Effects of Light, N and Defoliation on Biomass Allocation in Poa annua
Abstract
1. Introduction
2. Results
2.1. Experiment One
2.1.1. Shoot Mass
2.1.2. N Content/Concentration
2.1.3. Leaf Area/Specific Leaf Area/Leaf Area Ratio
2.1.4. Root Mass Fraction
2.1.5. Experiment Two
2.1.6. Root Mass
3. Discussion
3.1. Root Growth Is Mainly Controlled by Light Level
3.2. C Allocation within the Root System
3.3. N Uptake and Shoot Growth
3.4. Shoot to Root Ratio
4. Materials and Methods
4.1. Experiment One
4.2. Experiment Two
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farley, R.A.; Fitter, A.H. Temporal and spatial variation in soil resources in a deciduous woodland. J. Ecol. 1999, 87, 688–696. [Google Scholar] [CrossRef]
- Hodge, A.; Robinson, D.; Griffiths, B.S.; Fitter, A.H. Why plants bother: Root proliferation results in increased nitrogen capture from an organic patch when two grasses compete. Plant Cell Environ. 1999, 22, 811–820. [Google Scholar] [CrossRef]
- Linkohr, B.I.; Williamson, L.C.; Fitter, A.H.; Leyser, H.M.O. Nitrate and phosphate availability and distribution have different effects on root system architecture of Arabidopsis. Plant J. 2002, 29, 751–760. [Google Scholar] [CrossRef]
- Robinson, D.; Hodge, A.; Griffiths, B.S.; Fitter, A.H. Plant root proliferation in nitrogen-rich patches confers competitive advantage. Proc. R. Soc. B Biol. Sci. 1999, 266, 431. [Google Scholar] [CrossRef]
