Altering Nitrogen Sources Affects Growth Carbon Costs in Vachellia nilotica Growing in Nutrient-Deficient Grassland Soils
Abstract
:1. Introduction
2. Results
2.1. Soil Characteristics
2.2. Soil Bacteria and Plant Endophytic Bacterial Isolates Identification
2.3. Biomass and Mineral Nutrition
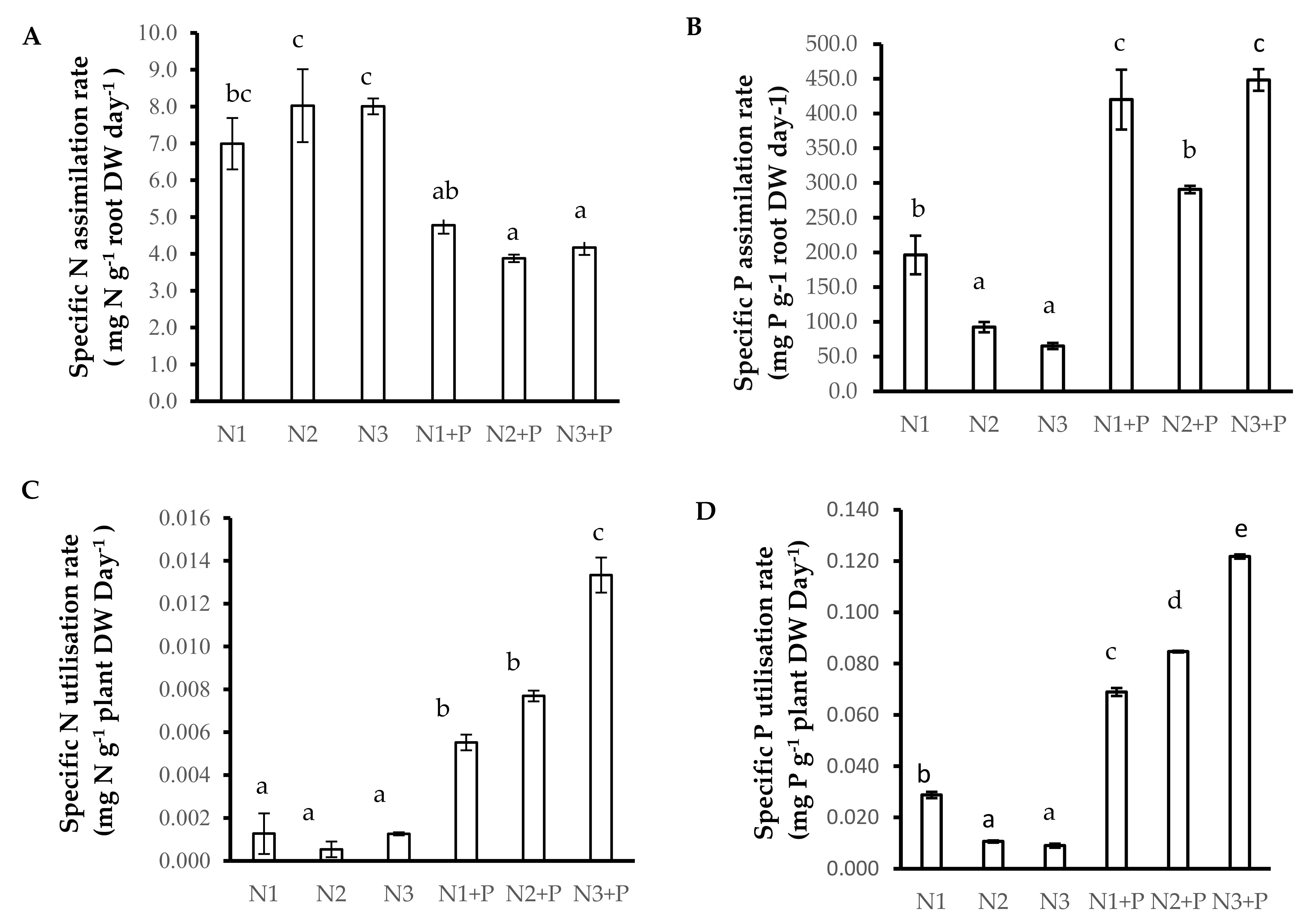
2.4. Growth Kinetics
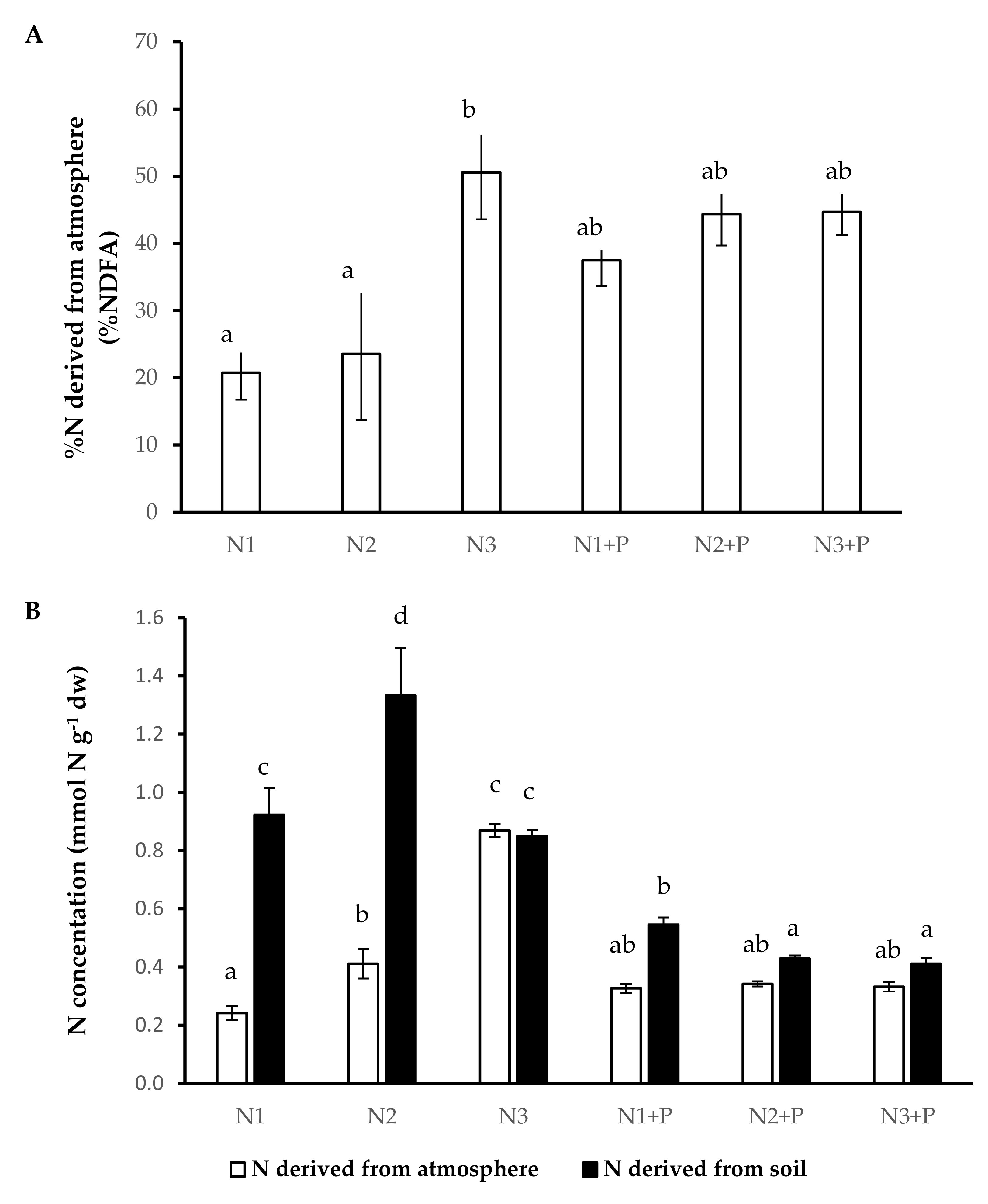
2.5. N and P Nutrition
2.6. N Source Preference
3. Discussion
4. Materials and Methods
4.1. Study Site
4.2. Experimental Design
4.3. Soil Characteristics Analysis and Bacterial Identification
4.4. Seed Germination and Growth Conditions
4.5. Plant Harvesting and Nutrient Analysis
4.6. Bacterial Extraction and Identification
4.7. Growth Calculations
4.7.1. Calculation of N Derived from the Atmosphere
4.7.2. Calculation of the Specific N/P Absorption Rate
4.7.3. Calculation of the Specific N/P Utilization Rate
4.7.4. Relative Growth Rate
4.7.5. Carbon Construction Costs
4.7.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Craine, J.M.; Morrow, C.; Stock, W.D. Nutrient concentration ratios and co-limitation in South African grasslands. New Phytol. 2008, 179, 829–836. [Google Scholar] [CrossRef]
- Smil, V. Global population and the nitrogen cycle. Sci. Am. 1997, 277, 76–81. [Google Scholar] [CrossRef]
- Ohyama, T. Nitrogen as a major essential element of plants. Nitrogen Assim. Plants 2010, 37, 1–17. [Google Scholar]
- Angus, J.; Bolger, T.; Kirkegaard, J.; Peoples, M. Nitrogen mineralisation in relation to previous crops and pastures. Soil Res. 2006, 44, 355–365. [Google Scholar] [CrossRef]
- Kumar, S.; Meena, R.S.; Datta, R.; Verma, S.K.; Yadav, G.S.; Pradhan, G.; Molaei, A.; Rahman, G.M.; Mashuk, H. Legumes for carbon and nitrogen cycling: An organic approach. In Carbon and Nitrogen Cycling in Soil; Springer: Berlin, Germany, 2020; pp. 337–375. [Google Scholar]
