Frontiers in the Standardization of the Plant Platform for High Scale Production of Vaccines
Abstract
:1. Introduction
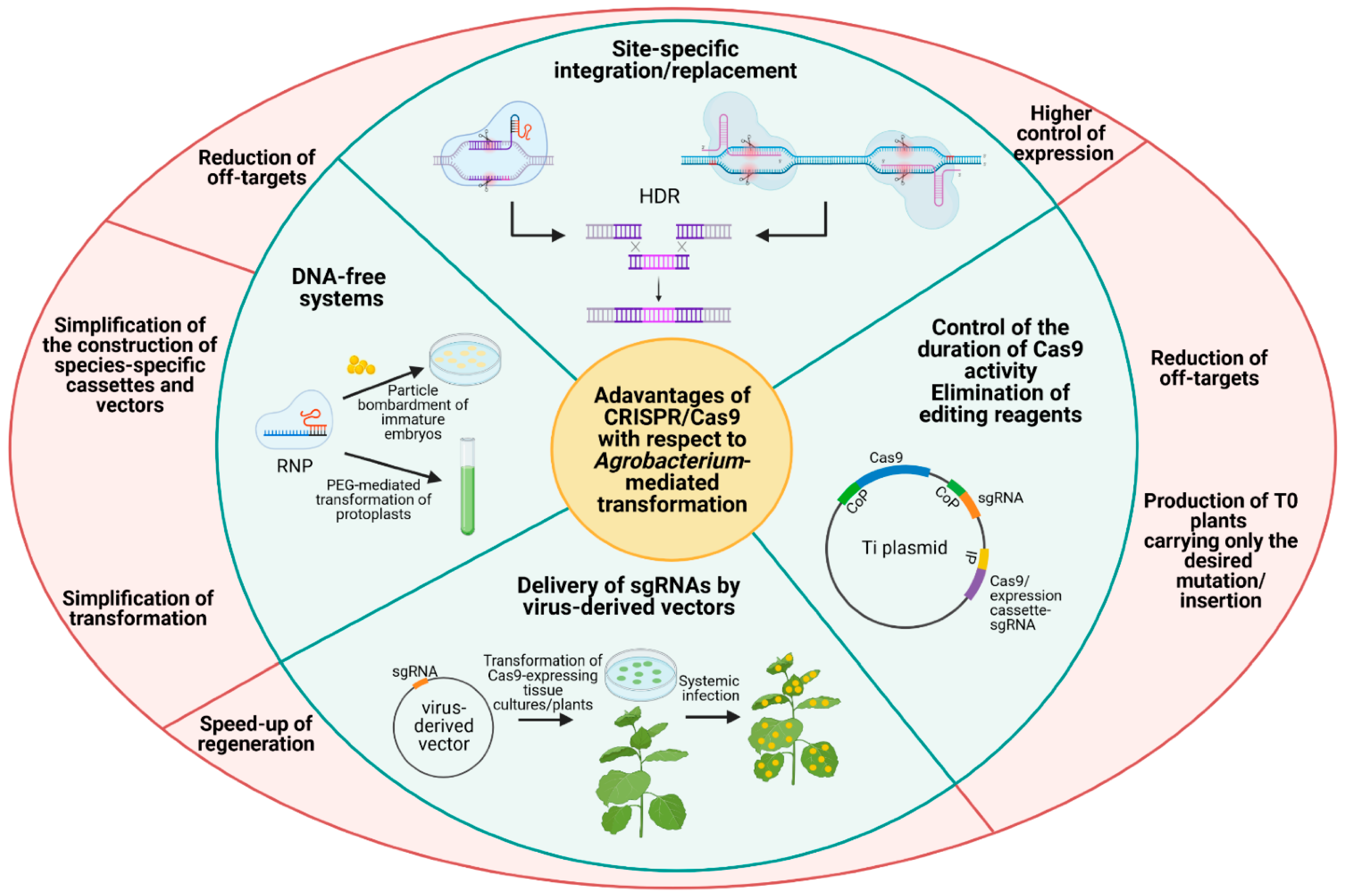
2. Transformation Technologies and CRISPR-Cas Genome Editing Methods
3. Suitable Plant Species for Vaccine Production
4. Virus-like Particles (VLPs) as Best Candidates for Vaccine Production in Plants
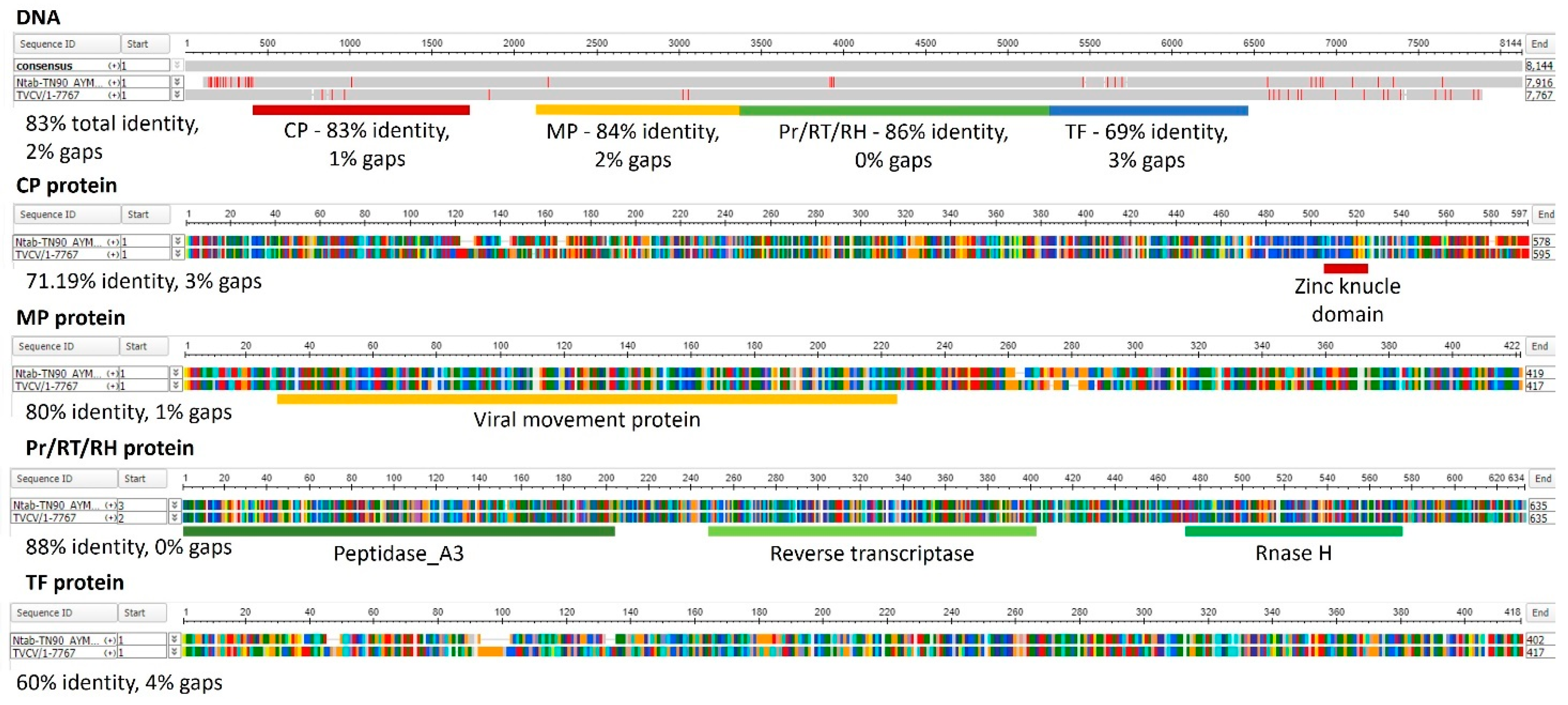
5. Taking Advantage of Pre-Existing VPL Structures in the Plant Genome
6. Simplified Industrial Production of Vaccines in Plant by Combining VLPs and Genome Editing
7. Other Advantages of Plant-Based Vaccine Production
8. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CP | coat protein |
| CRISPR | Cluster Regularly Interspersed Short Palindromic Repeats |
| CVP | chimeric virus particle |
| EPRE | Endogenous Pararetriviral Elements |
| GM | genetically modified |
| GMP | Good Manufacturing Practices |
| HDR | homologous-derived repair |
| NHEJ | nonhomologous end-joining repair |
| Ntab | Nicotiana tabacum |
| PAM | Protospacer Adjacent Motif |
| PEG | polyethylene glycol |
| sgRNA | single guide RNA |
| TPV | tobacco Pararetrovirus-like sequence |
| TVCV | Turnip vein-clearing virus |
| VLP | virus-like particle |
References
- Rappuoli, R. Reverse vaccinology. Curr. Opin. Microbiol. 2000, 3, 445–450. [Google Scholar] [CrossRef]
- Rappuoli, R. Bridging the knowledge gaps in vaccine design. Nat. Biotechnol. 2007, 25, 1361–1366. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant-based vaccines against viruses. Virol. J. 2014, 11, 205. [Google Scholar] [CrossRef]
- Takeyama, N.; Kiyono, H.; Yuki, Y. Plant-based vaccines for animals and humans: Recent advances in technology and clinical trials. Theor. Adv. Vaccines 2015, 3, 139–154. [Google Scholar] [CrossRef]
- Ward, B.J.; Makarkov, A.; Seguin, A.; Pillet, S.; Trepanier, S.; Dhaliwall, J.; Libman, M.D.; Vesikari, T.; Landry, N. Efficacy, immunogenicity, and safety of a plant-derived, quadrivalent, virus-like particle influenza vaccine in adults (18–64 years) and older adults (>/=65 years): Two multicentre, randomised phase 3 trials. Lancet 2020, 396, 1491–1503. [Google Scholar] [CrossRef]
- Ward, B.J.; Seguin, A.; Couillard, J.; Trepanier, S.; Landry, N. Phase III: Randomized observer-blind trial to evaluate lot-to-lot consistency of a new plant-derived quadrivalent virus like particle influenza vaccine in adults 18–49 years of age. Vaccine 2021, 39, 1528–1533. [Google Scholar] [CrossRef] [PubMed]
- Holtz, B.R.; Berquist, B.R.; Bennett, L.D.; Kommineni, V.J.M.; Munigunti, R.K.; White, E.L.; Wilkerson, D.C.; Wong, K.Y.I.; Ly, L.H.; Marcel, S. Commercial-scale biotherapeutics manufacturing facility for plant-made pharmaceuticals. Plant Biotechnol. J. 2015, 13, 1180–1190. [Google Scholar] [CrossRef] [Green Version]
- LeBlanc, Z.; Waterhouse, P.; Bally, J. Plant-Based Vaccines: The Way Ahead? Viruses 2021, 13, 5. [Google Scholar] [CrossRef]
- Halpin, C. Gene stacking in transgenic plants—The challenge for 21st century plant biotechnology. Plant Biotechnol. J. 2005, 3, 141–155. [Google Scholar] [CrossRef] [PubMed]
- De Muynck, B.; Navarre, C.; Boutry, M. Production of antibodies in plants: Status after twenty years. Plant Biotechnol. J. 2010, 8, 529–563. [Google Scholar] [CrossRef] [PubMed]
- Lau, O.S.; Sun, S.S.M. Plant seeds as bioreactors for recombinant protein production. Biotechnol. Adv. 2009, 27, 1015–1022. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Charpentier, E. Genome editing. The new frontier of genome engineering with CRISPR-Cas9. Science 2014, 346, 1258096. [Google Scholar] [CrossRef] [PubMed]
- Doudna, J.A.; Sontheimer, E.J. Methods in Enzymology. The use of CRISPR/Cas9, ZFNs, and TALENs in generating site-specific genome alterations. Methods Enzymol. 2014, 546, 2–549. [Google Scholar] [CrossRef]
- Schaeffer, S.M.; Nakata, P.A. CRISPR/Cas9-mediated genome editing and gene replacement in plants: Transitioning from lab to field. Plant Sci. 2015, 240, 130–142. [Google Scholar] [CrossRef] [PubMed]
- Mercx, S.; Smargiasso, N.; Chaumont, F.; De Pauw, E.; Boutry, M.; Navarre, C. Inactivation of the β(1,2)-xylosyltransferase and the α(1,3)-fucosyltransferase genes in Nicotiana tabacum BY-2 cells by a multiplex CRISPR/Cas9 strategy results in glycoproteins without plant-specific glycans. Front. Plant Sci. 2017, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Jansing, J.; Sack, M.; Augustine, S.M.; Fischer, R.; Bortesi, L. CRISPR/Cas9-mediated knockout of six glycosyltransferase genes in Nicotiana benthamiana for the production of recombinant proteins lacking beta-1,2-xylose and core alpha-1,3-fucose. Plant Biotechnol. J. 2019, 17, 350–361. [Google Scholar] [CrossRef] [Green Version]
