Effect of Isosteviol on Wheat Seed Germination and Seedling Growth under Cadmium Stress
Abstract
:1. Introduction
2. Results
2.1. Cd Stress Effect on Germination as Well as the Growth of Seedlings
2.2. Effects of Isosteviol on Seed Germination and Seedling Growth of Wheat under Cd Stress
2.3. Effects of Isosteviol on Chlorophyll Content in Wheat Leaves under Cd Stress
2.4. Effects of Isosteviol on MDA Content in Wheat Leaves under Cd Stress
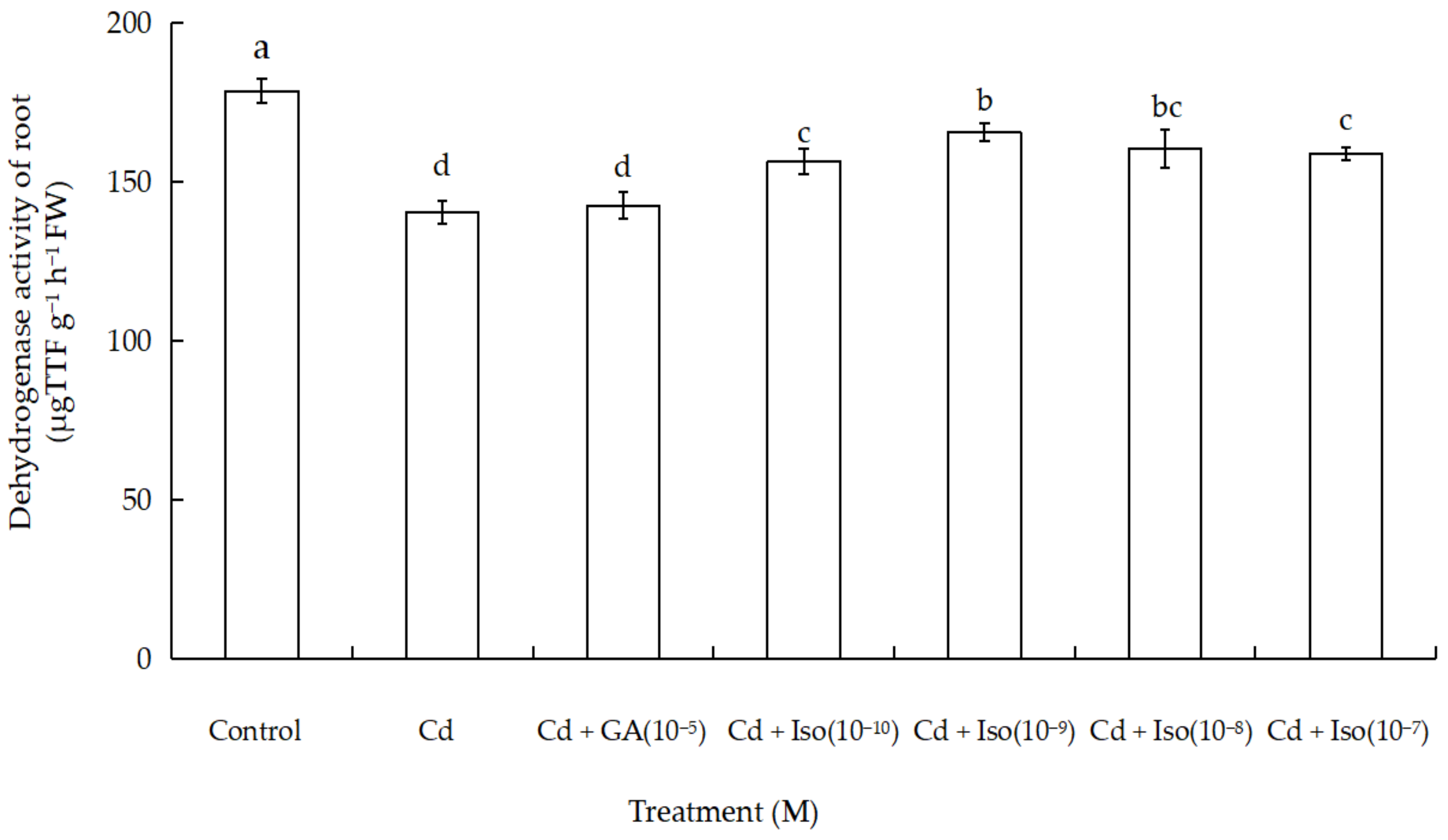
2.5. Effects of Isosteviol on Dehydrogenase Activity of Root in Wheat Seedlings under Cd Stress
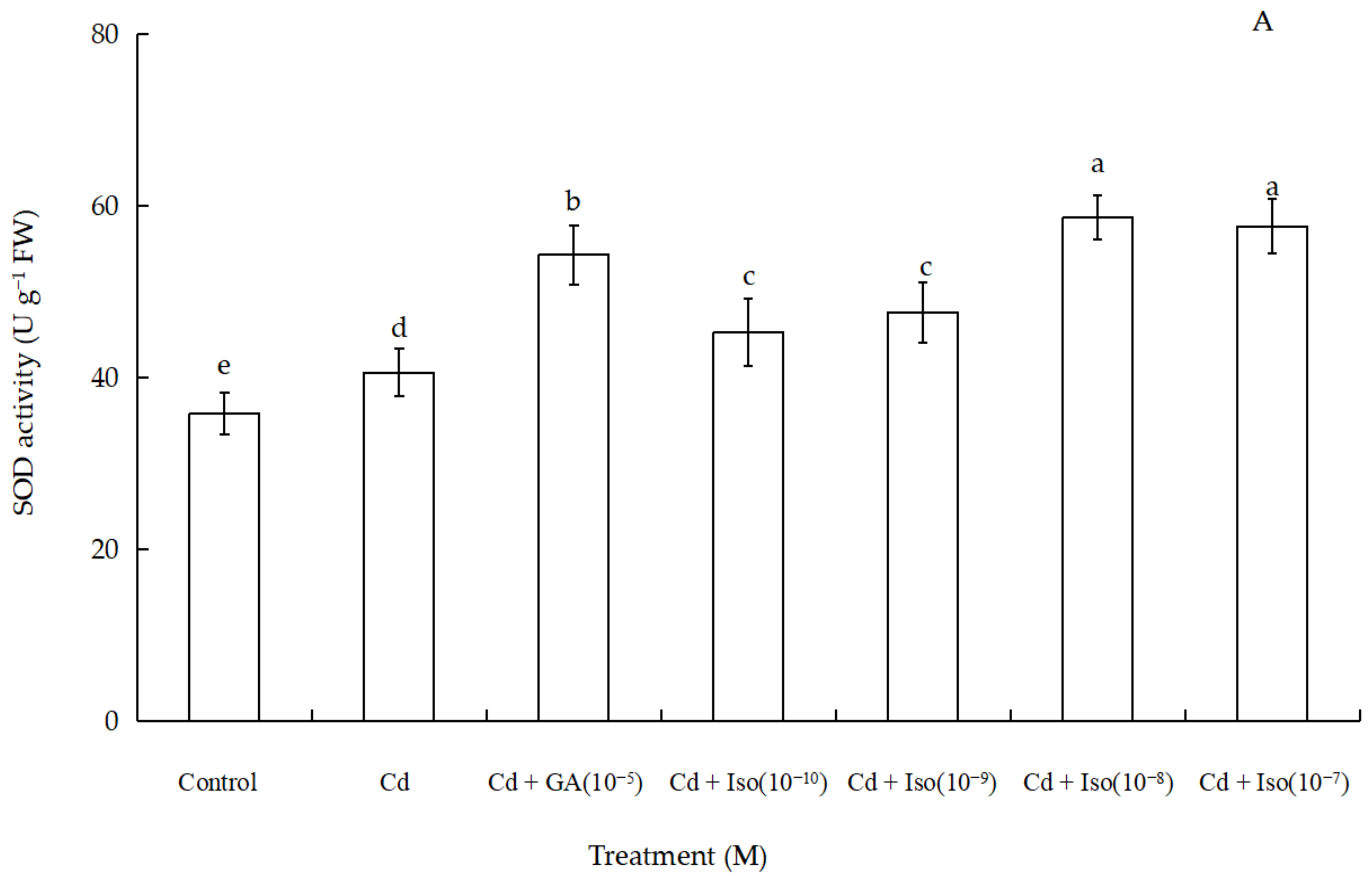
2.6. Effects of Isosteviol on Antioxidant Enzyme Activity of Wheat Seedlings under Cd Stress
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Test Materials
5.2. Experimental Design
5.2.1. Tests for Analyzing the Wheat Germination in the Presence of Cd Stress
- FG—final germination rate of the sample per group harvested;GI = ΣGt/Dt;
- GI—germination index;
- Gt—germinated seed number on day ‘t’;
- Dt—corresponding germination day number;
- t—germination time;VI = GI × S;
- VI—vigour index;
- GI—germination index;
- S—single plant length (cm).
5.2.2. Tests for Determining the Effect of Isosteviol in Wheat Seed Germination in the Presence of Cd Stress
5.3. Sampling and Sample Analysis
5.3.1. Determination of Chlorophyll
- A—absorbance at different wavelengths;
- W—fresh weight (g);
- V—volume of extract solution (mL).
