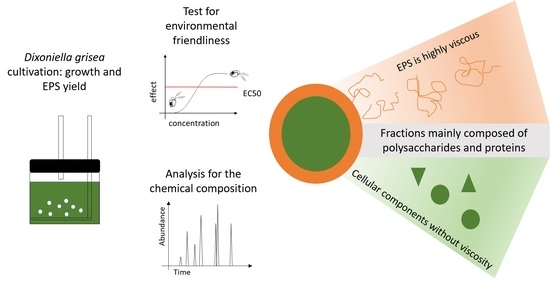
Potential of the Red Alga Dixoniella grisea for the Production of Additives for Lubricants
Abstract
:1. Introduction
2. Results
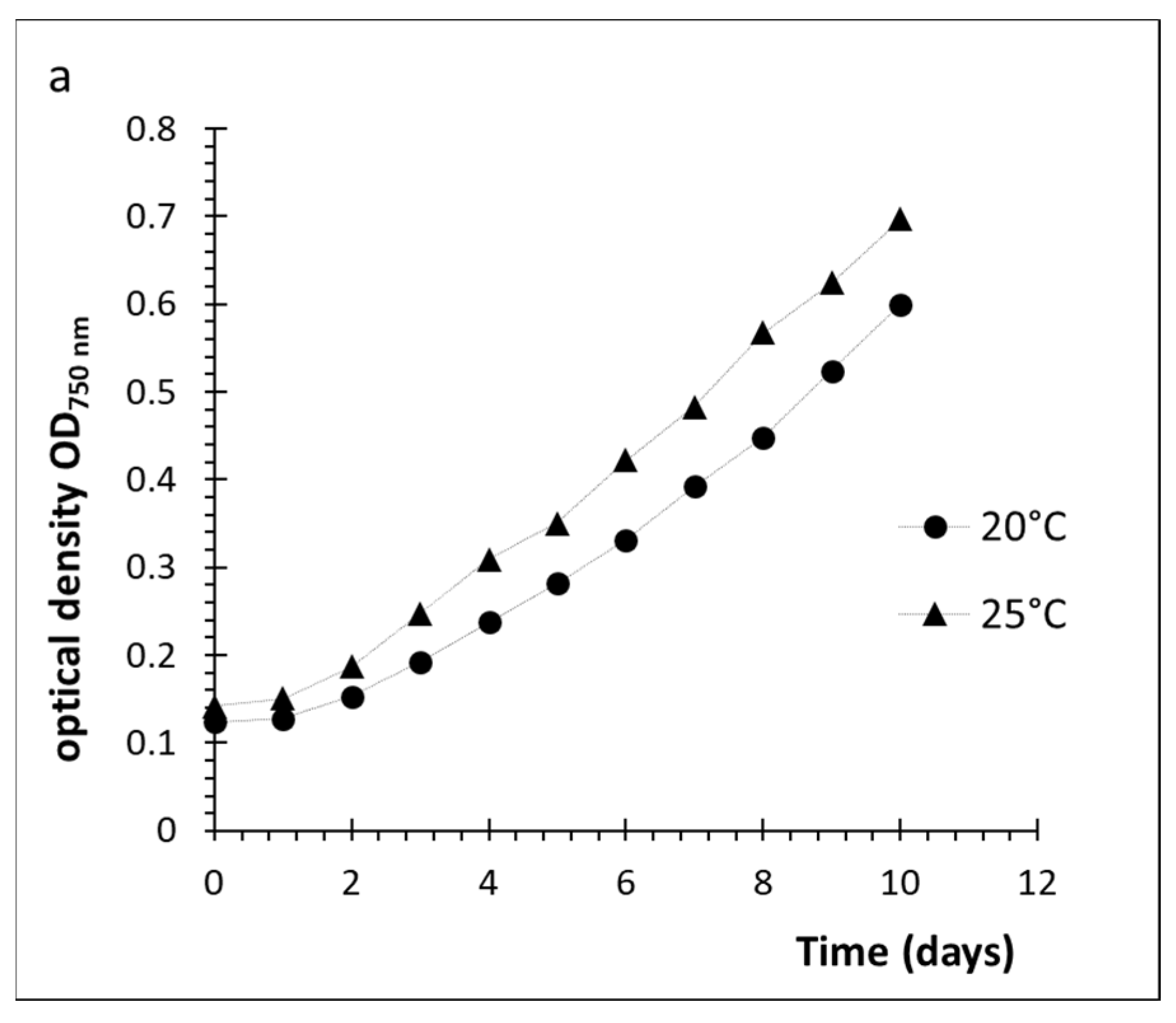
2.1. Cultivation of D. grisea UTEX 2320
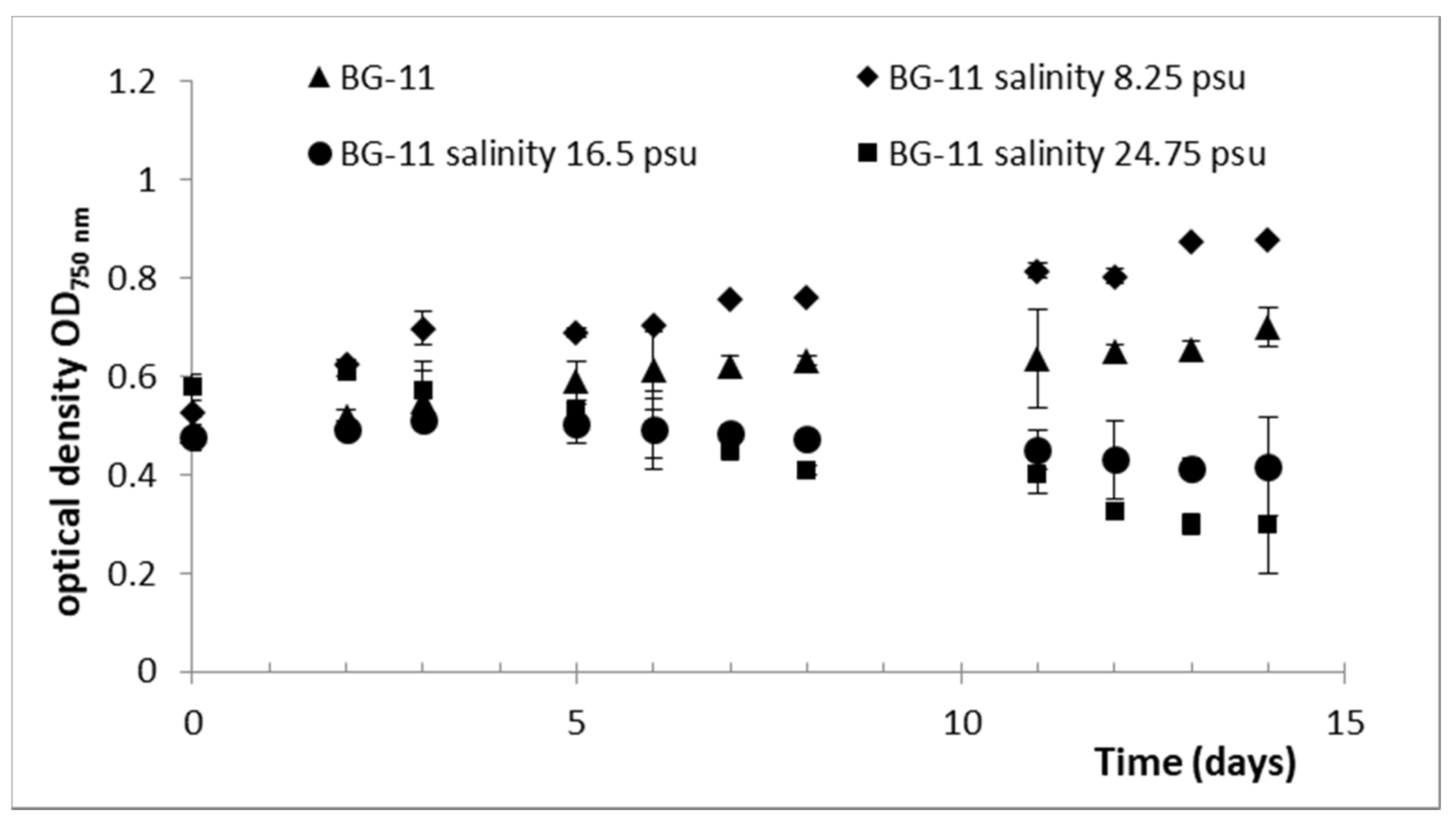
2.2. Effect of Culture Conditions and Harvesting Time
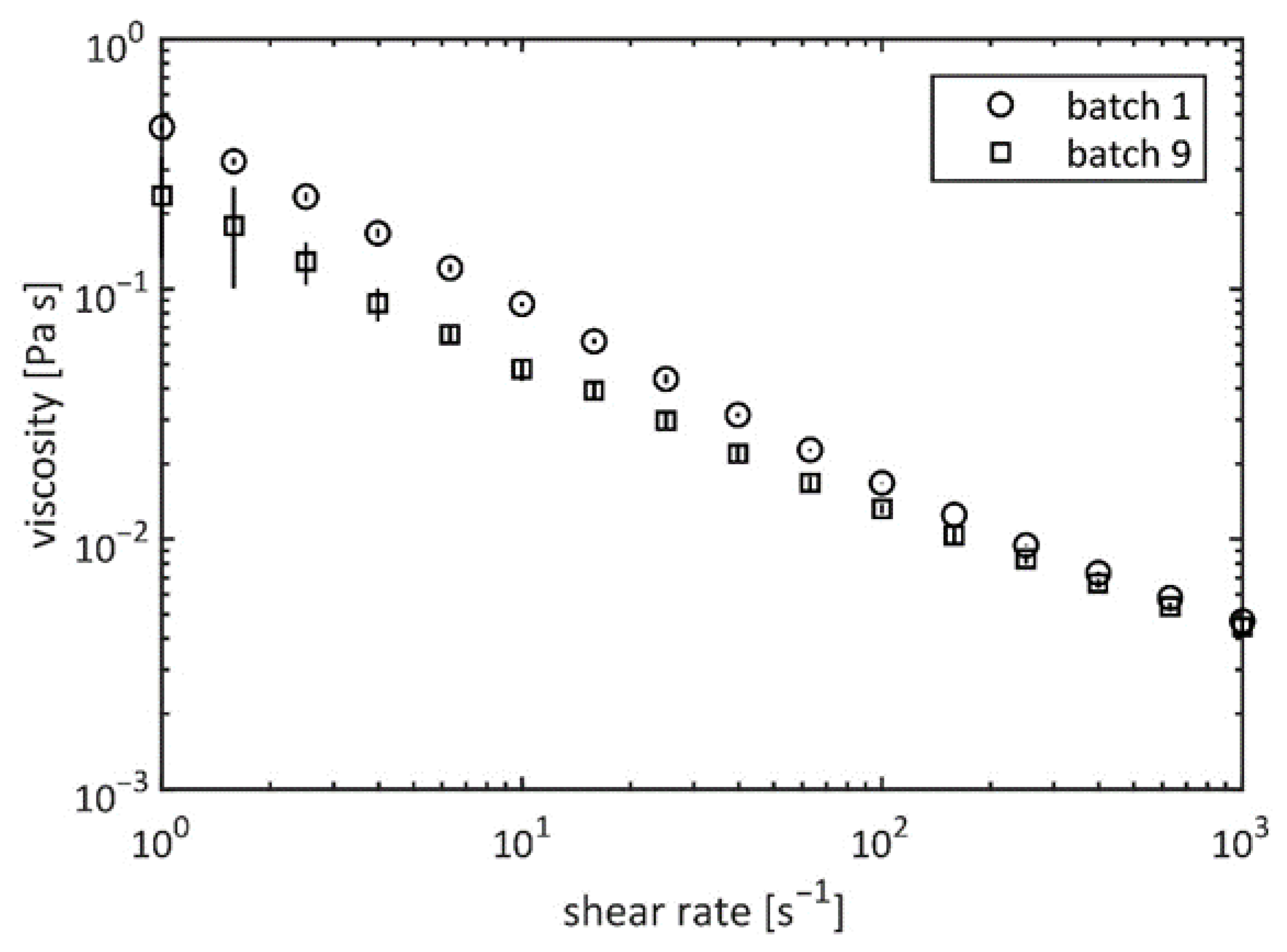
2.3. Viscosity
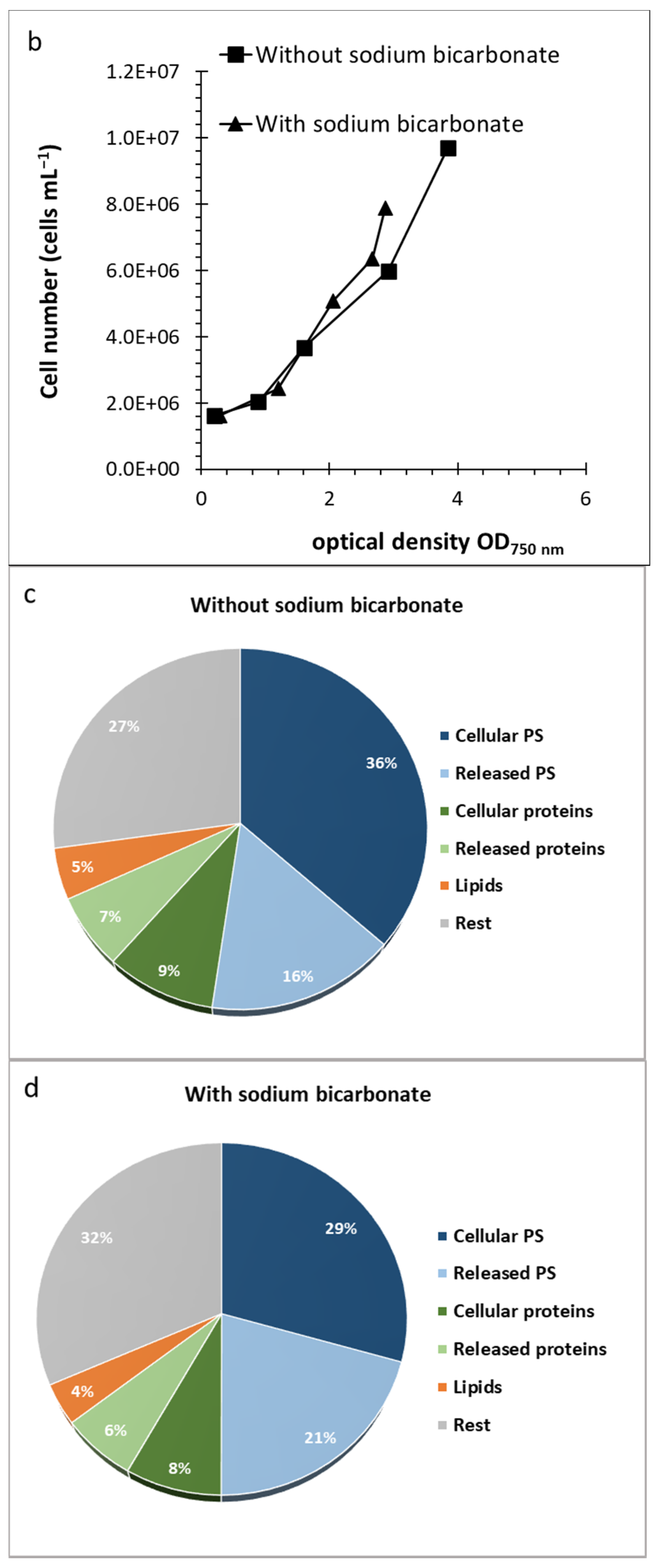
2.4. Composition of Algal Fractions
2.5. Ecotoxicological Effects of Algal Samples
3. Discussion
3.1. Challenges for Large-Scale Production of D. grisea
3.2. Molecular Composition of Dixoniella
3.3. Ecotoxicological Impact of D. grisea Fractions
3.4. Evaluation of Dixoniella for a Biorefinery Approach
4. Materials and Methods
4.1. Algae Growth Conditions
4.2. Sample Collection and Processing
4.2.1. Cell Growth and Cell Counts
4.2.2. Analysis of Polysaccharides in Medium and Cells after Centrifugation (Medium-C, Cells-C)
4.2.3. Analysis of Lipids in Cells after Centrifugation (Cells-C)
4.2.4. Analysis of Proteins in Medium and Cells after Centrifugation (Medium-C, Cells-C)
4.3. Viscosity
4.4. Analysis of Total Monosaccharides, Amino Acids, and Fatty Acids
4.4.1. Chemicals, Standards and Stocks
4.4.2. Extraction of D. grisea EPS and Cell Powder
4.4.3. Quantification of Monosaccharides and Fatty Acids by GC-MSD as TMSE Derivatives
4.4.4. Quantification of Proteins by HPLC-DAD as Their OPA and FMOC Derivatives
4.5. Eco-Toxicological Tests
4.5.1. Chemicals, Standards and Stocks
4.5.2. Test Systems and Organisms
4.5.3. Ecotoxicological Screening
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Vera, C.R.; Crespín, G.D.; Daranas, A.H.; Looga, S.M.; Lillsunde, K.E.; Tammela, P.; Perälä, M.; Hongisto, V.; Virtanen, J.; Rischer, H.; et al. Marine Microalgae: Promising source for new bioactive compounds. Mar. Drugs 2018, 16, 317. [Google Scholar] [CrossRef] [Green Version]
- Aslam, A.; Fazal, T.; Zaman, Q.; uz Shan, A.; Rehman, F.; Iqbal, J.; Rashid, N.; Ur Rehman, M.S. Biorefinery of Microalgae for Nonfuel Products. Microalgae Cultiv. Biofuels Prod. 2020, 197–209. [Google Scholar] [CrossRef]
- Chu, W.L.; Phang, S.M. Bioactive Compounds from Microalgae and Their Potential Applications as Pharmaceuticals and Nutraceuticals. In Grand Challenges in Algae Biotechnology; Hallmann, A., Rampelotto, P.H., Eds.; Springer Nature: Cham, Switzerland, 2019; pp. 429–469. [Google Scholar]
- European Union. Blue Bioeconomy Last Update: 2018WWW.EUMOFA.EU Situation Report and Perspectives; European Union: Luxembourg, 2018; ISBN 978-92-79-96713-9. [Google Scholar] [CrossRef]
- Quinchia, L.A.; Delgado, M.A.; Valencia, C.; Franco, J.M.; Gallegos, C. Natural and synthetic antioxidant additives for improving the performance of new biolubricant formulations. J. Agric. Food Chem. 2011, 59, 12917–12924. [Google Scholar] [CrossRef] [PubMed]
- Vijayendran, B.; Randall, M.; Schmid, E. Algal Oil Based Bio-Lubricants. U.S. Patent 9.458.407 B2, 4 October 2016. [Google Scholar]
- Koch, T.; Gläbe, R.; Sakka, Y.; Nentwig, N.; Filser, J.; Siol, A.; Köser, J.; Thöming, J.; Mesing, S.; Larek, R.; et al. Alternative additives for lubricants based on microalgae. Tribol. Schmier. 2020, 67, 41–46. [Google Scholar] [CrossRef]
- Ahmed, S.N.; Nassar, A.M. Lubricating Oil Additives. In Tribology-Lubricants and Lubrication; IntechOpen: London, UK, 2011; pp. 249–268. [Google Scholar] [CrossRef] [Green Version]