- Fransen, B.; De Kroon, H.; De Kovel, C.G.F.; Van Den Bosch, F. Disentangling the effects of root foraging and inherent growth rate on plant biomass accumulation in heterogeneous environments: A modelling study. Ann. Bot. 1999, 84, 305–311. [Google Scholar] [CrossRef][Green Version]
- Robinson, D. Root proliferation, nitrate inflow and their carbon costs during nitrogen capture by competing plants in patchy soil. Plant Soil 2001, 232, 41–50. [Google Scholar] [CrossRef]
- Rasmussen, C.R.; Weisbach, A.N.; Thorup-Kristensen, K.; Weiner, J. Size-asymmetric root competition in deep, nutrient-poor soil. J. Plant Ecol. 2017, 12, 78–88. [Google Scholar] [CrossRef]
- Poorter, H.; Nagel, O. The role of biomass allocation in the growth response of plants to different levels of light, CO2, nutrients and water: A quantitative review. Aust. J. Plant Physiol. 2000, 27, 595–607. [Google Scholar]
- Poorter, H.; Niklas, K.J.; Reich, P.B.; Oleksyn, J.; Poot, P.; Mommer, L. Biomass allocation to leaves, stems and roots: Meta-analyses of interspecific variation and environmental control. N. Phytol. 2012, 193, 30–50. [Google Scholar] [CrossRef] [PubMed]
- Brouwer, R. Nutritive influences on the distribution of dry matter in the plant. Neth. J. Agric. Sci. 1962, 10, 399–408. [Google Scholar] [CrossRef]
- Shipley, B.; Meziane, D. The balanced-growth hypothesis and the allometry of leaf and root biomass allocation. Funct. Ecol. 2002, 16, 326–331. [Google Scholar] [CrossRef]
- Thornley, J.H.M. A balanced quantitative model for root: Shoot ratios in vegetative plants. Ann. Bot. 1972, 431–441. [Google Scholar] [CrossRef]