- Mullen, R.W. Withcrop the production exception systems. of water, Although nitrogen is higher typically plant the species most limiting require adequate nutrient in nitrogen non-legume fertil-ization to maximize agronomic productivity, excess nitrogen application for crop production can have negative environmental effects when it is transported away from production fields. Pro-duction agriculture strives to strike a balance between adequate nitrogen supplementation and minimizing nitrogen losses in a way that is both agronomically and economically sound. Thus it is important to have a general understanding of nitrogen cycling within the soil. The nitrogen cycle is a dynamic collection of complex processes that are affected by a number of controllable. Soil Manag. Build. Stab. Base Agric. 2020, 154, 67. [Google Scholar]
- Mus, F.; Crook, M.B.; Garcia, K.; Costas, A.G.; Geddes, B.A.; Kouri, E.D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd, G.E.; Poole, P.S. Symbiotic nitrogen fixation and the challenges to its extension to nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maróti, G.; Kondorosi, É. Nitrogen-fixing Rhizobium-legume symbiosis: Are polyploidy and host peptide-governed symbiont differentiation general principles of endosymbiosis? Front. Microbiol. 2014, 5, 326. [Google Scholar] [PubMed] [Green Version]
- Sanginga, N.; Lyasse, O.; Singh, B. Phosphorus use efficiency and nitrogen balance of cowpea breeding lines in a low P soil of the derived savanna zone in West Africa. Plant Soil 2000, 220, 119. [Google Scholar] [CrossRef]
- Berg, G. Plant–microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.-S.P. Phosphorus homeostasis in legume nodules as an adaptive strategy to phosphorus deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef]
- Aerts, R.; Chapin, F.S., III. The mineral nutrition of wild plants revisited: A re-evaluation of processes and patterns. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 1999; Volume 30, pp. 1–67. [Google Scholar]