5.3.2. MDA Contents
- C—MDA concentration in µM;
- A—absorbance at different wavelengths.
5.3.3. Dehydrogenase Activity of Root
5.3.4. Antioxidant Enzyme Activity
5.4. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
Appendix A
| Treatment (M) | MDA Concentration (nmol g−1 FW) | Dehydrogenase Activity of Root (μg TTF g−1 h−1 FW) | SOD Activity (U g−1 FW) | POD Activity (U min−1 g−1 FW) | CAT Activity (U min−1 g−1 FW) |
|---|---|---|---|---|---|
| Control | 5.8 ± 0.9 d | 178.6 ± 3.9 a | 35.8 ± 2.4 e | 130.5 ± 4.6 f | 23.1 ± 0.9 a |
| Cd | 18.9 ± 0.7 a | 140.3 ± 3.7 d | 40.6 ± 2.8 d | 150.5 ± 7.8 e | 14.6 ± 2.1 de |
| Cd + GA (10−5) | 15.7 ± 1.2 c | 142.6 ± 4.2 d | 54.3 ± 3.4 b | 195.8 ± 6.4 b | 23.6 ± 1.0 a |
| Cd + Iso (10−10) | 18.0 ± 1.1 a | 156.3 ± 4.1 c | 45.2 ± 3.9 c | 180.5 ± 5.9 d | 15.2 ± 1.0 d |
| Cd + Iso (10−9) | 16.4 ± 0.7 b | 165.5 ± 2.7 b | 47.6 ± 3.5 c | 178.6 ± 9.5 d | 22.2 ± 1.5 ab |
| Cd + Iso (10−8) | 15.6 ± 1.0 c | 160.3 ± 6.0 bc | 58.6 ± 2.6 a | 210.3 ± 4.6 a | 21.9 ± 1.2 b |
| Cd + Iso (10−7) | 16.6 ± 1.1 b | 158.7 ± 2.1 c | 57.6 ± 3.2 a | 189.6 ± 7.2 c | 17.6 ± 0.9 c |
References
- Shamsi, I.H.; Wei, K.; Zhang, G.P.; Jilani, G.; Hassan, M.J. Interactive effects of cadmium and aluminum on growth and antioxidative enzymes in soybean. Biol. Plant. 2008, 52, 165–169. [Google Scholar] [CrossRef]
- Tuma, J.; Skalicky, M.; Tumova, L.; Flidr, J. Influence of cadmium dose and form on the yield of oat (Avena sativa L.) and the metal distribution in the plant. J. Elem. 2014, 19, 795–809. [Google Scholar] [CrossRef]
- Zhu, Q.S.; Zhang, J.; Yu, H.J.; Li, L.; Chen, X.; Jiang, M.Y.; Tan, M.P. Maize Cd-tolerant ZmVTE4 encoding γ-tocopherol-methyl-transferase alleviated Cd-toxicity through its product α-tocopherol. Environ. Exp. Bot. 2019, 158, 171–179. [Google Scholar] [CrossRef]
- Bae, J.; Benoit, D.L.; Watson, A.K. Effect of heavy metals on seed germination and seedling growth of common ragweed and roadside ground cover legumes. Environ. Pollut. 2016, 213, 112–118. [Google Scholar] [CrossRef] [PubMed]
- Jafarnejadi, A.R.; Homaee, M.; Sayyad, G.; Bybordi, M. Large scale spatial variability of accumulated cadmium in the wheat farm grains. Soil Sediment Contam. 2011, 20, 98–113. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, Y.; Hu, H.; Xu, Y.; Zhang, H. Comparative proteomic analysis of Cd-responsive proteins in wheat roots. Acta Physiol. Plant. 2011, 33, 349–357. [Google Scholar] [CrossRef]
- Li, C.S.; Wang, P.; Wu, G.L.; Wang, Y.N.; Cheng, Q.; Cai, Y.C.; Zhou, D.H.; Li, C.J.; Zhang, X.Y.; Tan, J.A.; et al. Additive and epistatic QTL on cadmium (Cd) tolerance associated with seed germinating ability in rice. J. Plant Growth Regul. 2020, 3, 1–9. [Google Scholar] [CrossRef]
- Gao, X.; Mohr, R.M.; McLaren, D.L.; Grant, C.A. Grain cadmium and zinc concentrations in wheat as affected by genotypic variation and potassium chloride fertilization. Field Crop. Res. 2011, 122, 95–103. [Google Scholar] [CrossRef]
- Wang, C.Q.; Song, H. Calcium protects Trifoliumrepens L. seedlings against cadmium stress. Plant Cell Rep. 2009, 28, 1341–1349. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Mostofa, M.G.; Nahar, K. Exogenous calcium alleviates cadmium-induced oxidative stress in rice (Oryza sativa L.) seedlings by regulating the antioxidant defense and glyoxalase systems. Braz. J. Bot. 2016, 39, 393–407. [Google Scholar] [CrossRef]