- Buyel, J.F.; Stoger, E.; Bortesi, L. Targeted genome editing of plants and plant cells for biomanufacturing. Transgenic Res. 2021, 30, 401–426. [Google Scholar] [CrossRef]
- Mett, V.; Farrance, C.E.; Green, B.J.; Yusibov, V. Plants as biofactories. Biologicals 2008, 36, 354–358. [Google Scholar] [CrossRef] [PubMed]
- Daniell, H.; Singh, N.D.; Mason, H.; Streatfield, S.J. Plant-made vaccine antigens and biopharmaceuticals. Trends Plant Sci. 2009, 14, 669–679. [Google Scholar] [CrossRef] [Green Version]
- Lico, C.; Santi, L.; Twyman, R.M.; Pezzotti, M.; Avesani, L. The use of plants for the production of therapeutic human peptides. Plant Cell Rep. 2012, 31, 439–451. [Google Scholar] [CrossRef]
- Taylor, N.J.; Fauquet, C.M. Microparticle bombardment as a tool in plant science and agricultural biotechnology. DNA Cell Biol. 2002, 21, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Gleba, Y.; Marillonnet, S.; Klimyuk, V. Engineering viral expression vectors for plants: The ’full virus’ and the ’deconstructed virus’ strategies. Curr. Opin. Plant Biol. 2004, 7, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Fu, J.; Ma, J.; Wang, X.; Gao, C.; Zhuang, C.; Wan, J.; Jiang, L. Isolation, culture, and transient transformation of plant protoplasts. Curr. Protoc. Cell Biol. 2014, 63, 2–8. [Google Scholar] [CrossRef]
- Jones, H.D.; Doherty, A.; Sparks, C.A. Transient transformation of plants. Methods Mol. Biol. 2009, 513, 131–152. [Google Scholar] [CrossRef] [PubMed]
- Krenek, P.; Samajova, O.; Luptovciak, I.; Doskocilova, A.; Komis, G.; Samaj, J. Transient plant transformation mediated by Agrobacterium tumefaciens: Principles, methods and applications. Biotechnol. Adv. 2015, 33, 1024–1042. [Google Scholar] [CrossRef]
- Sainsbury, F. Innovation in plant-based transient protein expression for infectious disease prevention and preparedness. Curr. Opin. Biotechnol. 2020, 61, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.H.; Yu, M.; Lai, E.M. Agrobacterium-mediated plant transformation: Biology and applications. Arab. Book 2017, 15, e0186. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Mason, H. Bean Yellow Dwarf Virus replicons for high-level transgene expression in transgenic plants and cell cultures. Biotechnol. Bioeng. 2006, 93, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lico, C.; Chen, Q.; Santi, L. Viral vectors for production of recombinant proteins in plants. J. Cell Physiol. 2008, 216, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Buyel, J.F.; Twyman, R.M.; Fischer, R. Very-large-scale production of antibodies in plants: The biologization of manufacturing. Biotechnol. Adv. 2017, 35, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Sack, M.; Rademacher, T.; Spiegel, H.; Boes, A.; Hellwig, S.; Drossard, J.; Stoger, E.; Fisher, R. From gene to harvest: Insights into upstream process development for the GMP production of a monoclonal antibody in transgenic tobacco plants. Plant Biotechnol. J. 2015, 13, 1094–1105. [Google Scholar] [CrossRef]
- Nessa, M.U.; Rahman, M.A.; Kabir, Y. Plant-Produced Monoclonal Antibody as Immunotherapy for Cancer. BioMed Res. Int. 2020, 2020, 3038564. [Google Scholar] [CrossRef]
- Jamal, A.; Ko, K.; Kim, H.S.; Choo, Y.K.; Joung, H.; Ko, K. Role of genetic factors and environmental conditions in recombinant protein production for molecular farming. Biotechnol. Adv. 2009, 27, 914–923. [Google Scholar] [CrossRef]
- Butaye, K.M.J.; Goderis, I.J.W.M.; Wouters, P.F.J.; Pues, J.M.T.G.; Delaurè, S.L.; Broekaert, W.F.; Depicker, A.; Cammue, B.P.A.; De Bolle, M.F.C. Stable high-level transgene expression in Arabidopsis thaliana using gene silencing mutants and matrix attachment regions. Plant J. 2004, 39, 440–449. [Google Scholar] [CrossRef] [PubMed]
- Khatodia, S.; Bhatotia, K.; Passricha, N.; Khurana, S.M.; Tuteja, N. The CRISPR/Cas Genome-Editing Tool: Application in Improvement of Crops. Front. Plant Sci. 2016, 7, 506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiao, A.; Cheng, Z.; Kong, L.; Zhu, Z.; Lin, S.; Gao, G.; Zhang, B. CasOT: A genome-wide Cas9/gRNA off-target searching tool. Bioinformatics 2014, 30, 1180–1182. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Bae, S.; Kim, J.S. Cas-Designer: A web-based tool for choice of CRISPR-Cas9 target sites. Bioinformatics 2015, 31, 4014–4016. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Xu, J.; Cheng, M.; Liao, X.; Peng, S. Review of CRISPR/Cas9 sgRNA Design Tools. Interdiscip. Sci. Comput. Life Sci. 2018, 10, 455–465. [Google Scholar] [CrossRef]