- Mitran, T.; Mani, P. Effect of organic amendments on rice yield trend, phosphorus use efficiency, uptake, and apparent balance in soil under long-term rice-wheat rotation. J. Plant Nutr. 2017, 40, 1312–1322. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Porder, S.; Houlton, B.Z.; Chadwick, O.A. Terrestrial phosphorus limitation: Mechanisms, implications, and nitrogen–phosphorus interactions. Ecol. Appl. 2010, 20, 5–15. [Google Scholar] [CrossRef] [Green Version]
- Zheng, M.; Li, D.; Lu, X.; Zhu, X.; Zhang, W.; Huang, J.; Fu, S.; Lu, X.; Mo, J. Effects of phosphorus addition with and without nitrogen addition on biological nitrogen fixation in tropical legume and non-legume tree plantations. Biogeochemistry 2016, 131, 65–76. [Google Scholar] [CrossRef]
- Mortimer, P.E.; Le Roux, M.R.; Pérez-Fernández, M.A.; Benedito, V.A.; Kleinert, A.; Xu, J.; Valentine, A.J. The dual symbiosis between arbuscular mycorrhiza and nitrogen fixing bacteria benefits the growth and nutrition of the woody invasive legume Acacia cyclops under nutrient limiting conditions. Plant Soil 2013, 366, 229–241. [Google Scholar] [CrossRef]
- Smith, S.; Read, D. Mycorrhizal Symbiosis Third Edition Introduction. Mycorrhizal Symbiosis; Academic Press: London, UK, 2008; pp. 1–9. [Google Scholar]
- Jia, Y.; Gray, V.M.; Straker, C.J. The influence of Rhizobium and arbuscular mycorrhizal fungi on nitrogen and phosphorus accumulation by Vicia faba. Ann. Bot. 2004, 94, 251–258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sithole, N.; Pérez-Fernández, M.; Magadlela, A. Nutritional status of soils from KwaZulu-Natal modulate symbiotic interactions and plant performance in Vigna unguiculata L.(Walp). Appl. Soil Ecol. 2019, 142, 1–7. [Google Scholar] [CrossRef]
- Minchin, F.R.; Witty, J.F. Respiratory/carbon costs of symbiotic nitrogen fixation in legumes. In Plant Respiration; Springer: Berlin, Germany, 2005; pp. 195–205. [Google Scholar]