- Li, S.W.; Leng, Y.; Feng, L.; Zeng, X.Y. Involvement of abscisic acid in regulating antioxidative defense systems and IAA-oxidase activity and improving adventitious rooting in mung bean [Vigna radiata (L.) Wilczek] seedlings under cadmium stress. Environ. Sci. Pollut. Res. 2014, 21, 525–537. [Google Scholar] [CrossRef]
- Ergün, N.; Öncel, I. Effects of some heavy metals and heavy metal hormone interactions on wheat (Triticum aestivum L. cv. Gun 91) seedlings. Afr. J. Agric. Res. 2012, 7, 1518–1523. [Google Scholar] [CrossRef]
- Guo, B.; Liang, Y.C.; Zhu, Y.G.; Zhao, F.J. Role of salicylic acid in alleviating oxidative damage in rice roots (Oryza sativa) subjected to cadmium stress. Environ. Pollut. 2007, 147, 743–749. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Fujita, Y.; Noutoshi, Y.; Takahashi, F.; Narusaka, Y.; Yamaguchi-Shinozaki, F.; Shinozaki, K. Crosstalk between abiotic and biotic stress responses: A current view from the points of convergence in the stress signaling networks. Curr. Opin. Plant Biol. 2006, 9, 436–442. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.Q.; Xiao, X.Y.; Guo, Z.H.; Peng, C.; Zeng, P.; Wang, X.Y. Co-application of indole-3-acetic acid/gibberellin and oxalic acid for phytoextraction of cadmium and lead with Sedum alfredii Hance from contaminated soil. Chemosphere 2021, 285, 131420. [Google Scholar] [CrossRef] [PubMed]
- Wasuntarawat, C.; Temcharoen, P.; Toskulkao, C.; Mungkornkarn, P.; Suttajit, M.; Glinsukon, T. Developmental toxicity of steviol, a metabolite of stevioside, in the hamster. Drug Chem. Toxicol. 1998, 21, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Franck, B. Key building blocks of natural product biosynthesis and their significance in chemistry and medicine. Angew. Chem. Int. Ed. 1979, 18, 429–439. [Google Scholar] [CrossRef]
- Mosettig, E.; Nes, W.R. Stevioside. II. The structure of the aglucon. J. Org. Chem. 1955, 20, 884–899. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Nevmerzhitskaya, Y.Y.; Miftakhova, I.G.; Strobykina, A.S.; Mikhailov, A.L.; Strobykina, I.Y.; Mironov, V.F. Diterpenoid steviol derivatives regulate the growth of winter wheat and improve its frost resistance. Dokl. Biol. Sci. 2010, 435, 411–414. [Google Scholar] [CrossRef]
- Timofeeva, O.A.; Nevmerzhitskaya, Y.Y.; Mikhaylov, A.L.; Schaimullina, G.K.; Mironov, V.F. Stevioside prevents oxidative stress in wheat seedlings. Dokl. Biol. Sci. 2015, 465, 293–295. [Google Scholar] [CrossRef]
- Stoyanova, S.; Geuns, J.; Hideg, É.; Wim, V.D.E. The food additives inulin and stevioside counteract oxidative stress. Int. J. Food Sci. Nutr. 2011, 62, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Savchenko, T.; Tikhonov, K. Oxidative stress-induced alteration of plant central metabolism. Life 2021, 11, 304. [Google Scholar] [CrossRef] [PubMed]
- Solanki, R.; Dhankhar, R. Biochemical changes and adaptive strategies of plants under heavy metal stress. Biologia 2011, 66, 195–204. [Google Scholar] [CrossRef]
- Kumari, M.K.; Varaprasad, D.; Narasimham, D.; Paramesh, K.; Chandrasekhar, T. Impacts of cadmium and manganses on in vitro seed germination and seedling growth of horsegram. Indian J. Plant Sci. 2016, 5, 119–125. [Google Scholar]