- Thornley, J.H.M. Modelling shoot: Root relations: The only way forward? Ann. Bot. 1998, 81, 165–171. [Google Scholar] [CrossRef]
- Dewar, R.C. A root-shoot partitioning model based on carbon-nitrogen-water interactions and Munch phloem flow. Funct. Ecol. 1993, 7, 356–368. [Google Scholar] [CrossRef]
- Wilson, J.B. A review of evidence on the control of shoot: Root ratio, in relation to models. Ann. Bot. 1988, 61, 433–449. [Google Scholar] [CrossRef]
- Andrews, M.; Sprent, J.I.; Raven, J.A.; Eady, P.E. Relationships between shoot to root ratio, growth and leaf soluble protein concentration of Pisum sativum, Phaseolus vulgaris and Triticum aestivum under different nutrient deficiencies. Plant Cell Environ. 1999, 22, 949–958. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Sprent, J.I. Environmental effects on dry matter partitioning between shoot and root of crop plants: Relations with growth and shoot protein concentration. Ann. Appl. Biol. 2001, 138, 57–68. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Lea, P.J.; Sprent, J.I. A role for shoot protein in shoot-root dry matter allocation in higher plants. Ann. Bot. 2006, 97, 3–10. [Google Scholar] [CrossRef]
- Irving, L.J.; Vaughan, J.K.E.; Ong, G.; Schwier, N.; Hama, T.; Cameron, D.D. Differential carbon allocation to nitrogen-rich patches in Poa annua precedes root proliferation but has no immediate benefit to N uptake. J. Plant Physiol. 2019, 234–235, 54–59. [Google Scholar] [CrossRef]