- Rozov, S.M.; Permyakova, N.V.; Deineko, E.V. The Problem of the Low Rates of CRISPR/Cas9-Mediated Knock-ins in Plants: Approaches and Solutions. Int. J. Mol. Sci. 2019, 20, 3371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Xia, L. Precise gene replacement in plants through CRISPR/Cas genome editing technology: Current status and future perspectives. aBIOTECH 2020, 1, 58–73. [Google Scholar] [CrossRef] [Green Version]
- Wada, N.; Ueta, R.; Osakabe, Y.; Osakabe, K. Precision genome editing in plants: State of the art in CRISPR/Cas9-based genome engineering. BMC Plant Biol. 2020, 20, 234. [Google Scholar] [CrossRef]
- Li, J.; Li, H.; Chen, J.; Yan, L.; Xia, L. Toward precision genome editing in crop plants. Mol. Plant 2020, 13, 811–813. [Google Scholar] [CrossRef]
- Kantor, A.; McClements, M.E.; MacLaren, R.E. CRISPR-Cas9 DNA base-editing and prime editing. Int. J. Mol. Sci. 2020, 21, 6240. [Google Scholar] [CrossRef]
- Anzalone, A.V.; Randolph, P.B.; Davis, J.R.; Sousa, A.A.; Koblan, L.W.; Levy, J.M.; Chen, P.J.; Wilson, C.; Newby, G.A.; Raguram, A.; et al. Search and replace genome editing without double strand breaks or donor DNA. Nature 2019, 576, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Montecillo, J.A.V.; Chu, L.L.; Bae, H. CRISPR-Cas9 System for Plant Genome Editing: Current Approaches and Emerging Developments. Agronomy 2020, 10, 1033. [Google Scholar] [CrossRef]
- Woo, J.; Kim, J.; Kwon, S.; Corvalan, C.; Cho, S.W.; Kim, H.; Kim, S.G.; Kim, S.T.; Choe, S.; Kim, J.S. DNA-free genome editing in plants with preassembled CRISPR-Cas9 ribonucleoproteins. Nat. Biotechnol. 2015, 33, 1162–1164. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Koprowski, H. Plant biopharming of monoclonal antibodies. Virus Res. 2005, 111, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Besufekad, Y.; Malaiyarsa, P. Production of Monoclonal Antibodies in Transgenic Plants. J. Adv. Biol. Biotechnol. 2017, 12, 1–8. [Google Scholar] [CrossRef]
- Issaro, N.; Wang, D.; Liu, M.; Tassaneetrithep, B.; Phawong, C.; Rattanarojpong, T.; Jiang, C. Transgenic carrot plant-made vaccines against human infectious diseases. J. Innov. Pharma. Biol. Sci. 2018, 5, 43–48. [Google Scholar]
- Okai, S.; Sezgin, M. Transgenic plants for the production of immunogenic proteins. Bioengineering 2018, 5, 151–161. [Google Scholar] [CrossRef]
- Molina-Hidalgo, F.J.; Vazquez-Vilar, M.; D’Andrea, L.; Demurtas, O.C.; Fraser, P.; Giuliano, G.; Bock, R.; Orzáez, D.; Goossens, A. Engineering Metabolism in Nicotiana Species: A Promising Future. Trends Biotechnol. 2020, 39, 901–913. [Google Scholar] [CrossRef]
- Chen, Q.; Davis, K.R. The potential of plants as a system for the development and production of human biologics. F1000Research 2016, 5, 912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sparrow, P.A.C.; Irwin, J.A.; Dale, P.J.; Twyman, R.M.; Ma, J.K.C. Pharma-Planta: Road testing the developing regulatory guidelines for plant-made pharmaceuticals. Transgenic Res. 2007, 16, 147–161. [Google Scholar] [CrossRef] [PubMed]
- Breyer, D.; De Schrijver, A.; Goossens, M.; Pauwels, K.; Herman, P. Biosafety of Molecular Farming in Genetically Modified Plants. In Molecular Farming in Plants: Recent Advances and Future Prospects; Wang, A., Ma, S., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 259–274. [Google Scholar] [CrossRef]
- Hitzeroth, I.J.; Chabeda, A.; Whitehead, M.P.; Graf, M.; Rybicki, E.P. Optimizing a Human Papillomavirus Type 16 L1-Based Chimaeric Gene for Expression in Plants. Front. Bioeng. Biotechnol. 2018, 6, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Habibi-Pirkoohi, M.; Malekzadeh-Shafaroudi, S.; Marashi, H.; Mohkami, A. Expression of an epitope-based recombinant vaccine against Foot and Moueth Disease (FMDV) in tobacco plant (Nicotiana tabacum). J. Plant Mol. Breed. 2019, 7, 1–9. [Google Scholar] [CrossRef]
- Park, Y.; An, D.J.; Choe, S.; Lee, Y.; Park, M.; Park, S.; Gu, S.; Min, K.; Kim, N.H.; Lee, S.; et al. Development of Recombinant Protein-Based Vaccine Against Classical Swine Fever Virus in Pigs Using Transgenic Nicotiana benthamiana. Front. Plant Sci. 2019, 10, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diamos, A.G.; Hunter, J.G.L.; Pardhe, M.D.; Rosenthal, S.H.; Sun, H.; Foster, B.C.; DiPalma, M.P.; Chen, Q.; Mason, H.S. High Level Production of Monoclonal Antibodies Using an Optimized Plant Expression System. Font. Bioeng. Biotechnol. 2020, 7, 472. [Google Scholar] [CrossRef]