- Juel, M.A.I.; Chowdhury, Z.U.M.; Ahmed, T. Heavy metal speciation and toxicity characteristics of tannery sludge. Int. Conf. Mech. Eng. 2016, 1754, 343–347. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, C.Q.; Li, S.G.; Li, B.; Li, Q.Q.; Chen, G.D.; Chen, W.L.; Wang, F. Cadmium adsorption, chelation and compartmentalization limit root-to-shoot translocation of cadmium in rice (Oryza sativa L.). Environ. Sci. Pollut. Res. 2017, 24, 11319–11330. [Google Scholar] [CrossRef] [PubMed]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant. Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]
- Kuriakose, S.V.; Prasad, M.N.V. Cadmium stress affects seed germination and seedling growth in Sorghum bicolor (L.) Moench by changing the activities of hydrolyzing enzymes. Plant Growth Regul. 2008, 54, 143–156. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, L.; Zheng, S.; Xie, J.; Bi, Y. Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J. Plant Physiol. 2010, 167, 1130–1136. [Google Scholar] [CrossRef]
- Rizvi, A.; Zaidi, A.; Ameen, F.; Ahmed, B.; Alkahtanic, M.D.F.; Khan, M.S. Heavy metal induced stress on wheat: Phytotoxicity and microbiological management. RSC Adv. 2020, 10, 38379–38403. [Google Scholar] [CrossRef]
- Liu, X.F.; Huang, X.; Xu, H.S. Syntheses and physiological testing of new PGRS. J. Wuhan Univ. 1994, 2, 74–78, 98. [Google Scholar]
- Ghorbanli, M.; Kaveh, S.H.; Sepehr, M.F. Effects of cadmium and gibberellin on growth and photosynthesis of Glycine Max. Photosynthetica 2000, 37, 627–631. [Google Scholar] [CrossRef]
- Meng, H.B.; Hua, S.J.; Shamsi, I.H.; Jilani, G.; Li, Y.L.; Jiang, L.X. Cadmium-induced stress on the seed germination and seedling growth of Brassica napus L., and its alleviation through exogenous plant growth regulators. Plant Growth Regul. 2009, 58, 47–59. [Google Scholar] [CrossRef]
- Nevmerzhitskaya, Y.Y.; Timofeeva, O.A.; Mikhaylov, A.L.; Strobykina, A.S.; Strobykina, I.Y.; Mironov, V.F. Stevioside increases the resistance of winter wheat to low temperatures and heavy metals. Dokl. Biol. Sci. 2013, 452, 287–290. [Google Scholar] [CrossRef] [PubMed]
- Sakuraba, Y.; Yokono, M.; Akimoto, S.; Tanaka, R.; Tanaka, A. Deregulated chlorophyll b synthesis reduces the energy transfer rate between photosynthetic pigments and induces photodamage in Arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 1055–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golan, K.; Rubinowska, K.; Kmieć, K.; Kot, I.; Górska-Drabik, E.; Lagowska, B.; Michałek, W. Impact of scale insect infestation on the content of photosynthetic pigments and chlorophyll fluorescence in two host plant species. Arthropod-Plant Interact. 2015, 9, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Dobroviczká, T.; Pirelová, B.; Mészáros, P.; Blehová, A.; Matuíková, I. Effects of cadmium and arsenic ions on content of photosynthetic pigments in the leaves of Glycine max (L.) Merrill. Pak. J. Bot. 2013, 45, 105–110. [Google Scholar] [CrossRef]
- Farooqa, M.A.; Alia, S.; Hameedb, A.; Bharwanaa, S.A.; Rizwana, M.; Ishaqueb, W.; Faridc, M.; Mahmoodb, K.; Iqbalb, Z. Cadmium stress in cotton seedlings: Physiological, photosynthesis and oxidative damages alleviated by glycinebetaine. S. Afr. J. Bot. 2016, 104, 61–68. [Google Scholar] [CrossRef]
- Filek, M.; Kościelniak, J.; Łabanowska, M.; Bednarska, E.; Bidzińska, E. Selenium-induced protection of photosynthesis activity in rape (brassica napus) seedlings subjected to cadmium stress. fluorescence and epr measurements. Photosynth. Res. 2010, 105, 27–37. [Google Scholar] [CrossRef]