- Barcelos, M.C.S.; Vespermann, K.A.C.; Pelissari, F.M.; Molina, G. Current status of biotechnological production and applications of microbial exopolysaccharides. Crit. Rev. Food Sci. Nutr. 2020, 60, 1475–1495. [Google Scholar] [CrossRef] [PubMed]
- Rhein-Knudsen, N.; Ale, M.T.; Meyer, A.S. Seaweed Hydrocolloid Production: An Update on Enzyme Assisted Extraction and Modification Technologies. Mar. Drugs 2015, 13, 3340–3359. [Google Scholar] [CrossRef]
- Viana, A.G.; Noseda, M.D.; Duarte, M.E.R.; Cerezo, A.S. Alkali modification of carrageenans. Part V. The iota–nu hybrid carrageenan from Eucheuma denticulatum and its cyclization. Carbohydr. Polym. 2004, 58, 455–460. [Google Scholar] [CrossRef]
- Cicinskas, E.; Kalitnik, A.A.; Karetin, Y.A.; Saravana, M.; Mohan, G.; Achary, A.; Kravchenko, A.O. Immunomodulating Properties of Carrageenan from Tichocarpus crinitus. Inflammation 2020, 43, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Ale, M.T.; Mikkelsen, J.D.; Meyer, A.S. Important determinants for fucoidan bioactivity: A critical review of structure-function relations and extraction methods for fucose-containing sulfated polysaccharides from brown seaweeds. Mar. Drugs 2011, 9, 2106–2130. [Google Scholar] [CrossRef] [Green Version]
- Borah, D.; Nainamalai, S.; Gopalakrishnan, S.; Rout, J.; Alharbi, N.S.; Alharbi, S.A.; Nooruddin, T. Biolubricant potential of exopolysaccharides from the cyanobacterium Cyanothece epiphytica. Appl. Microbiol. Biotechnol. 2018, 102, 3635–3647. [Google Scholar] [CrossRef]
- Gasljevic, K.; Hall, K.; Chapman, D.; Matthys, E.F. Drag-reducing polysaccharides from marine microalgae: Species productivity and drag reduction effectiveness. J. Appl. Phycol. 2008, 20, 299–310. [Google Scholar] [CrossRef]
- Spinelli, L.S.; Aquino, A.S.; Lucas, E.; d’Almeida, A.R.; Leal, R.; Martins, A.L. Adsorption of Polymers Used in Drilling Fluids on the Inner Surfaces of Carbon Steel Pipes Luciana. Polym. Eng. Sci. 2008, 1886–1891. [Google Scholar] [CrossRef]
- Wijffels, R.H.; Barbosa, M.J.; Eppink, M.H.M. Microalgae for the production of bulk chemicals and biofuels. Biofuels Bioprod. Biorefining 2010, 4, 287–295. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Yoo, C.; Jun, S.; Ahn, C.; Oh, H. Bioresource Technology Comparison of several methods for effective lipid extraction from microalgae. Bioresour. Technol. 2010, 101, S75–S77. [Google Scholar] [CrossRef] [PubMed]
- Bastiaens, L.; Van Roy, S.; Thomassen, G.; Elst, K. Biorefinery of algae: Technical and economic considerations. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Munoz, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 327–345. ISBN 9780081010273. [Google Scholar]
- Netanel Liberman, G.; Ochbaum, G.; Mejubovsky-Mikhelis, M.; Bitton, R.; Arad, S.M. Physico-chemical characteristics of the sulfated polysaccharides of the red microalgae Dixoniella grisea and Porphyridium aerugineum. Int. J. Biol. Macromol. 2020, 145, 1171–1179. [Google Scholar] [CrossRef] [PubMed]
- Xiao, R.; Zheng, Y. Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol. Adv. 2016, 34, 1225–1244. [Google Scholar] [CrossRef]
- Kumar, D.; Kaštánek, P.; Adhikary, S.P. Exopolysaccharides from cyanobacteria and microalgae and their commercial application. Curr. Sci. 2018, 115, 234–241. [Google Scholar] [CrossRef]
- Keeling, P.J. Diversity and evolutionary history of plastids and their hosts. Am. J. Bot. 2004, 91, 1481–1493. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paul, A.K.; Borugadda, V.B.; Goud, V.V. In-Situ Epoxidation of Waste Cooking Oil and Its Methyl Esters for Lubricant Applications: Characterization and Rheology. Lubricants 2021, 9, 27. [Google Scholar] [CrossRef]