- Vessey, J.K.; Layzell, D. Regulation of assimilate partitioning in soybean: Initial effects following change in nitrate supply. Plant Physiol. 1987, 83, 341–348. [Google Scholar] [CrossRef]
- Minchin, P.E.H.; Lacointe, A. New understanding on phloem physiology and possible consequences for modelling long-distance carbon transport. New Phytol. 2005, 166, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Minchin, P.E.H. Source-sink coupling in young barley plants and control of phloem loading. J. Exp. Bot. 2002, 53, 1671–1676. [Google Scholar] [CrossRef]
- Minchin, P.E.H.; Thorpe, M.R.; Farrar, J.F. A simple mechanistic model of phloem transport which explains sink priority. J. Exp. Bot. 1993, 44, 947–955. [Google Scholar] [CrossRef]
- Minchin, P.E.H.; Farrar, J.F.; Thorpe, M.R. Partitioning of carbon in split root systems of barley: Effect of temperature of the root. J. Exp. Bot. 1994, 45, 1103–1109. [Google Scholar] [CrossRef]
- Bloom, A.J.; Sukrapanna, S.S.; Warner, R.L. Root respiration associated with ammonium and nitrate absorption and assimilation by barley. Plant Physiol. 1992, 99, 1294–1301. [Google Scholar] [CrossRef] [PubMed]
- Lattanzi, F.A.; Schnyder, H.; Thornton, B. The sources of carbon and nitrogen supplying leaf growth. Assessment of the role of stores with compartmental models. Plant Physiol. 2005, 137, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Robin, A.; Irving, L.; Khaembah, E.; Matthew, C. Modelling carbon fluxes as an aid to understanding perennial ryegrass (Lolium perenne) root dynamics. Agronomy 2018, 8, 236. [Google Scholar] [CrossRef]