- Castilho, A.; Neumann, L.; Daskalova, S.; Mason, H.S.; Steinkellner, H.; Altmann, F.; Strasser, R. Engineering of sialylated mucin-type O-glycosylation in plants. J. Biol. Chem. 2012, 287, 36518–36526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kallolimath, S.; Castilho, A.; Strasser, R.; Grünwald-Gruber, C.; Altmann, F.; Strubl, S.; Galuska, C.E.; Zlatina, K.; Galuska, S.P.; Werner, S.; et al. Engineering of complex protein sialylation in plants. Proc. Natl. Acad. Sci. USA 2016, 113, 9498–9503. [Google Scholar] [CrossRef] [Green Version]
- Alderborn, A.; Sundström, J.; Soeria-Atmadja, D.; Sandberg, M.; Andersson, H.C.; Hammerling, U. Genetically modified plants for non-food or non-feed purposes: Straightforward screening for their appearance in food and feed. Food Chem. Toxicol. 2010, 48, 453–464. [Google Scholar] [CrossRef]
- Chichester, J.A.; Jones, R.M.; Green, B.J.; Stow, M.; Miao, F.; Moonsammy, G.; Streatfield, S.J.; Yusibov, V. Safety and immunogenicity of a plant-produced recombinant hemagglutinin-based influenza vaccine (HAI-05) derived from A/Indonesia/05/2005 (H5N1) influenza virus: A phase 1 randomized, double-blind, placebo-controlled, dose-escalation study in healthy adults. Viruses 2012, 4, 3227–3244. [Google Scholar] [CrossRef]
- Landry, N.; Ward, B.J.; Trepanier, S.; Montomoli, E.; Dargis, M.; Lapini, G.; Vezina, L.P. Preclinical and clinical development of plant-made virus-like particle vaccine against avian H5N1 influenza. PLoS ONE 2010, 5, e15559. [Google Scholar] [CrossRef] [Green Version]
- Cummings, J.F.; Guerrero, M.L.; Moon, J.E.; Waterman, P.; Nielsen, R.K.; Jefferson, S.; Gross, F.L.; Hancock, K.; Katz, J.M.; Yusibov, V. Safety and immunogenicity of a plant-produced recombinant monomer hemagglutinin-based influenza vaccine derived from influenza A (H1N1)pdm09 virus:a phase 1 dose-escalation study in healthy adults. Vaccines 2014, 32, 2251–2259. [Google Scholar] [CrossRef]
- Kapusta, J.; Modelska, A.; Figlerowicz, M.; Pniewski, T.; Letellier, M.; Lisowa, O.; Yusibov, V.; Koprowski, H.; Plucienniczak, A.; Legocki, A.B. A plant-derived edible vaccine against hepatitis B virus. FASEB J. 1999, 13, 1796–1799. [Google Scholar] [CrossRef]
- Monreal-Escalante, E.; Ramos-Vega, A.A.; Salazar-González, J.A.; Banuelos-Hernandez, B.; Angulo, C.; Rosales-Mendoza, S. Expression of the VP40 antigen from the Zaire ebolavirus in tobacco plants. Planta 2017, 246, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Yanez, R.J.R.; Lamprecht, R.; Granadillo, M.; Weber, B.; Torrens, I.; Rybicki, E.P.; Hitzeroth, I.I. Expression optimization of a cell membrane-penetrating human papillomavirus type 16 therapeutic vaccine candidate in Nicotiana benthamiana. PLoS ONE 2017, 12, e0183177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrica, M.O.; van Eerde, A.; Tucureanu, C.; Onu, A.; Paruch, L.; Caras, I.; Vlase, E.; Steen, H.; Haugslien, S.; Alonzi, D.; et al. Hepatitis C virus E2 envelope glycoprotein produced in Nicotiana benthamiana triggers humoral response with virus-neutralizing activity in vaccinated mice. Plant Biotechnol. J. 2021, 1–13. [Google Scholar] [CrossRef]
- Sierro, N.; Battey, J.N.D.; Ouadi, S.; Bakaher, N.; Bovet, L.; Wiling, A.; Goepfert, S.; Peitsch, M.C.; Ivanov, N.V. The tobacco genome sequence and its comparison with those of tomato and potato. Nat. Commun. 2014, 5, 3833. [Google Scholar] [CrossRef]
- Edwards, K.D.; Fernandez-Pozo, N.; Drake-Stowe, K.; Humphry, M.; Evans, A.D.; Bombarely, A.; Allen, F.; Hurst, R.; White, B.; Kernodle, S.P.; et al. A reference genome for Nicotiana tabacum enables map-based cloning of homeologous loci implicated in nitrogen utilization efficiency. BMC Genom. 2017, 18, 448. [Google Scholar] [CrossRef]
- Bombarely, A.; Rosli, H.G.; Vrebalov, J.; Moffett, P.; Mueller, L.A.; Martin, G.B. A Draft Genome Sequence of Nicotiana benthamiana to Enhance Molecular Plant-Microbe Biology Research. Mol. Plant-Microbe Interact. 2012, 24, 1523–1530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nekrasov, V.; Staskawicz, B.; Weigel, D.; Jones, J.D.G.; Kamoun, S. Targeted mutagenesis in the model plant Nicotiana benthamiana using Cas9 RNA-guided endonuclease. Nat. Biotechnol. 2013, 31, 691–693. [Google Scholar] [CrossRef]
- Gao, J.; Wang, G.; Ma, S.; Xie, X.; Wu, X.; Zhang, X.; Wu, Y.; Zhao, P.; Xia, Q. CRISPR/Cas9-mediated targeted mutagenesis in Nicotiana tabacum. Plant Mol. Biol. 2015, 87, 99–110. [Google Scholar] [CrossRef]
- Kang, M.; Ahn, H.; Rothe, E.; Baldwin, I.T.; Kim, S.G. A robust genome-editing method for wild plant species Nicotiana attenuata. Plant Biotechnol. Rep. 2015, 14, 585–598. [Google Scholar] [CrossRef]