- Ryan, M.; Tibbett, M.; Edmonds-Tibbett, T.; Suriyagoda, L.; Lambers, H.; Cawthray, G.; Pang, J. Carbon trading for phosphorus gain: The balance between rhizosphere carboxylates and arbuscular mycorrhizal symbiosis in plant phosphorus acquisition. Plant Cell Environ. 2012, 35, 2170–2180. [Google Scholar] [CrossRef] [PubMed]
- Magadlela, A.; Pérez-Fernández, M.A.; Kleinert, A.; Dreyer, L.L.; Valentine, A.J. Source of inorganic N affects the cost of growth in a legume tree species (Virgilia divaricata) from the Mediterrean-type Fynbos ecosystem. J. Plant Ecol. 2016, 9, 752–761. [Google Scholar] [CrossRef] [Green Version]
- Elmerich, C. One Hundred Years Discovery of Nitrogen-Fixing Rhizobacteria. Biol. Nitr. Fixat. 2015, 2015, 897–912. [Google Scholar]
- Khan, A.A.; Jilani, G.; Akhtar, M.S.; Naqvi, S.M.S.; Rasheed, M. Phosphorus solubilizing bacteria: Occurrence, mechanisms and their role in crop production. J. Agric. Biol. Sci. 2009, 1, 48–58. [Google Scholar]
- Mehta, P.; Walia, A.; Kulshrestha, S.; Chauhan, A.; Shirkot, C.K. Efficiency of plant growth-promoting P-solubilizing Bacillus circulans CB7 for enhancement of tomato growth under net house conditions. J. Basic Microbiol. 2015, 55, 33–44. [Google Scholar] [CrossRef]
- Ali, A.; Akhtar, N.; Khan, B.A.; Khan, M.S.; Rasul, A.; Khalid, N.; Waseem, K.; Mahmood, T.; Ali, L. Acacia nilotica: A plant of multipurpose medicinal uses. J. Med. Plants Res. 2012, 6, 1492–1496. [Google Scholar]
- Amadou, I.; Soulé, M.; Salé, A. An Overview on the Importance of Acacia nilotica (L.) Willd. Ex Del.: A Review. Asian J. Res. Agric. For. 2020, 5, 12–18. [Google Scholar] [CrossRef] [Green Version]
- Brockwell, J.; Searle, S.D.; Jeavons, A.C.; Waayers, M. Nitrogen Fixation in Acacias: An Untapped Resource for Sustainable Plantations, Farm Forestry and Land Reclamation. 2005. Available online: https://ageconsearch.umn.edu/record/114065/files/115.pdf (accessed on 2 April 2014).