- Ryle, G.J.A.; Powell, C.E. Defoliation and regrowth in the graminaceous plant: The role of current assimilate. Ann. Bot. 1975, 39, 297–310. [Google Scholar] [CrossRef]
- Gonzalez-Dugo, V.; Durand, J.L.; Gastal, F.; Bariac, T.; Poincheval, J. Restricted root-to-shoot translocation and decreased sink size are responsible for limited nitrogen uptake in three grass species under water deficit. Environ. Exp. Bot. 2012, 75, 258–267. [Google Scholar] [CrossRef]
- Granato, T.C.; Raper, C.D. Proliferation of maize (Zea mays L.) roots in response to localized supply of nitrate. J. Exp. Bot. 1989, 40, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.; Rorison, I.H. A comparison of the responses of Lolium perenne (L.), Holcus lanatus (L.) and Deschampsia elexuosa (L.) Trin. to a localized supply of nitrogen. N. Phytol. 1983, 94, 363. [Google Scholar] [CrossRef]
- Devienne-Barret, F.; Justes, E.; Machet, J.M.; Mary, B. Integrated control of nitrate uptake by crop growth rate and soil nitrate availability under field conditions. Ann. Bot. 2000, 86, 995–1005. [Google Scholar] [CrossRef]
- Van Vuuren, M.M.; Robinson, D.; Griffiths, B.S. Nutrient inflow and root proliferation during the exploitation of a temporally and spatially discrete source of nitrogen in soil. Plant Soil 1996, 178, 185–192. [Google Scholar] [CrossRef]
- Hodge, A.; Berta, G.; Doussan, C.; Merchan, F.; Crespi, M. Plant root growth, architecture and function. Plant Soil 2009, 321, 153–187. [Google Scholar] [CrossRef]
- Evans, J.R. Photosynthesis and nitrogen relationship in leaves of C3 plants. Oecologia 1989, 78, 9–19. [Google Scholar] [CrossRef] [PubMed]