- Schachtsiek, J.; Felix, S. Nicotine-free, nontransgenic tobacco (Nicotiana tabacum L.) edited by CRISPR-Cas9. Plant Biotechnol. J. 2019, 17, 2228–2230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.; Wijewardhana, C.; Mann, J.F.S. Virus-Like Particle, Liposome, and Polymeric Particle-Based Vaccines against HIV-1. Front. Immunol. 2018, 9, 345. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benne, N.; van Duijn, J.; Kuiper, J.; Jiskoot, W.; Slutter, B. Orchestrating immune responses: How size, shape and rigidity affect the immunogenicity of particulate vaccines. J. Control Release 2016, 234, 124–134. [Google Scholar] [CrossRef]
- Chen, T.H.; Hu, C.C.; Liao, J.T.; Lee, Y.L.; Huang, Y.W.; Lin, N.S.; Hsu, Y.H. Production of Japanese Encephalitis Virus Antigens in Plants Using Bamboo Mosaic Virus-Based Vector. Front. Microbiol. 2017, 8, 788. [Google Scholar] [CrossRef]
- Hodgins, B.; Pillet, S.; Landry, N.; Ward, B.J. Prime-pull vaccination with a plant-derived virus-like particle influenza vaccine elicits a broad immune response and protects aged mice from death and frailty after challenge. Immun. Ageing 2019, 16, 27. [Google Scholar] [CrossRef]
- Villagrana-Escareño, M.V.; Reynaga-Hernández, E.; Galicia-Cruz, O.G.; Durán-Meza, A.L.; De la Cruz-González, V.; Hernández-Carballo, C.Y.; Ruíz-García, J. VLPsDerived from the CCMV Plant Virus Can Directly Transfect and Deliver Heterologous Genes for Translation into Mammalian Cells. BioMed Res. Int. 2019, 2019, 4630891. [Google Scholar] [CrossRef]
- Wang, C.; Beiss, V.; Steinmetz, N.F. Cowpea Mosaic Virus Nanoparticles and Empty Virus-Like Particles Show Distinct but Overlapping Immunostimulatory Properties. J. Virol. 2019, 93, e00129-19. [Google Scholar] [CrossRef]
- D’Aoust, M.A.; Couture, M.M.; Charland, N.; Trepanier, S.; Landry, N.; Ors, F.; Vezina, L.P. The production of hemagglutinin-based virus-like particles in plants: A rapid, efficient and safe response to pandemic influenza. Plant Biotechnol. J. 2010, 8, 607–619. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, N.; Streatfield, S.J.; Yusibov, V. Virus-like particles as a highly efficient vaccine platform: Diversity of targets and production systems and advances in clinical development. Vaccine 2012, 31, 58–83. [Google Scholar] [CrossRef] [PubMed]
- Makarkov, A.I.; Golizeh, M.; Ruiz-Lancheros, E.; Gopal, A.A.; Costas-Cancelas, I.N.; Chierzi, S.; Pillet, S.; Charland, N.; Landry, N.; Rouiller, I.; et al. Plant-derived virus-like particle vaccines drive cross-presentation of influenza A hemagglutinin peptides by human monocyte-derived macrophages. Vaccines 2019, 4, 17. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Wang, H.; Deng, F. Advances and challenges in enveloped virus-like particles (VLP)-based vaccines. J. Immunol. Sci. 2018, 2, 36–41. [Google Scholar]
- Balke, I.; Zeltins, A. Use of plant viruses and virus-like particles for the creation of novel vaccines. Adv. Drug Deliv. Rev. 2019, 145, 119–129. [Google Scholar] [CrossRef]
- Bamogo, P.K.A.; Brugidou, C.; Sereme, D.; Tiendrebeogo, F.; Djigma, F.W.; Simpore, J.; Lacombe, S. Virus-based pharmaceutical production in plants: An opportunity to reduce health problems in Africa. Virol. J. 2019, 16, 167. [Google Scholar] [CrossRef]
- Syomin, B.V.; Ilyin, Y.V. Virus-Like Particles as an Instrument of Vaccine Production. Mol. Biol. 2019, 53, 323–334. [Google Scholar] [CrossRef]
- Lei, X.; Cai, X.; Yang, Y. Genetic engineering strategies for construction of multivalent chimeric VLPs vaccines. Expert Rev. Vaccines 2020, 19, 235–246. [Google Scholar] [CrossRef] [Green Version]
- Quan, F.S.; Basak, S.; Chu, K.B.; Kim, S.S.; Kang, S.M. Progress in the development of virus-like particle vaccines against respiratory viruses. Expert Rev. Vaccines 2020, 19, 11–24. [Google Scholar] [CrossRef]
- Zeltins, A. Viral nanoparticles: Principles of construction and characterization. In Viral Nanotechnology; Khudyakov, Y.E., Pumpens, P., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 93–119. [Google Scholar]
- Steele, J.F.C.; Peyret, H.; Saunders, K.; Castells-Graells, R.; Marsian, J.; Meschcheriakova, Y.; Lomonossoff, G.P. Synthetic plant virology for nanobiotechnology and nanomedicine. WIREs Nanomed. Nanobiotechnol. 2017, 9, e1447. [Google Scholar] [CrossRef] [Green Version]
- Eiben, S.; Koch, C.; Altintoprak, K.; Southan, A.; Tovar, G.; Laschat, S.; Weiss, I.M.; Wege, C. Plant virus-based materials for biomedical applications: Trends and prospects. Adv. Drug Deliv. Rev. 2019, 145, 96–118. [Google Scholar] [CrossRef]
- Ibrahim, A.; Odon, V.; Kormelink, R. Plant Viruses in Plant Molecular Pharming: Toward the Use of Enveloped Viruses. Front. Plant Sci. 2019, 10, 803. [Google Scholar] [CrossRef]