- Arad, S.; Rapoport, L.; Moshkovich, A.; Van Moppes, D.; Karpasas, M.; Golan, R.; Golan, Y. Superior biolubricant from a species of red microalga. Langmuir 2006, 22, 7313–7317. [Google Scholar] [CrossRef]
- Lin, W.; Mashiah, R.; Seror, J.; Kadar, A.; Dolkart, O.; Pritsch, T.; Goldberg, R.; Klein, J. Lipid-hyaluronan synergy strongly reduces intrasynovial tissue boundary friction. Acta Biomater. 2019, 83, 314–321. [Google Scholar] [CrossRef]
- Eteshola, E.; Karpasas, M.; Arad, S.M.; Gottlieb, M. Red microalga exopolysaccharides: 2. Study of the rheology, morphology and thermal gelation of aqueous preparations. Acta Polym. 1998, 49, 549–556. [Google Scholar] [CrossRef]
- Singh, A.; Corvelli, M.; Unterman, S.A.; Wepasnick, K.A.; Mcdonnell, P.; Elissee, J.H. Enhanced lubrication on tissue and biomaterial surfaces through peptide-mediated binding of hyaluronic acid. Nat. Mater. 2014, 13, 988–995. [Google Scholar] [CrossRef] [PubMed]
- Arad, S.M.; van Moppes, D. Novel Sulfated Polysaccharides of Red Microalgae: Basics and Applications. Handb. Microalgal Cult. Appl. Phycol. Biotechnol. 2013, 406–416. [Google Scholar] [CrossRef]
- Raposo, M.F.D.J.; De Morais, A.M.M.B.; De Morais, R.M.S.C. Influence of sulphate on the composition and antibacterial and antiviral properties of the exopolysaccharide from Porphyridium cruentum. Life Sci. 2014, 101, 56–63. [Google Scholar] [CrossRef]
- Singh, S.; Kate, B.N.; Banecjee, U.C. Bioactive compounds from cyanobacteria and microalgae: An overview. Crit. Rev. Biotechnol. 2005, 25, 73–95. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Patidar, S.K. Microalgae harvesting techniques: A review. J. Environ. Manag. 2018, 217, 499–508. [Google Scholar] [CrossRef]
- Arad, S.; Levy-Ontman, O. Red microalgal cell-wall polysaccharides: Biotechnological aspects. Curr. Opin. Biotechnol. 2010, 21, 358–364. [Google Scholar] [CrossRef]
- Li, S.-Y.; Shabtai, Y.; Arad, S.M. Production and composition of the sulphated cell wall polysaccharide of Porphyridium (Rhodophyta) as affected by CO2 concentration. Phycologia 2000, 39, 332–336. [Google Scholar] [CrossRef]
- Arad, S.M.; Lerental, Y.B.; Dubinsky, O. Effect of nitrate and sulfate starvation on polysaccharide formation in Rhodella reticulata. Bioresour. Technol. 1992, 42, 141–148. [Google Scholar] [CrossRef]
- Dubinsky, O.; Simon, B.; Karamanos, Y.; Geresh, S.; Barak, Z.; Arad, S.M. Composition of the cell-wall polysaccharide produced by the unicellular red alga Rhodella reticulata. Plant Physiol. Biochem. 1992, 30, 409–414. [Google Scholar]
- Capek, P.; Matulová, M.; Combourieu, B. The extracellular proteoglycan produced by Rhodella grisea. Int. J. Biol. Macromol. 2008, 43, 390–393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shrestha, R.P.; Weinstein, Y.; Bar-Zvi, D.; Arad, S. A glycoprotein noncovalently associated with cell-wall polysaccharide of the red microalga Porphyridium sp. (Rhodophyta). J. Phycol. 2004, 40, 568–580. [Google Scholar] [CrossRef]
- Solymosi, K.; Böddi, B. Optical properties of bud scales and protochlorophyll(ide) forms in leaf primordia of closed and opened buds. Tree Physiol. 2006, 26, 1075–1085. [Google Scholar] [CrossRef] [PubMed]
- Heaney-Kieras, J.; Chapman, D. Structural studies on the extracellular polysaccharide of the red alga Porphyridium cruentum. Carbohydr. Res. 1976, 52, 169–177. [Google Scholar] [CrossRef]