- Wang, J.; Lin, L.; Luo, L.; Liao, M.; Lv, X.; Wang, Z.H.; Liang, D.; Xia, H.; Wang, X.; Lai, Y.S.; et al. The effects of abscisic acid (ABA) addition on cadmium accumulation of two ecotypes of Solanum photeinocarpum. Environ. Monit. Assess. 2016, 188, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Mirzaee, M.; Moieni, A.; Ghanati, F. Effects of drought stress on the lipid peroxidation and antioxidant enzyme activities in two canola (Brassica napus L.) cultivars. J. Agric. Sci. Technol. 2013, 15, 593–602. [Google Scholar] [CrossRef]
- Pishkari, N.; Habibi-Rezaei, M.; Taghavi, F.; Amanlou, M.; Sheibani, N.; Saso, L.; Moosavi-Movahedi, A.A. The correlation between ROS generation and LPO process as the function of methylparaben concentrations during hemoglobin fructation. J. Iran. Chem. Soc. 2020, 17, 1249–1255. [Google Scholar] [CrossRef]
- Goodarzi, M.; Moosavi-Movahedi, A.A.; Habibi-Rezaei, M.; Shourian, M.; Ghourchian, H.; Ahmad, F.; Sheibani, N. Hemoglobin fructation promotes heme degradation through the generation of endogenous reactive oxygen species. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 130, 561–567. [Google Scholar] [CrossRef]
- Metwally, A.; Finkemeier, I.; Georgi, M.; Dietz, K.J. Salicylic acid alleviates the cadmium toxicity in barley seedlings. Plant Physiol. 2003, 132, 272–281. [Google Scholar] [CrossRef] [Green Version]
- Ali, E.; Maodzeka, A.; Hussain, N.; Shamsi, I.H.; Jiang, L. The alleviation of cadmium toxicity in oilseed rape (Brassica napus) by the application of salicylic acid. Plant Growth Regul. 2015, 75, 641–655. [Google Scholar] [CrossRef]
- Moussa, H.R.; El-Gamal, S.M. Role of salicylic acid in regulation of cadmium toxicity in wheat (Triticum aestivum L.). J. Plant Nutr. 2010, 33, 1460–1471. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Pandey, P.; Rajpoot, R.; Rani, A.; Gautam, A.; Dubey, R.S. Exogenous application of calcium and silica alleviates cadmium toxicity by suppressing oxidative damage in rice seedlings. Protoplasma 2015, 252, 959–975. [Google Scholar] [CrossRef]
- Ye, W.L.; Wu, F.; Zhang, G.Y.; Fang, Q.; Lu, H.J.; Hu, H.X. Calcium decreases cadmium concentration in root but facilitates cadmium translocation from root to shoot in rice. J. Plant Growth Regul. 2020, 39, 422–429. [Google Scholar] [CrossRef]
- Yan, M. Seed priming stimulate germination and early seedling growth of Chinese cabbage under drought stress. S. Afr. J. Bot. 2015, 99, 88–92. [Google Scholar] [CrossRef]
- Kumchai, J.; Huang, J.Z.; Lee, C.Y.; Chen, F.C.; Chin, S.W. The induction of antioxidant enzyme activities in cabbage seedlings by heavy metal stress. World Acad. Sci. Eng. Technol. 2013, 73, 424–429. [Google Scholar]
- Adhikari, S.; Ghosh, S.; Azahar, I.; Adhikari, A.; Shaw, A.K.; Konar, S.; Roy, S.; Hossain, Z. Sulfate improves cadmium tolerance by limiting cadmium accumulation, modulation of sulfur metabolism and antioxidant defense system in maize. Environ. Exp. Bot. 2018, 153, 143–162. [Google Scholar] [CrossRef]
- Nowicka, B.; Pluciński, B.; Kuczyńska, P.; Kruk, J. Prenyl lipid antioxidants participate in response to acute stress induced by heavy metals in green microalga Chlamydomonas reinhardtii. Environ. Exp. Bot. 2016, 123, 98–107. [Google Scholar] [CrossRef]
- Kieffffer, P.; Dommes, J.; Hoffffmann, L.; Hausman, J.F.; Renaut, J. Quantitative changes in protein expression of cadmium-exposed poplar plants. Proteomics 2008, 8, 2514–2530. [Google Scholar] [CrossRef] [PubMed]