- Ferreira, T.C.; Aguilar, J.V.; Souza, L.A.; Justino, G.C.; Aguiar, L.F.; Camargos, L.S. pH effects on nodulation and biological nitrogen fixation in Calopogonium mucunoides. Braz. J. Bot. 2016, 39, 1015–1020. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.; Williams, M.; Sistani, K. N 2 fixation by soybean-Bradyrhizobium combinations under acidity, low P and high Al stresses. Plant Soil 1991, 131, 293–300. [Google Scholar] [CrossRef]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef] [PubMed]
- Boonkerd, N. Symbiotic association between Frankia and actinorhizal plants. In Nitrogen Fixation with Non-Legumes; Springer: Berlin, Germany, 1998; pp. 327–331. [Google Scholar]
- AbdElgawad, H.; Abuelsoud, W.; Madany, M.M.; Selim, S.; Zinta, G.; Mousa, A.S.; Hozzein, W.N. Actinomycetes Enrich Soil Rhizosphere and Improve Seed Quality as well as Productivity of Legumes by Boosting Nitrogen Availability and Metabolism. Biomolecules 2020, 10, 1675. [Google Scholar] [CrossRef] [PubMed]
- Chaudhary, M.I.; Adu-Gyamfi, J.J.; Saneoka, H.; Nguyen, N.T.; Suwa, R.; Kanai, S.; El-Shemy, H.A.; Lightfoot, D.A.; Fujita, K. The effect of phosphorus deficiency on nutrient uptake, nitrogen fixation and photosynthetic rate in mashbean, mungbean and soybean. Acta Physiol. Plant. 2008, 30, 537–544. [Google Scholar] [CrossRef]
- Thuynsma, R.; Valentine, A.; Kleinert, A. Phosphorus deficiency affects the allocation of below-ground resources to combined cluster roots and nodules in Lupinus albus. J. Plant Physiol. 2014, 171, 285–291. [Google Scholar] [CrossRef] [PubMed]
- Jeffries, P.; Gianinazzi, S.; Perotto, S.; Turnau, K.; Barea, J.-M. The contribution of arbuscular mycorrhizal fungi in sustainable maintenance of plant health and soil fertility. Biol. Fertil. Soils 2003, 37, 1–16. [Google Scholar] [CrossRef]
- Patra, A.; Abbadie, L.; Clays-Josserand, A.; Degrange, V.; Grayston, S.; Loiseau, P.; Louault, F.; Mahmood, S.; Nazaret, S.; Philippot, L. Effects of grazing on microbial functional groups involved in soil N dynamics. Ecol. Monogr. 2005, 75, 65–80. [Google Scholar] [CrossRef] [Green Version]
- Widdig, M.; Schleuss, P.M.; Weig, A.R.; Guhr, A.; Biederman, L.A.; Borer, E.T.; Crawley, M.J.; Kirkman, K.P.; Seabloom, E.; Wragg, P. Nitrogen and phosphorus additions alter the abundance of phosphorus-solubilizing bacteria and phosphatase activity in grassland soils. Front. Environ. Sci. 2019, 7, 185. [Google Scholar] [CrossRef] [Green Version]
- Ward, D.; Kirkman, K.; Tsvuura, Z. An African grassland responds similarly to long-term fertilization to the Park Grass experiment. PLoS ONE 2017, 12, e0177208. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.; Kirkman, K.P.; Tsvuura, Z.; Morris, C.; Fynn, R.W. Are there common assembly rules for different grasslands? Comparisons of long-term data from a subtropical grassland with temperate grasslands. J. Vegetat. Sci. 2020, 31, 780–791. [Google Scholar] [CrossRef]
- Bilskie, J.; Campbell Scientific, Inc. Soil Water Status: Content and Potential; Campbell Scientific, Inc.; App Note: 2S-1; 2001. Available online: https://s.campbellsci.com/documents/us/technical-papers/soilh20c.pdf (accessed on 2 April 2014).