- Luyten, H.; Vereijken, J.; Buecking, M. Using Proteins as Additives in Foods: An Introduction. In Proteins in Food Processing; Yada, R.Y., Ed.; Woodhead Publishing Limited: Cambridge, UK, 2004; ISBN 9781855737235. [Google Scholar]
- Minami, I.; Mori, S.; Isogai, Y.; Hiyoshi, S.; Inayama, T.; Nakayama, S. Molecular Design of Environmentally Adapted Lubricants: Antiwear Additives Derived from Natural Amino Acids. Tribol. Trans. 2010, 53, 713–721. [Google Scholar] [CrossRef]
- Lee, S.; Røn, T.; Pakkanen, K.I.; Linder, M. Hydrophobins as aqueous lubricant additive for a soft sliding contact. Colloids Surf. B Biointerfaces 2015, 125, 264–269. [Google Scholar] [CrossRef] [Green Version]
- Karmakar, G.; Ghosh, P. Green additives for lubricating oil. ACS Sustain. Chem. Eng. 2013, 1, 1364–1370. [Google Scholar] [CrossRef]
- Novoveská, L.; Ross, M.E.; Stanley, M.S.; Pradelles, R.; Wasiolek, V.; Sassi, J.-F. Microalgal Carotenoids: A Review of Production, Current Markets, Regulations, and Future Direction. Mar. Drugs 2019, 17, 640. [Google Scholar] [CrossRef] [Green Version]
- Saini, D.K.; Pabbi, S.; Shukla, P. Cyanobacterial pigments: Perspectives and biotechnological approaches. Food Chem. Toxicol. 2018, 120, 616–624. [Google Scholar] [CrossRef]
- Yuliarita, E.; Zulys, A. Utilization of natural compounds (chlorophyll and carotene extracts) as an octane-boosting additive in gasoline. IOP Conf. Ser. Mater. Sci. Eng. 2019, 496, 012048. [Google Scholar] [CrossRef] [Green Version]
- Carmichael, W.W. Algal toxins. Adv. Bot. Res. 1986, 12, 47–101. [Google Scholar]
- Chen, B.; You, W.; Huang, J.; Yu, Y.; Chen, W. Isolation and antioxidant property of the extracellular polysaccharide from Rhodella reticulata. World J. Microbiol. Biotechnol. 2010, 26, 833–840. [Google Scholar] [CrossRef]
- Najdenski, H.M.; Gigova, L.G.; Iliev, I.I.; Pilarski, P.S.; Lukavský, J.; Tsvetkova, I.V.; Ninova, M.S.; Kussovski, V.K. Antibacterial and antifungal activities of selected microalgae and cyanobacteria. Int. J. Food Sci. Technol. 2013, 48, 1533–1540. [Google Scholar] [CrossRef]
- Talyshinsky, M.M.; Souprun, Y.Y.; Huleihel, M.M. Anti-viral activity of red microalgal polysaccharides against retroviruses. Cancer Cell Int. 2002, 2, 14–17. [Google Scholar] [CrossRef] [Green Version]
- Toshkova, R.; Gardeva, E.; Minkova, K.; Panova, T.; Zvetkova, E. Immunopotential and mitogenic properties of marine microalgal polysaccharides extracted from Porphyridium cruentum and Dixoniella grisea (Rhodophyta). Comptes Rendus L’Academie Bulg. Sci. 2009, 62, 589–594. [Google Scholar]
- Khattar, J.I.S.; Singh, D.P.; Jindal, N.; Kaur, N.; Singh, Y.; Rahi, P.; Gulati, A. Isolation and characterization of exopolysaccharides produced by the cyanobacterium Limnothrix redekei PUPCCC 116. Appl. Biochem. Biotechnol. 2010, 162, 1327–1338. [Google Scholar] [CrossRef]
- Eggert, A.; Raimund, S.; Michalik, D.; West, J.; Karsten, U. Ecophysiological performance of the primitive red alga Dixoniella grisea (Rhodellophyceae) to irradiance, temperature and salinity stress: Growth responses and the osmotic role of mannitol. Phycologia 2007, 46, 22–28. [Google Scholar] [CrossRef]
- Geitler, L. Beitrage zur epiphytischen Algenflora des Neusiedler Sees. Osterr. Bot. Ztg. 1970, 118, 17–29. [Google Scholar] [CrossRef]
- Deason, T.R.; Butler, G.L.; Rhyne, C. Rhodella reticulata sp. nov.: A new coccoid rhodophytan alga (Porphyridiales). J. Phycol. 1983, 19, 104–111. [Google Scholar] [CrossRef]