- Shoeb, E.; Hefferon, K. Future of cancer immunotherapy using plant virus-based nanoparticles. Future Sci. 2019, 5, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balke, I.; Zeltins, A. Recent Advances in the Use of Plant Virus-Like Particles as Vaccines. Viruses 2020, 12, 270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, R.M.; Chichester, J.A.; Mett, V.; Jaje, J.; Tottey, S.; Manceva, S.; Casta, L.J.; Gibbs, S.K.; Musiychuk, K.; Shamloul, M.; et al. A plant-produced Pfs25 VLP malaria vaccine candidate induces persistent transmission blocking antibodies against Plasmodium falciparum in immunized mice. PLoS ONE 2013, 8, e79538. [Google Scholar] [CrossRef] [PubMed]
- Chichester, J.A.; Green, B.J.; Jones, R.M.; Shoji, Y.; Miura, K.; Long, C.A.; Lee, C.K.; Ockenhouse, C.F.; Morin, M.J.; Streatfield, S.J.; et al. Safety and immunogenicity of a plant-produced Pfs25 virus-like particle as a transmission blocking vaccine against malaria: A Phase 1 dose-escalation study in healthy adults. Vaccines 2018, 36, 5865–5871. [Google Scholar] [CrossRef] [PubMed]
- Langley, J.; Pastural, E.; Halperin, S.; McNeil, S.; ElSherif, M.; MacKinnon-Cameron, D.; Ye, L.; Grange, C.; Thibodeau, V.; Cailhier, J.F.; et al. A Randomized Controlled Study to Evaluate the Safety and Reactogenicity of a Novel rVLP-Based Plant Virus Nanoparticle Adjuvant Combined with Seasonal Trivalent Influenza Vaccine Following Single Immunization in Healthy Adults 18–50 Years of Age. Vaccines 2020, 8, 393. [Google Scholar] [CrossRef]
- Duan, L.; Zheng, Q.; Zhang, H.; Niu, Y.; Lou, Y.; Wang, H. The SARS-CoV-2 Spike Glycoprotein Biosynthesis, Structure, Function, and Antigenicity: Implications for the Design of Spike-Based Vaccine Immunogens. Front. Immunol. 2020, 11, 576622. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, K.; Massoud, T.F.; Paulmurugan, R. SARS-CoV-2 Vaccine Development: An Overview and Perspectives. ACS Pharmacol. Transl. Sci. 2020, 3, 844–858. [Google Scholar] [CrossRef]
- Yang, J.; Wang, W.; Chen, Z.; Lu, S.; Yang, F.; Bi, Z.; Bao, L.; Mo, F.; Li, X.; Huang, Y.; et al. A vaccine targeting the RBD of the S protein of SARS-CoV-2 induces protective immunity. Nature 2020, 586, 572–577. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- Shang, J.; Ye, G.; Shi, K.; Wan, Y.; Luo, C.; Aihara, H.; Geng, Q.; Auerbach, A.; Li, F. Structural basis of receptor recognition by SARS-CoV-2. Nature 2020, 581, 221–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Capell, T.; Twyman, R.M.; Armario-Najera, V.; Ma, J.K.; Schillberg, S.; Christou, P. Potential Applications of Plant Biotechnology against SARS-CoV-2. Trends Plant Sci. 2020, 25, 635–643. [Google Scholar] [CrossRef]
- Lockhart, B.E.; Menke, J.; Dahal, G.; Olszewski, N.E. Characterization and genomic analysis of tobacco vein clearing virus, a plant pararetrovirus that is transmitted vertically and related to sequences integrated in the host genome. J. Gen. Virol. 2000, 81, 1579–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mette, M.F.; Kanno, T.; Aufsatz, W.; Jakowitsch, J.; van der Winden, J.; Matzke, M.A.; Matzke, A.J. Endogenous viral sequences and their potential contribution to heritable virus resistance in plants. EMBO J. 2002, 21, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Staginnus, C.; Richert-Poggeler, R. Endogenous pararetroviruses: Two faced travelers in the plant genome. Trends Plant Sci. 2006, 11, 1360–1385. [Google Scholar] [CrossRef] [PubMed]
- Chiba, S.; Kondo, H.; Tani, A.; Saisho, D.; Sakamoto, W.; Kanematsu, S.; Suzuki, N. Widespread Endogenization of Genome Sequences of Non-Retroviral RNA Viruses into Plant Genomes. PLoS Pathog. 2001, 7, e1002146. [Google Scholar] [CrossRef] [Green Version]
- Teycheney, P.Y.; Geering, A.D. Endogenous viral sequences in plant genomes. In Recent Advances in Plant Virology; Caranta, C., Aranda, M.A., Tepfer, M., Lopez-Moya, J.J., Eds.; Caister Academic Press: Norfolk, UK, 2011; pp. 343–362. ISBN 978-1-904455-75-2. [Google Scholar]
- Mushegian, A.R.; Elena, S.F. Evolution of plant virus movement proteins from the 30K superfamily and of their homologs integrated in plant genomes. Virology 2015, 476, 304–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, H.; Fukuhara, T.; Kitazawa, H.; Kormelink, R. Virus Latency and the Impact on Plants. Front. Microbiol. 2019, 19, 2764. [Google Scholar] [CrossRef] [PubMed]
- Harper, G.; Hull, R.; Lockhart, B.; Olszewski, N. Viral sequences integrated into plant genomes. Annu. Rev. Phytopathol. 2002, 40, 119–136. [Google Scholar] [CrossRef]