- Bashri, G.; Prasad, S.M. Exogenous IAA differentially affects growth, oxidative stress and antioxidants system in Cd stressed Trigonella foenum-graecum L. seedlings: Toxicity alleviation by up-regulation of ascorbate-glutathione cycle. Ecotoxicol. Environ. Saf. 2016, 132, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Cochrane, A. Salt and waterlogging stress impacts on seed germination and early seedling growth of selected endemic plant species from Western Australia. Plant Ecol. 2018, 219, 633–647. [Google Scholar] [CrossRef]
- Gerona, M.E.B.; Deocampo, M.P.; Egdane, J.A.; Ismail, A.M.; Dionisio-Sese, M.L. Physiological responses of contrasting rice genotypes to salt stress at reproductive stage. Rice Sci. 2019, 26, 207–219. [Google Scholar] [CrossRef]
- Deng, B.L.; Yang, K.J.; Zhang, Y.F.; Li, Z.T. Can heavy metal pollution defend seed germination against heat stress? Effect of heavy metals (Cu2+, Cd2+ and Hg2+) on maize seed germination under high temperature. Environ. Pollut. 2016, 216, 46–52. [Google Scholar] [CrossRef]
- Hishida, M.; Ascencio-Valle, F.; Fujiyama, H.; Orduño-Cruz, A.; Endo, T.; Larrinaga-Mayoral, J.Á. Antioxidant enzyme responses to salinity stress of Jatropha curcas and J. cinerea at seedling stage. Russ. J. Plant Physiol. 2014, 61, 53–62. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavaddeur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef]
- Williams, L.E.; Pittman, J.K.; Hall, J.L. Emerging mechanisms for heavy metal transport in plants. Biochim. Biophys. Acta Biomembr. 2000, 1465, 104–126. [Google Scholar] [CrossRef]
- Grotz, N.; Fox, T.; Connolly, E.; Park, W.; Guerinot, M.L.; Eide, D. Identifification of a family of zinc transporter genes from Arabidopsis that respond to zinc defificiency. Proc. Natl. Acad. Sci. USA 1998, 95, 7220–7224. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Hu, H.; Xu, Y.; Li, X.X.; Zhang, H.J. Differential proteomic analysis of cadmium-responsive proteins in wheat leaves. Biol. Plant. 2011, 55, 586–590. [Google Scholar] [CrossRef]
- Avent, A.G.; Hanson, J.R.; Oliveira, B.H.D. Hydrolysis of the diterpenoid glycoside, stevioside. Phytochemistry 1990, 29, 2712–2715. [Google Scholar] [CrossRef]
- Mei, L.Y.; Liao, M.A.; Ren, Y.J.; Zhou, X.L. Effects of concentrated H2SO4 and IBA on breaking dormancy and germination index of P. acinosa seeds. Med. Plant 2012, 3, 13–15. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzym. 1987, 148, 350–382. [Google Scholar] [CrossRef]
- Heath, R.L.; Packer, L. Photoperoxidation in isolated chloroplast I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 1968, 25, 189–198. [Google Scholar] [CrossRef]
- Luo, H.H.; Yong, H.H.; Zhang, Y.L.; Zhang, W.F. Effects of water stress and rewatering on photosynthesis, root activity, and yield of cotton with drip irrigation under mulch. Photosynthetica 2016, 54, 65–73. [Google Scholar] [CrossRef]
- Dhindsa, R.S.; Dhindsa, P.P.; Thorpe, T.A. Leaf senescence correlated with increased levels of membrane permeability and lipid-peroxidation and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 1981, 32, 93–101. [Google Scholar] [CrossRef]
- Beauchamp, C.; Fridovich, I. Superoxide dismutase: Improved assays and an assay applicable to acrylamide gels. Anal. Biochem. 1971, 44, 276–287. [Google Scholar] [CrossRef]
- Chance, B.; Maehly, C. Assay of catalase and peroxidases. Methods Enzymol. 1955, 11, 764–775. [Google Scholar]