- Fresnel, J.; Billard, C.; Hindak, F.; Pekárková, B. New observations on Porphyridium griseum Geitler=Rhodella grisea (Geitler) Comb-Nova (Porphyridiales, Rhodophyceae). Plant Syst. Evol. 1989, 164, 253–262. [Google Scholar] [CrossRef]
- Frank, M.P.; Powers, R.W. Simple and rapid quantitative high-performance liquid chromatographic analysis of plasma amino acids. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007, 852, 646–649. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sundaram Palaniswamy, A.M. Determination of Amino Acid Composition of Cell Culture Media and Protein Hydrosylate Standard; Agilent Technologies, Inc.: Santa Clara, CA, USA, 2017; p. 10. [Google Scholar]
- Medeiros, P.M.; Simoneit, B.R.T. Analysis of sugars in environmental samples by gas chromatography-mass spectrometry. J. Chromatogr. A 2007, 1141, 271–278. [Google Scholar] [CrossRef]
- Becker, M.; Liebner, F.; Rosenau, T.; Potthast, A. Ethoximtion-silylation approach for mono- and disaccharide analysis and characterization of their identification parameters by GC/MS. Talanta 2013, 115, 642–651. [Google Scholar] [CrossRef]
- Xia, Y.G.; Sun, H.M.; Wang, T.L.; Liang, J.; Yang, B.Y.; Kuang, H.X. A modified GC-MS analytical procedure for separation and detection of multiple classes of carbohydrates. Molecules 2018, 23, 1284. [Google Scholar] [CrossRef] [Green Version]
- Bernaerts, T.M.M.; Kyomugasho, C.; Van Looveren, N.; Gheysen, L.; Foubert, I.; Hendrickx, M.E.; Van Loey, A.M. Molecular and rheological characterization of different cell wall fractions of Porphyridium cruentum. Carbohydr. Polym. 2018, 195, 542–550. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Chen, C.; Zhao, S.; Sun, M.; Gao, Z.; Wu, H.; Fan, J. Efficient accumulation of high-value bioactive substances by carbon to nitrogen ratio regulation in marine microalgae Porphyridium purpureum. Bioresour. Technol. 2020, 309, 123362. [Google Scholar] [CrossRef]
- Cohen, E.; Arad, S.M. A closed system for outdoor cultivation of Porphyridium. Biomass 1989, 18, 59–67. [Google Scholar] [CrossRef]
- Fernández, F.G.A.; Reis, A.; Wijffels, R.H.; Barbosa, M.; Verdelho, V.; Llamas, B. The role of microalgae in the bioeconomy. New Biotechnol. 2021, 61, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Christenson, L.; Sims, R. Production and harvesting of microalgae for wastewater treatment, biofuels, and bioproducts. Biotechnol. Adv. 2011, 29, 686–702. [Google Scholar] [CrossRef]
- Muylaert, K.; Bastiaens, L.; Vandamme, D.; Gouveia, L. Harvesting of microalgae: Overview of process options and their strengths and drawbacks. In Microalgae-Based Biofuels and Bioproducts: From Feedstock Cultivation to End-Products; Gonzalez-Fernandez, C., Munoz, R., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 9780081010273. [Google Scholar]
- Pierre, G.; Delattre, C.; Dubessay, P.; Jubeau, S.; Vialleix, C.; Cadoret, J.P.; Probert, I.; Michaud, P. What is in store for EPS microalgae in the next decade? Molecules 2019, 24, 4296. [Google Scholar] [CrossRef] [Green Version]
- Araujo, G.S.; Matos, L.J.B.L.; Fernandes, J.O.; Cartaxo, S.J.M.; Gonçalves, L.R.B.; Fernandes, F.A.N.; Farias, W.R.L. Extraction of lipids from microalgae by ultrasound application: Prospection of the optimal extraction method. Ultrason. Sonochem. 2013, 20, 95–98. [Google Scholar] [CrossRef] [PubMed]
- Van Wychen, S.; Laurens, L.M.L. Determination of Total Carbohydrates in Algal Biomass-Laboratory Analytical Procedure (LAP); National Renewable Energy Lab(NREL): Golden, CO, USA, 2015. [Google Scholar]