- Ogren, E. Convexity of the photosynthetic light-response curve in relation to intensity and direction of light during growth. Plant Physiol. 1993, 101, 1013–1019. [Google Scholar] [CrossRef] [PubMed]
- Makino, A.; Osmond, B. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiol. 1991, 96, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Nikinmaa, E.; Hölttä, T.; Hari, P.; Kolari, P.; Mäkelä, A.; Sevanto, S.; Vesala, T. Assimilate transport in phloem sets conditions for leaf gas exchange. Plant Cell Environ. 2013, 36, 655–669. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- McDonald, M.S. A simple and improved method for the determination of microgram quantities of nitrogen in plant material. Ann. Bot. 1978, 42, 363–366. [Google Scholar] [CrossRef]
- Loftus, G.R.; Masson, M.E.J. Using confidence intervals in within-subject designs. Psychon. Bull. Rev. 2013, 1, 476–490. [Google Scholar] [CrossRef] [PubMed]
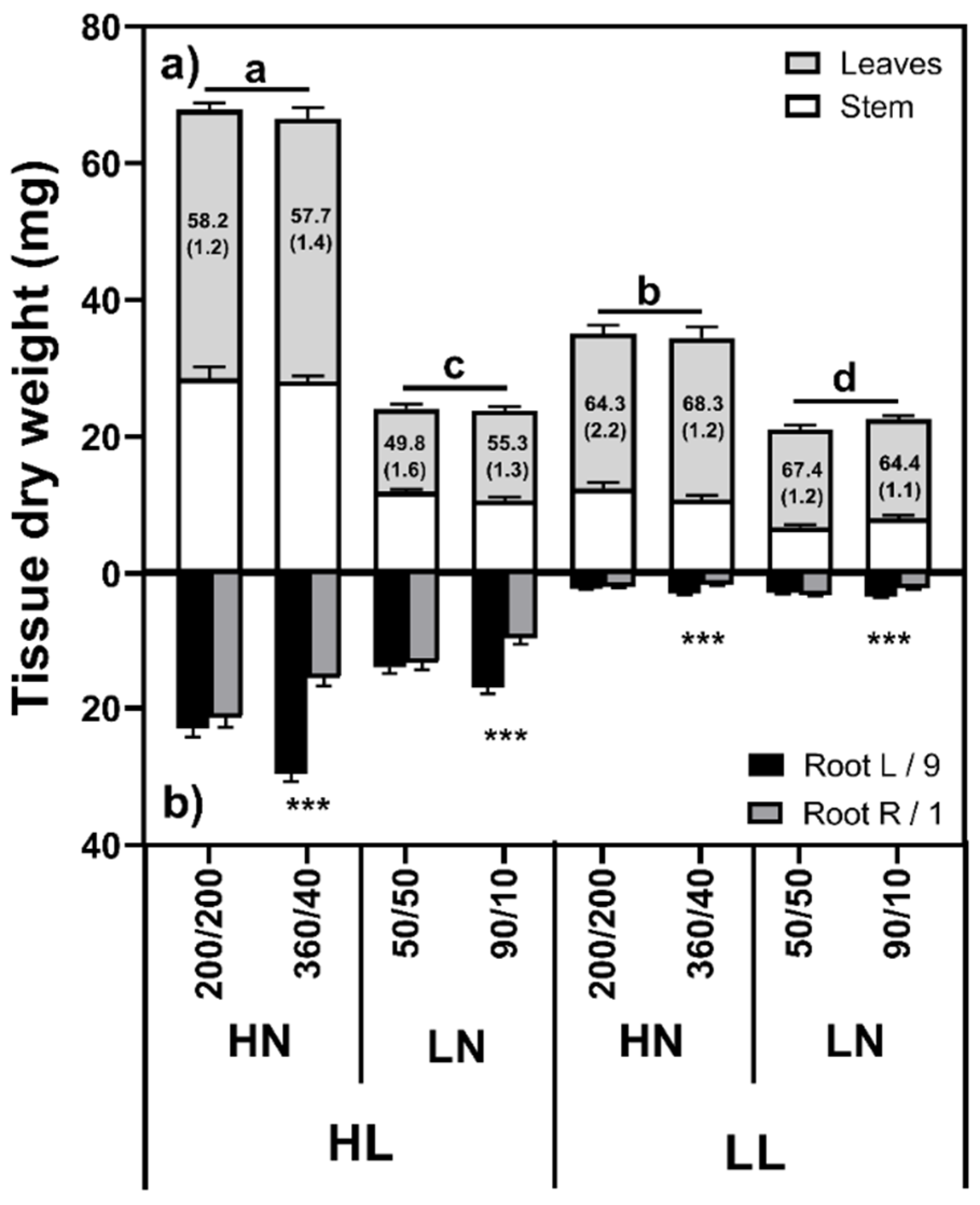
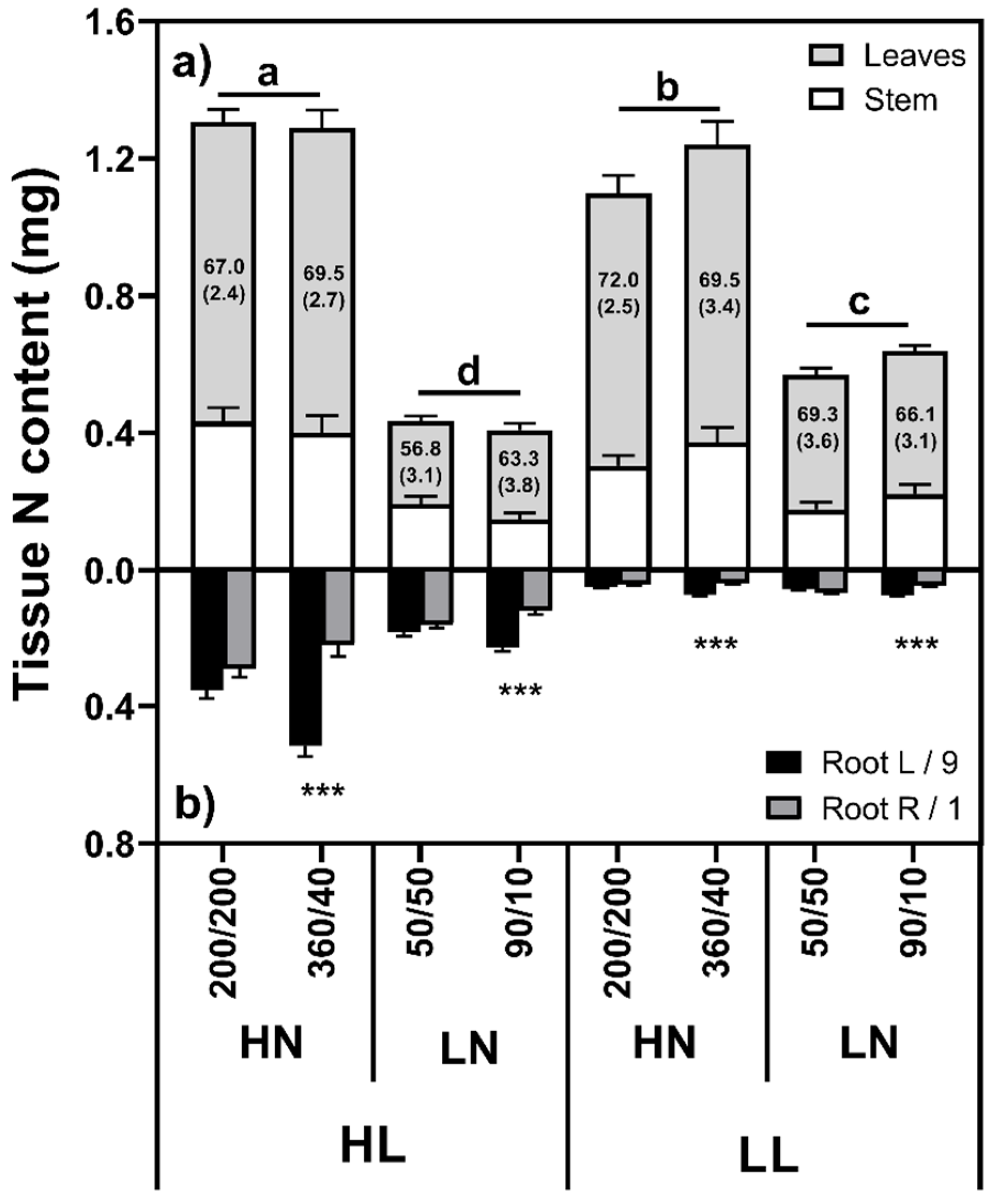
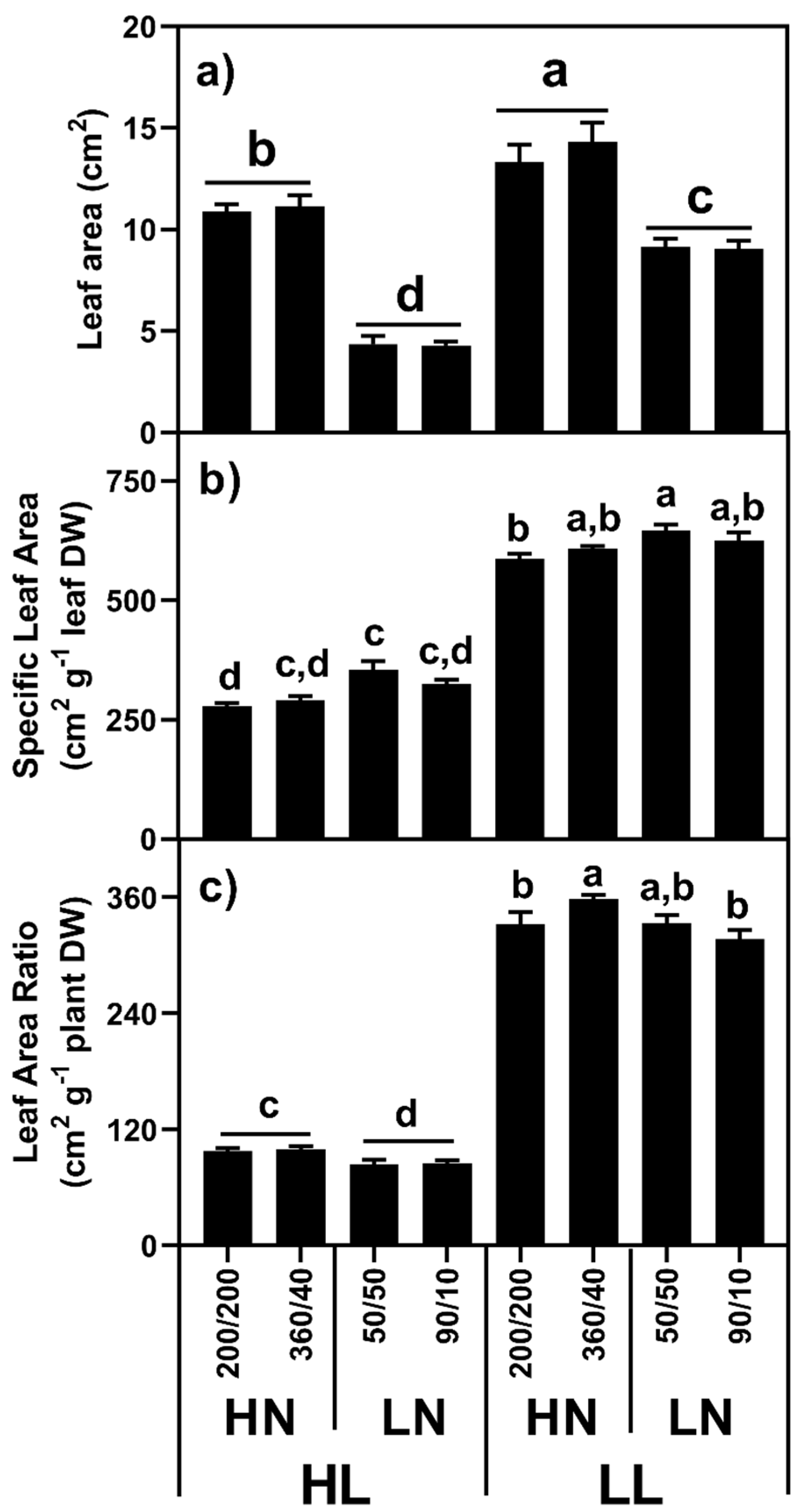
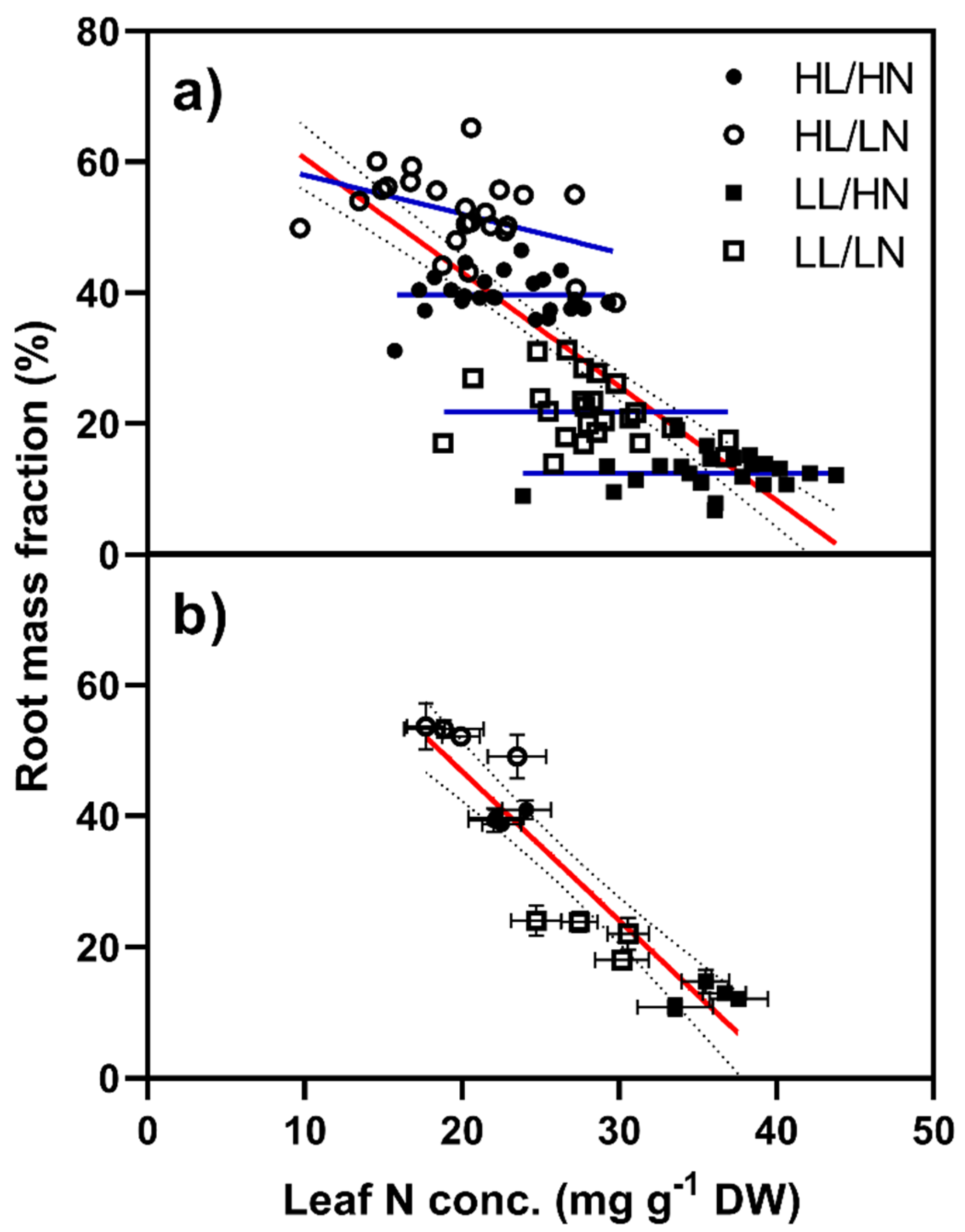
| Light | Defoliation | N Distribution | Shoot | L/9 | R/1 | p-Value | L:R Ratio | RMF (%) |
|---|---|---|---|---|---|---|---|---|
| High | None | Equal | 68.6 (2.4) a | 23.4 | 22.2 (1.0) | 0.651 | 52:48 (2) | 39.5 (1.4) a |
| Unequal | 72.4 (3.5) a | 26.7 | 17.2 (0.7) | 0.006 | 61:39 (2) | 37.7 (1.0) a | ||
| Weak | Equal | 49.6 (1.5) b | 13.9 | 14.9 (0.9) | 0.224 | 47:53 (3) | 36.4 (1.2) a | |