- Bertsch, C.; Beuve, M.; Dolja, V.V.; Wirth, M.; Pelsy, F.; Herrbach, E.; Lemaire, O. Retention of the virus-derived sequences in the nuclear genome of grapevine as a potential pathway to virus resistance. Biol. Direct 2009, 4, 21. [Google Scholar] [CrossRef] [Green Version]
- Feschotte, C.; Gilbert, C. Endogenous viruses: Insights into viral evolution and impact on host biology. Nat. Rev. Genet. 2012, 13, 283–296. [Google Scholar] [CrossRef] [Green Version]
- Geering, A.; Maumus, F.; Copetti, D.; Choise, N.; Zwickl, D.J.; Zytnicki, M.; McTaggart, A.R.; Scalabrin, S.; Vezzulli, S.; Wing, R.A.; et al. Endogenous florendoviruses are major components of plant genomes and hallmarks of virus evolution. Nat. Commum. 2014, 5, 5269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diop, S.I.; Geering, A.D.W.; Alfama-Depauw, F.; Loaec, M.; Teycheney, P.Y.; Maumus, F. Tracheophyte genomes keep track of the deep evolution of the Caulimoviridae. Sci. Rep. 2017, 8, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefeuvre, P.; Harkins, G.W.; Lett, J.M.; Briddon, R.W.; Chase, M.W.; Moury, B.; Martin, D.P. Evolutionary Time-Scale of the Begomoviruses: Evidence from Integrated Sequences in the Nicotiana Genome. PLoS ONE 2011, 6, e19193. [Google Scholar] [CrossRef] [PubMed]
- Werner, S.; Marillonnet, S.; Hause, G.; Klimyuk, V.; Gleba, Y. Immunoabsorbent nanoparticles based on a tobamovirus displaying protein A. Proc. Nat. Acad. Sci. USA 2006, 103, 17678–17683. [Google Scholar] [CrossRef] [Green Version]
- Werner, S.; Marillonnet, S.; Klimyuk, V.; Gleba, Y. Plant Viral Particles Comprising a Plurality of Fusion Proteins Consisting of a Plant Viral Coat Protein, a Peptide Linker and a Recombinant Protein and Use of Such Plant Viral Particles for Protein Purification. U.S. Patent No. US 2009/0062514 A1, 5 March 2009. [Google Scholar]
- Clamp, M.; Cuff, J.; Searle, S.M.; Barton, G.J. The Jalview Java alignment editor. Bioinformatics 2004, 20, 426–427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, J.; Anishchenko, I.; Park, H.; Peng, Z.; Ovchinnikov, S.; Baker, D. Improved protein structure prediction using predicted interresidue orientations. Proc. Nat. Acad. Sci. USA 2020, 117, 1496–1503. [Google Scholar] [CrossRef]
- Sayle, R.; Milner-White, E.J. RasMol: Biomolecular graphics for all. Trends Biochem. Sci. 1995, 20, 374. [Google Scholar] [CrossRef]
- Lowder, L.G.; Zhang, D.; Baltes, N.J.; Paul, J.W., III; Tang, X.; Zheng, X.; Voytas, D.F.; Hsieh, T.F.; Zhang, Y.; Qi, Y. A CRISPR/Cas9 Toolbox for Multiplexed Plant Genome Editing and Transcriptional Regulation. Plant Physiol. 2015, 169, 971–985. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Lowder, L.G.; Zhang, T.; Malzahn, A.A.; Zheng, X.; Voytas, D.F.; Zhong, Z.; Chen, Y.; Ren, Q.; Li, Q.; et al. A CRISPR-Cpf1 system for efficient genome editing and transcriptional repression in plants. Nat. Plants 2017, 3, 17018. [Google Scholar] [CrossRef] [PubMed]
- de Melo, B.P.; Lourenco-Tessutti, I.T.; Roca Paixao, J.F.; Noriega, D.D.; Mattar Silva, M.C.; de Almeida-Engler, J.; Batista Fontes, E.P.; Grossi-de-Sa, M.S. Transcriptional modulation of AREB-1 by CRISPRa improves plant physiological performance under severe water deficit. Sci. Rep. 2020, 10, 16231. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wu, X.; Markel, K.; Malzahn, A.A.; Kundagrami, N.; Stretenovic, S.; Zhang, Y.; Cheng, Y.; Shih, P.M.; Qi, Y. CRISPR-Act3.0 for highly efficient multiplexed gene activation in plants. Nat. Plants 2021, 7, 942–953. [Google Scholar] [CrossRef]
- Peyret, H. A protocol for the gentle purification of virus-like particles produced in plants. J. Virol. Methods 2015, 225, 59–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Zyl, A.R.; Hitzeroth, I.I. Purification of Virus-Like Particles (VLPs) from Plants. Methods Mol. Biol. 2016, 1404, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.; Wei, X.; Crooks, P.A.; Koprowski, H. Elimination of alkaloids from plant-derived human monoclonal antibody. J. Immunol. Methods 2004, 286, 79–85. [Google Scholar] [CrossRef]
- Buyel, J.F. Plant Molecular Farming-Integration and Exploitation of Side Streams to Achieve Sustainable Biomanufacturing. Front. Plant Sci. 2019, 9, 1893. [Google Scholar] [CrossRef]
- Shillberg, S.; Raven, N.; Spiegel, H.; Resche, S.; Buntru, M. Critical Analysis of the Commercial Potential of Plants for the Production of Recombinant Proteins. Front. Plant Sci. 2019, 10, 720. [Google Scholar] [CrossRef]
