 1.5) c | (13.8 ± 3.9) c | (2.0 ± 0.3) b | (9.1 ± 1.2) b | |
| IB C7 | (1.6 ± 0.2) ab | (3.0 ± 0.6) bc | (6.7 ± 2.0) c | (14.3 ± 3.0) c | (1.8 ± 0.3) b | (10.6 ± 1.5) b | |
| With plants | Without bacteria | (1.8 ± 0.1) b | (2.1 ± 0.3) b | (2.7 ± 0.2) b | (3.3 ± 0.4) b | (1.9 ± 0.2) b | (8.5 ± 0.6) b |
| UOM 3 | (2.3 ± 0.4) bc | (4.6 ± 0.4) d | (19.3 ± 2.9) d | (25.5 ± 2.9) d | (3.9 ± 0.5) c | (29.9 ± 2.3) d | |
| IB C7 | (3.0 ± 0.3) c | (3.3 ± 0.4) c | (21.9 ± 2.4) d | (29.5 ± 3.8) d | (4.1 ± 0.4) c | (20.8 ± 2.4) c | |
| Variants of Experiments | Chlorophyll (μg/cm2) | Flavonoids (a.u.) | NBI (a.u.) | Proline (μg/g) |
|---|---|---|---|---|
| 10 days after germination | ||||
| Control | 35.1 ± 1.0 e | 0.56 ± 0.02 a | 62.7 ± 2.0 f | 26.4 ± 3.3 a |
| Oil | 16.3 ± 1.0 a | 0.90 ± 0.02 e | 18.1 ± 1.0 a | 79.3 ± 2.8 c |
| Oil + UOM 3 | 18.4 ± 1.0 ab | 0.89 ± 0.02 e | 20.7 ± 1.0 b | 29.5 ± 2.9 a |
| Oil + IB C7 | 18.2 ± 1.0 ab | 0.87 ± 0.02 e | 20.9 ± 1.0 b | 35.5 ± 3.2 a |
| 34 days after germination | ||||
| Control | 32.0 ± 0.6 d | 0.65 ± 0.02 b | 49.2 ± 1.4 e | 52.9 ± 4.5 b |
| Oil | 18.9 ± 0.7 b | 0.74 ± 0.01 c | 24.7 ± 1.0 c | 115.9 ± 4.2 d |
| Oil + UOM 3 | 22.1 ± 0.9 c | 0.81 ± 0.01 d | 27.3 ± 0.8 c | 61.9 ± 2.7 b |
| Oil + IB C7 | 24.1 ± 0.5 c | 0.79 ± 0.01 d | 30.5 ± 0.6 d | 80.7 ± 3.4 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuzina, E.; Rafikova, G.; Vysotskaya, L.; Arkhipova, T.; Bakaeva, M.; Chetverikova, D.; Kudoyarova, G.; Korshunova, T.; Chetverikov, S. Influence of Hydrocarbon-Oxidizing Bacteria on the Growth, Biochemical Characteristics, and Hormonal Status of Barley Plants and the Content of Petroleum Hydrocarbons in the Soil. Plants 2021, 10, 1745. https://doi.org/10.3390/plants10081745
Kuzina E, Rafikova G, Vysotskaya L, Arkhipova T, Bakaeva M, Chetverikova D, Kudoyarova G, Korshunova T, Chetverikov S. Influence of Hydrocarbon-Oxidizing Bacteria on the Growth, Biochemical Characteristics, and Hormonal Status of Barley Plants and the Content of Petroleum Hydrocarbons in the Soil. Plants. 2021; 10(8):1745. https://doi.org/10.3390/plants10081745
Chicago/Turabian StyleKuzina, Elena, Gulnaz Rafikova, Lidiya Vysotskaya, Tatyana Arkhipova, Margarita Bakaeva, Dar’ya Chetverikova, Guzel Kudoyarova, Tatyana Korshunova, and Sergey Chetverikov. 2021. "Influence of Hydrocarbon-Oxidizing Bacteria on the Growth, Biochemical Characteristics, and Hormonal Status of Barley Plants and the Content of Petroleum Hydrocarbons in the Soil" Plants 10, no. 8: 1745. https://doi.org/10.3390/plants10081745
APA StyleKuzina, E., Rafikova, G., Vysotskaya, L., Arkhipova, T., Bakaeva, M., Chetverikova, D., Kudoyarova, G., Korshunova, T., & Chetverikov, S. (2021). Influence of Hydrocarbon-Oxidizing Bacteria on the Growth, Biochemical Characteristics, and Hormonal Status of Barley Plants and the Content of Petroleum Hydrocarbons in the Soil. Plants, 10(8), 1745. https://doi.org/10.3390/plants10081745