- Aagot, N.; Nybroe, O.; Nielsen, P.; Johnsen, K. An altered pseudomonas diversity is recovered from soil by using nutrient-poorpseudomonas-selective soil extract media. Appl. Environ. Microbiol. 2001, 67, 5233–5239. [Google Scholar] [CrossRef] [Green Version]
- Zungu, N.S.; Egbewale, S.O.; Olaniran, A.O.; Pérez-Fernández, M.; Magadlela, A. Soil nutrition, microbial composition and associated soil enzyme activities in KwaZulu-Natal grasslands and savannah ecosystems soils. Appl. Soil Ecol. 2020, 155, 103663. [Google Scholar] [CrossRef]
- Nielsen, K.L.; Eshel, A.; Lynch, J.P. The effect of phosphorus availability on the carbon economy of contrasting common bean (Phaseolus vulgaris L.) genotypes. J. Exp. Bot. 2001, 52, 329–339. [Google Scholar]
- Ågren, G.I.; Franklin, O. Root: Shoot ratios, optimization and nitrogen productivity. Ann. Bot. 2003, 92, 795–800. [Google Scholar] [CrossRef] [Green Version]
- Mortimer, P.; Archer, E.; Valentine, A. Mycorrhizal C costs and nutritional benefits in developing grapevines. Mycorrhiza 2005, 15, 159–165. [Google Scholar] [CrossRef]
- Peng, S.; Eissenstat, D.M.; Graham, J.H.; Williams, K.; Hodge, N.C. Growth depression in mycorrhizal citrus at high-phosphorus supply (analysis of carbon costs). Plant Physiol. 1993, 101, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.; Percival, F.; Merino, J.; Mooney, H. Estimation of tissue construction cost from heat of combustion and organic nitrogen content. Plant Cell Environ. 1987, 10, 725–734. [Google Scholar]

| Treatment Trials | ||||||
|---|---|---|---|---|---|---|
| Parameter | N1 | N2 | N3 | N1 + P | N2 + P | N3 + P |
| Biomass (g) | ||||||
| Total plant | 0.90 ± 0.11 ab | 0.51 ± 0.03 a | 0.53 ± 0.04 a | 1.50 ± 0.36 ac | 1.88 ± 0.25 bc | 2.38 ± 0.33 c |
| Leaves | 0.36 ± 0.05 b | 0.17 ± 0.01 a | 0.13 ± 0.02 a | 0.49 ± 0.15 bd | 0.70 ± 0.10 cd | 0.87 ± 0.09 c |
| Shoot | 0.28 ± 0.04 ab | 0.14 ± 02 a | 0.19 ± 0.02 a | 0.50 ± 0.10 ac | 0.58 ± 0.10 bc | 0.74 ± 0.18 c |
| Roots | 0.27 ± 0.04 ab | 0.20 ± 0.02 a | 0.21 ± 0.01 ab | 0.51 ± 0.13 bc | 0.60 ± 0.05 c | 0.76 ± 0.10 c |
| Root: shoot ratio | 0.43 ± 0.05 a | 0.66 ± 0.05 a | 0.69 ± 0.06 b | 0.53 ± 0.09 ab | 0.49 ± 0.03 ab | 0.49 ± 0.05 ab |
| Mineral nutrition | ||||||
| Total plant N (mmol N g−1) | 1.16 ± 0.12 a | 1.74 ± 0.21 b | 1.72 ± 0.05 b | 0.87 ± 0.04 a | 0.77 ± 0.02 a | 0.74 ± 0.04 a |
| Standard corrected 15N/14N | 2.88 ± 0.27 c | 2.69 ± 0.62 ac | 0.83 ± 0.23 ab | 1.73 ± 0.37 ac | 1.25 ± 0.34 ab | 1.23 ± 0.17 ab |
| Total plant P (µmol P g−1) | 32.52 ± 4.60 b | 19.98 ± 1.62 ab | 13.92 ± 0.93 a | 75.76 ± 7.76 d | 57.08 ± 1.03 c | 79.16 ± 2.74 d |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sithole, N.; Tsvuura, Z.; Kirkman, K.; Magadlela, A. Altering Nitrogen Sources Affects Growth Carbon Costs in Vachellia nilotica Growing in Nutrient-Deficient Grassland Soils. Plants 2021, 10, 1762. https://doi.org/10.3390/plants10091762
Sithole N, Tsvuura Z, Kirkman K, Magadlela A. Altering Nitrogen Sources Affects Growth Carbon Costs in Vachellia nilotica Growing in Nutrient-Deficient Grassland Soils. Plants. 2021; 10(9):1762. https://doi.org/10.3390/plants10091762
Chicago/Turabian StyleSithole, Nonkululeko, Zivanai Tsvuura, Kevin Kirkman, and Anathi Magadlela. 2021. "Altering Nitrogen Sources Affects Growth Carbon Costs in Vachellia nilotica Growing in Nutrient-Deficient Grassland Soils" Plants 10, no. 9: 1762. https://doi.org/10.3390/plants10091762