- Diamos, A.G.; Mason, H.S. High-level expression and enrichment of norovirus virus-like particles in plants using modified geminiviral vectors. Protein Expr. Purif. 2018, 151, 86–92. [Google Scholar] [CrossRef]
- Sheets, R.; Kang, H.N.; Meyer, H.; Knezevic, I. Who informal consultation on development of guidelines for assuring the quality s, efficacy of DNAv. WHO informal consultation on the guidelines for evaluation of the quality, safety, and efficacy of DNA vaccines, Geneva, Switzerland, December 2019. NPJ Vaccines 2020, 5, 52. [Google Scholar] [CrossRef] [PubMed]
- Gleba, Y.; Klimyuk, V.; Marillonnet, S. Magnifection—A new platform for expressing recombinant vaccines in plants. Vaccine 2005, 23, 2042–2048. [Google Scholar] [CrossRef]
- Klimyuk, V.; Pogue, G.; Herz, S.; Butler, J.; Haydon, H. Production of recombinant antigens and antibodies in Nicotiana benthamiana using ‘magnifection’ technology: GMP-compliant facilities for small- and large-scale manufacturing. Curr. Top. Microbiol. Immunol. 2014, 375, 127–154. [Google Scholar] [CrossRef] [PubMed]
- Cimica, V.; Galarza, J.M. Adjuvant formulations for virus-like particle (VLP) based vaccines. Clin. Immunol. 2017, 183, 99–108. [Google Scholar] [CrossRef]
- Woods, N.; Niwasabutra, K.; Acevedo, R.; Igoli, J.; Altwaijry, N.A.; Tusiimire, J.; Gray, A.I.; Watson, D.G.; Ferro, V.A. Natural Vaccine Adjuvants and Immunopotentiators Derived from Plants, Fungi, Marine Organisms, and Insects. In Immunopotentiators in Modern Vaccines, 2nd ed.; Academic Press: Cambridge, MA, USA, 2017; Volume 11, pp. 211–229. [Google Scholar] [CrossRef]
- Czyz, M.; Dembczynski, R.; Marecik, R.; Pniewski, T. Stability of S-HBsAg in long-term stored lyophilised plant tissue. Biologicals 2016, 44, 69–72. [Google Scholar] [CrossRef]
- Lua, L.H.; Connors, N.K.; Sainsbury, F.; Chuan, Y.P.; Wibowo, N.; Middelberg, A.P. Bioengineering virus-like particles as vaccines. Biotechnol. Bioeng. 2014, 111, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Onions, D.; Egan, W.; Jarrett, R.; Novicki, D.; Gregersen, J.P. Validation of the safety of MDCK cells as a substrate for the production of a cell-derived influenza vaccine. Biologicals 2010, 38, 544–551. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, I.; Stacey, G.; Petricciani, J.; Sheets, R. Substrates WHOSGoC. Evaluation of cell substrates for the production of biologicals: Revision of WHO recommendations. Report of the WHO Study Group on Cell Substrates for the Production of Biologicals, 22-23 April 2009, Bethesda, USA. Biologicals 2010, 38, 162–169. [Google Scholar] [CrossRef]
- Sprink, T.; Eriksson, D.; Schiemann, J.; Hartung, F. Regulatory hurdles for genome editing: Process- vs. product-based approaches in different regulatory contexts. Plant Cell Rep. 2016, 35, 1493–1506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EFSA Panel on Genetically Modified Organisms (EFSA GMO Panel); Naegeli, H.; Bresson, J.L.; Dalmay, T.; Dewhurst, I.C.; Epstein, M.M.; Firbank, L.G.; Guerche, P.; Hejatko, J.; Moreno, F.J.; et al. Applicability of the EFSA Opinion on site-directed nucleases type 3 for the safety assessment of plants developed using site-directed nucleases type 1 and 2 and oligonucleotide-directed mutagenesis. EFSA J. 2020, 18, e06299. [Google Scholar] [CrossRef]
- Turnbull, C.; Lillemo, M.; Hvoslef-Eide, T.A.K. Global Regulation of Genetically Modified Crops Amid the Gene Edited Crop Boom—A Review. Front. Plant Sci. 2021, 12, 630396. [Google Scholar] [CrossRef]
- Zhang, D.; Hussain, A.; Manghwar, H.; Xie, K.; Xie, S.; Zhao, S.; Larkin, R.M.; Qing, P.; Jin, S.; Ding, F. Genome editing with the CRISPR-Cas system: An art, ethics and global regulatory perspective. Plant Biotechnol. J. 2020, 18, 1651–1669. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citiulo, F.; Crosatti, C.; Cattivelli, L.; Biselli, C. Frontiers in the Standardization of the Plant Platform for High Scale Production of Vaccines. Plants 2021, 10, 1828. https://doi.org/10.3390/plants10091828
Citiulo F, Crosatti C, Cattivelli L, Biselli C. Frontiers in the Standardization of the Plant Platform for High Scale Production of Vaccines. Plants. 2021; 10(9):1828. https://doi.org/10.3390/plants10091828
Chicago/Turabian StyleCitiulo, Francesco, Cristina Crosatti, Luigi Cattivelli, and Chiara Biselli. 2021. "Frontiers in the Standardization of the Plant Platform for High Scale Production of Vaccines" Plants 10, no. 9: 1828. https://doi.org/10.3390/plants10091828
APA StyleCitiulo, F., Crosatti, C., Cattivelli, L., & Biselli, C. (2021). Frontiers in the Standardization of the Plant Platform for High Scale Production of Vaccines. Plants, 10(9), 1828. https://doi.org/10.3390/plants10091828