- Silva, G.P.; Sales, J.F.; Nascimento, K.J.T.; Rodriguesb, A.A.; Cameloc, G.N.; Borges, E.E.D.L. Biochemical and physiological changes in Dipteryx alata Vog. seeds during germination and accelerated aging. S. Afr. J. Bot. 2020, 131, 84–92. [Google Scholar] [CrossRef]
- Fathi, G.A.; Gharineh, H.; Barzali, M.; Siadat, S.A.A.; Tamadon-Rastegar, M. Evaluation of water deficit stress on seedling growth, antioxidant enzyme activity and yield of four cultivars of cotton. Int. J. Agric. Innov. Res. 2014, 3, 610–617. [Google Scholar]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef] [Green Version]



| Cd2+ Concentration (µM) | FG Rate (%) | GI | VI | SL (cm) | RL (cm) | FRN |
|---|---|---|---|---|---|---|
| Control | 96.3 ± 1.3 a | 44.4 ± 0.1 a | 528.4 ± 2.9 a | 5.5 ± 1.0 a | 6.4 ± 1.0 a | 5.2 ± 0.7 a |
| 1 | 96.2 ± 1.1 a | 44.1 ± 0.6 a | 516.0 ± 1.1 b | 5.2 ± 0.9 ab | 6.5 ± 1.2 a | 5.0 ± 0.6 ab |
| 10 | 91.3 ± 5.3 b | 35.3 ± 0.4 b | 388.3 ± 5.6 c | 5.0 ± 1.1 b | 6.0 ± 1.3 b | 4.9 ± 0.7 b |
| 100 | 86.0 ± 2.7 c | 35.5 ± 0.2 b | 266.3 ± 1.1 d | 4.3 ± 0.6 c | 3.2 ± 0.9 c | 4.7 ± 0.9 c |
| 1000 | 82.7 ± 0.9 d | 33.3 ± 0.9 c | 23.3 ± 2.0 e | 0.5 ± 0.1 d | 0.2 ± 0.1 d | 1.3 ± 0.1 d |
| Treatment (M) | FG Rate (%) | GI | VI | SL (cm) | RL (cm) | FRN |
|---|---|---|---|---|---|---|
| Control | 96.1 ± 1.7 a | 43.6 ± 0.4 a | 562.4 ± 3.6 a | 5.4 ± 1.1 b | 7.5 ± 1.2 a | 5.3 ± 0.6 a |
| Cd | 91.7 ± 3.2 c | 37.2 ± 0.7 d | 431.5 ± 6.5 g | 4.9 ± 0.3 cd | 6.7 ± 0.4 c | 4.3 ± 0.3 d |
| Cd + GA (10−5) | 95.3 ± 2.8 ab | 41.5 ± 0.9 b | 522.9 ± 4.2 b | 5.8 ± 1.0 a | 6.8 ± 0.6 c | 4.4 ± 0.2 cd |
| Cd + Iso (10−10) | 92.0 ± 1.6 c | 37.6 ± 0.6 d | 451.2 ± 2.7 f | 5.1 ± 0.8 c | 6.9 ± 1.2 bc | 4.5 ± 0.4 c |
| Cd + Iso (10−9) | 93.8 ± 4.0 b | 39.6 ± 0.2 c | 491.2 ± 3.4 d | 4.9 ± 0.6 cd | 7.5 ± 1.1 a | 4.6 ± 0.7 bc |
| Cd + Iso (10−8) | 94.3 ± 2.2 b | 39.5 ± 0.3 c | 513.5 ± 5.5 c | 5.4 ± 0.5 b | 7.6 ± 0.7 a | 4.7 ± 0.6 b |
| Cd + Iso (10−7) | 93.3 ± 3.8 bc | 38.5 ± 0.5 cd | 465.9 ± 4.7 e | 5.1 ± 0.9 c | 7.0 ± 0.5 b | 4.4 ± 0.3 cd |
| Treatment (M) | Chlorophyll (mg g−1) | Chlorophyll a/b | ||
|---|---|---|---|---|
| Chlorophyll a | Chlorophyll b | Chlorophyll a + b | ||
| Control | 1.7 ± 0.3 a | 0.7 ± 0.1 a | 2.4 ± 0.2 a | 2.4 ± 0.2 ab |
| Cd | 0.9 ± 0.2 d | 0.4 ± 0.04 bc | 1.3 ± 0.2 e | 2.3 ± 0.2 ab |
| Cd + GA (10−5) | 1.2 ± 0.1 b | 0.5 ± 0.06 b | 1.7 ± 0.1 b | 2.4 ± 0.3 ab |
| Cd + Iso (10−10) | 0.9 ± 0.1 d | 0.4 ± 0.03 bc | 1.3 ± 0.1 e | 2.3 ± 0.2 ab |
| Cd + Iso (10−9) | 1.0 ± 0.1 c | 0.4 ± 0.04 bc | 1.4 ± 0.1 d | 2.5 ± 0.1 a |
| Cd + Iso (10−8) | 1.1 ± 0.04 bc | 0.5 ± 0.1 b | 1.6 ± 0.2 bc | 2.2 ± 0.1 ab |
| Cd + Iso (10−7) | 1.1 ± 0.1 bc | 0.4 ± 0.03 bc | 1.5 ± 0.1 c | 2.7 ± 0.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, L.; Gao, B. Effect of Isosteviol on Wheat Seed Germination and Seedling Growth under Cadmium Stress. Plants 2021, 10, 1779. https://doi.org/10.3390/plants10091779
Zhang L, Gao B. Effect of Isosteviol on Wheat Seed Germination and Seedling Growth under Cadmium Stress. Plants. 2021; 10(9):1779. https://doi.org/10.3390/plants10091779
Chicago/Turabian StyleZhang, Liang, and Bingbing Gao. 2021. "Effect of Isosteviol on Wheat Seed Germination and Seedling Growth under Cadmium Stress" Plants 10, no. 9: 1779. https://doi.org/10.3390/plants10091779
APA StyleZhang, L., & Gao, B. (2021). Effect of Isosteviol on Wheat Seed Germination and Seedling Growth under Cadmium Stress. Plants, 10(9), 1779. https://doi.org/10.3390/plants10091779