- Mathimani, T.; Mallick, N. A comprehensive review on harvesting of microalgae for biodiesel–Key challenges and future directions. Renew. Sustain. Energy Rev. 2018, 91, 1103–1120. [Google Scholar] [CrossRef]
- Niemi, C.; Lage, S.; Gentili, F.G. Comparisons of analysis of fatty acid methyl ester (FAME) of microalgae by chromatographic techniques. Algal Res. 2019, 39, 101449. [Google Scholar] [CrossRef]
- Gaignard, C.; Gargouch, N.; Dubessay, P.; Delattre, C.; Pierre, G.; Laroche, C.; Fendri, I.; Abdelkafi, S.; Michaud, P. New horizons in culture and valorization of red microalgae. Biotechnol. Adv. 2019, 37, 193–222. [Google Scholar] [CrossRef] [PubMed]
- Gaignard, C.; Laroche, C.; Pierre, G.; Dubessay, P.; Delattre, C.; Gardarin, C.; Gourvil, P.; Probert, I.; Dubuffet, A.; Michaud, P. Screening of marine microalgae: Investigation of new exopolysaccharide producers. Algal Res. 2019, 44. [Google Scholar] [CrossRef]
- Aguilera, A.; Souza-Egipsy, V.; San Martín-Úriz, P.; Amils, R. Extraction of extracellular polymeric substances from extreme acidic microbial biofilms. Appl. Microbiol. Biotechnol. 2008, 78, 1079–1088. [Google Scholar] [CrossRef] [PubMed]
- Paidi, M.K.; Agarwal, P.; More, P.; Agarwal, P.K. Chemical Derivatization of Metabolite Mass Profiling of the Recretohalophyte Aeluropus lagopoides Revealing Salt Stress Tolerance Mechanism. Mar. Biotechnol. 2017, 19, 207–218. [Google Scholar] [CrossRef]
- Ivanova, J.G.; Kabaivanova, L.V.; Petkov, G.D. Temperature and Irradiance Effects on Rhodella reticulata Growth and Biochemical Characteristics. Russ. J. Plant Physiol. 2015, 62, 690–695. [Google Scholar] [CrossRef]
- Soanen, N.; Da Silva, E.; Gardarin, C.; Michaud, P.; Laroche, C. Improvement of exopolysaccharide production by Porphyridium marinum. Bioresour. Technol. 2016, 213, 231–238. [Google Scholar] [CrossRef]
- Patel, A.K.; Laroche, C.; Marcati, A.; Ursu, A.V.; Jubeau, S.; Marchal, L.; Petit, E.; Djelveh, G.; Michaud, P. Separation and fractionation of exopolysaccharides from Porphyridium cruentum. Bioresour. Technol. 2013, 145, 345–350. [Google Scholar] [CrossRef]
- Villay, A.; Laroche, C.; Roriz, D.; El Alaoui, H.; Delbac, F.; Michaud, P. Optimisation of culture parameters for exopolysaccharides production by the microalga Rhodella violacea. Bioresour. Technol. 2013, 146, 732–735. [Google Scholar] [CrossRef] [PubMed]
- Adda, M.; Merchuk, J.C.; Malis, S. Effect of Nitrate on Growth and production of Cell wall polysaccharide by the unicellular red alga Porphyridium. Biomas 1986, 10, 131–140. [Google Scholar] [CrossRef]
- Ikawa, M. Algal polyunsaturated fatty acids and effects on plankton ecology and other organisms. UNH Cent. Freshwat. Biol. Res 2004, 6, 17–44. [Google Scholar]
- McCracken, M.D.; Middaugh, R.E.; Middaugh, R.S. A chemical characterization of an algal inhibitor obtained from chlamydomonas. Hydrobiologia 1980, 70, 271–276. [Google Scholar] [CrossRef]
- Bosma, R.; Miazek, K.; Willemsen, S.M.; Vermuë, M.H.; Wijffels, R.H. Growth inhibition of Monodus subterraneus by free fatty acids. Biotechnol. Bioeng. 2008, 101, 1108–1114. [Google Scholar] [CrossRef]
- McKee, M.S.; Köser, J.; Focke, O.; Filser, J. A new test system for unraveling the effects of soil components on the uptake and toxicity of silver nanoparticles (NM-300K) in simulated pore water. Sci. Total Environ. 2019, 673, 613–621. [Google Scholar] [CrossRef]