| Unequal | 52.3 (1.7) b | 14.1 | 9.6 (0.8) | <0.001 | 60:40 (4) | 30.9 (1.4) b | ||
| Strong | Equal | 47.7 (1.6) b | 10.3 | 11.6 (0.6) | 0.507 | 46:54 (3) | 30.7 (1.7) b | |
| Unequal | 49.2 (1.0) b | 13.7 | 7.7 (0.5) | 0.005 | 65:35 (3) | 29.8 (1.4) b | ||
| Low | None | Equal | 28.6 (1.7) c | 2.0 | 2.1 (0.2) | 0.616 | 48:52 (6) | 12.2 (0.7) c |
| Unequal | 29.6 (2.1) c | 2.3 | 1.4 (0.2) | 0.001 | 62:38 (4) | 11.0 (0.5) c | ||
| Weak | Equal | 8.7 (0.3) d | 0.9 | 0.7 (0.1) | 0.824 | 54:46 (5) | 15.1 (1.3) c | |
| Unequal | 8.1 (0.3) d | 0.8 | 0.7 (0.1) | 0.356 | 56:44 (5) | 15.4 (1.2) c | ||
| Strong | Equal | 7.4 (0.4) e | 0.7 | 0.7 (0.1) | 0.227 | 49:51 (3) | 15.9 (0.8) c | |
| Unequal | 6.9 (0.4) e | 0.7 | 0.6 (<0.1) | 0.083 | 54:46 (2) | 15.7 (0.7) c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Irving, L.J.; Mori, S. Effects of Light, N and Defoliation on Biomass Allocation in Poa annua. Plants 2021, 10, 1783. https://doi.org/10.3390/plants10091783
Irving LJ, Mori S. Effects of Light, N and Defoliation on Biomass Allocation in Poa annua. Plants. 2021; 10(9):1783. https://doi.org/10.3390/plants10091783
Chicago/Turabian StyleIrving, Louis John, and Sayuki Mori. 2021. "Effects of Light, N and Defoliation on Biomass Allocation in Poa annua" Plants 10, no. 9: 1783. https://doi.org/10.3390/plants10091783
APA StyleIrving, L. J., & Mori, S. (2021). Effects of Light, N and Defoliation on Biomass Allocation in Poa annua. Plants, 10(9), 1783. https://doi.org/10.3390/plants10091783