- Yogarajalakshmi, P.; Venugopal Poonguzhali, T.; Ganesan, R.; Karthi, S.; Senthil-Nathan, S.; Krutmuang, P.; Radhakrishnan, N.; Mohammad, F.; Kim, T.J.; Vasantha-Srinivasan, P. Toxicological screening of marine red algae Champia parvula (C. Agardh) against the dengue mosquito vector Aedes aegypti (Linn.) and its non-toxicity against three beneficial aquatic predators. Aquat. Toxicol. 2020, 222, 105474. [Google Scholar] [CrossRef]
- Echa. Guidance on Information Requirements and Chemical Safety Assessment Chapter R.7b: Endpoint Specific Guidance November 2012; European Chemical Agency: Helsinki, Finland, 2012; Volume 2012, ISBN 9789292447618. [Google Scholar] [CrossRef]
- Guihéneuf, F.; Stengel, D.B. Towards the biorefinery concept: Interaction of light, temperature and nitrogen for optimizing the co-production of high-value compounds in Porphyridium purpureum. Algal Res. 2015, 10, 152–163. [Google Scholar] [CrossRef]
- Silva, M.B.F.; Azero, E.G.; Teixeira, C.M.L.L.; Andrade, C.T. Influence of culture conditions on the production of extracellular polymeric substances (EPS) by Arthrospira platensis. Bioresour. Bioprocess. 2020, 7, 47. [Google Scholar] [CrossRef]
- Aussant, J.; Guihéneuf, F.; Stengel, D.B. Impact of temperature on fatty acid composition and nutritional value in eight species of microalgae. Appl. Microbiol. Biotechnol. 2018, 102, 5279–5297. [Google Scholar] [CrossRef]
- Li, S.; Ji, L.; Shi, Q.; Wu, H.; Fan, J. Advances in the production of bioactive substances from marine unicellular microalgae Porphyridium spp. Bioresour. Technol. 2019, 292, 122048. [Google Scholar] [CrossRef] [PubMed]
- Gujar, A.; Cui, H.; Ji, C.; Kubar, S.M.; Li, R. Optimizing culture system to promote cell growth and optimizing culture system to promote cell growth and polysaccharides contents of Porphyridium cruentum. Fresenius Environ. Bull. 2020, 29, 6738–6747. [Google Scholar]
- Liu, L.; Pohnert, G.; Wei, D. Extracellular metabolites from industrial microalgae and their biotechnological potential. Mar. Drugs 2016, 14, 191. [Google Scholar] [CrossRef]
- Gilbert-lópez, B.; Mendiola, J.A.; Fontecha, J.; van den Broek, L.A.M.; Sijtsma, L.; Cifuentes, A.; Herrero, M.; Ibáñez, E. Downstream processing of Isochrysis galbana: A step towards microalgal biorefinery. Green Chem. 2015, 17, 4599–4609. [Google Scholar] [CrossRef] [Green Version]
- Rippka, R.; Deruelles, J.; Waterbury, J.B.; Herdman, M.; Stanier, R.Y. Generic assignments, strain histories and properties of pure cultures of cyanobacteria. J. Gen. Microbiol. 1979, 111, 1–61. [Google Scholar] [CrossRef] [Green Version]
- Van Wychen, S.; Laurens, L.M.L. Determination of Total Solids and Ash in Algal Biomass: Laboratory Analytical Procedure (LAP); Tech. Rep. NREL/TP-5100-60957; National Renewable Energy Lab (NREL): Golden, CO, USA, 2015. [Google Scholar]
- loropZavřel, T.; Červený, J.; Sinetova, M.A. Measurement of Chhyll. Bio-Protocol 2015, 5, 1–5. [Google Scholar]
- Zavřel, T.; Očenášová, P.; Sinetova, M.; Červený, J. Determination of Storage (Starch/Glycogen) and Total Saccharides Content in Algae and Cyanobacteria by a Phenol-Sulfuric Acid Method. Bio-Protocol 2018, 8, e2966. [Google Scholar] [CrossRef]
- Byreddy, A.R.; Gupta, A.; Barrow, C.J.; Puri, M. A quick colorimetric method for total lipid quantification in microalgae. J. Microbiol. Methods 2016, 125, 28–32. [Google Scholar] [CrossRef]
- Lowry, O.H.; Rosebrough, N.J.; Farr, L.A.; Randall, R.J. Protein measurement with the folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Delattre, C.; Pierre, G.; Laroche, C.; Michaud, P. Production, extraction and characterization of microalgal and cyanobacterial exopolysaccharides. Biotechnol. Adv. 2016, 34, 1159–1179. [Google Scholar] [CrossRef]
- El Jay, A. Effects of organic solvents and solvent-atrazine interactions on two algae, Chlorella vulgaris and Selenastrum capricornutum. Arch. Environ. Contam. Toxicol. 1996, 31, 84–90. [Google Scholar] [CrossRef]
- Stratton, G.W. The influence of solvent type on solvent-pesticide interactions in bioassays. Arch. Environ. Contam. Toxicol. 1985, 14, 651–658. [Google Scholar] [CrossRef]
- Bowman, M.C.; Oiler, W.L.; Cairns, T.; Gosnell, A.B.; Oliver, K.H. Stressed bioassay systems for rapid screening of pesticide residues. Part I: Evaluation of bioassay systems. Arch. Environ. Contam. Toxicol. 1981, 10, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, T.; Desai, K.; Patel, D.; Lawani, D.; Bahaley, P.; Joshi, P.; Kothari, V. Effect of various solvents on bacterial growth in context of determining MIC of various antimicrobials. Internet J. Microbiol. 2008, 7, 1–8. [Google Scholar]
- OECD. Test No. 202: Daphnia sp. Acute Immobilisation Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2004. [Google Scholar] [CrossRef]
- Baumann, J.; Sakka, Y.; Bertrand, C.; Köser, J.; Filser, J. Adaptation of the Daphnia sp. acute toxicity test: Miniaturization and prolongation for the testing of nanomaterials. Environ. Sci. Pollut. Res. 2014, 21, 2201–2213. [Google Scholar] [CrossRef]
- Houx, N.W.H.; Dekker, A.; Van Kammen-Polman, A.M.M.; Ronday, R. Environmental Contamination n d Toxicology Acute Toxicity Test for Terrestrial Hazard Assessment with Exposure of Folsomia candida to Pesticides in an Aqueous Medium. Arch. Environ. Contam. Toxicol 1996, 30, 9–14. [Google Scholar] [CrossRef]
- Engelke, M.; Köser, J.; Hackmann, S.; Zhang, H.; Mädler, L.; Filser, J. A miniaturized solid contact test with Arthrobacter globiformis for the assessment of the environmental impact of silver nanoparticles. Environ. Toxicol. Chem. 2014, 33, 1142–1147. [Google Scholar] [CrossRef] [PubMed]
- Roembke, J.; Knacker, T. Aquatic toxicity test for enchytraeids. Hydrobiologia 1989, 180, 235–242. [Google Scholar] [CrossRef]



| Batch Number | Age of the Culture (Weeks) | Adaptation of Culture Condition |
|---|---|---|
| 1 | 3 | none |
| 2 | 3 | none |
| 3 | 4.5 | none |
| 4 | 3 | none |
| 5 | 4 | light intensity: 70 µmol photons m−2 s−1 |
| 6 | 4 | additional 24 mM NaHCO3 |
| 7 | 4 | none |
| 8 | 4 | none |
| 9 | 1.5 | none |
| For Derivatization Only | For Total Work Up | ||||||
|---|---|---|---|---|---|---|---|
| EPS | MEDIA | CELLS | EPS | MEDIA | CELLS | ||
| Erythritol | TMSE Derivatization | 90–96 | 73–76 | 86–91 | 0.5–2 | 93–97 | 0–2 |
| Oleic Acid | TMSE Derivatization | 93–98 | 72–76 | 74–78 | 3.5–5 | 14–20 | 63–71 |
| Norvaline | OPA Derivatization | 77–87 | 93–102 | 88–93 | 10 | 30.5 | 75 |
| Sarcosine | FMOC Derivatization | 89–93 | 94–98 | 94–104 | 19 | 79 | 39 |
| Batch Number | Fatty Acids [mg g−1] | Glycerol [mg g−1] | Monosaccharides [mg g−1] | Single Monosaccharides | Monoasaccharide Derivatives |
|---|---|---|---|---|---|
| 1 | 3.62 ± 0.04 | 4.52 ± 0.87 | 55.15 ± 6.09 | Ara, Glc | Gal or Glc as oximes |
| 3 | 24.63 ± 0.22 | 6.16 ± 0.95 | 40.33 ± 4.56 | Ara, Rib, Xyl, Man, Glc | Gal or Glc alkylated or as alcohols and oximes |
| 4 | 179.5 ± 1.79 | Not detected | 25.64 ± 2.74 | Xyl | as alkylated sugar |
| 5 | 93.26 ± 1.07 | 80.34 ± 8.57 | 25.82 ± 2.76 | Man, Gal | alkylated or as alcohols |
| 6 | 60.13 ± 0.69 | 184.7 ± 20.09 | 3.85 ± 0.42 | Gal | Gal or Glc alkylated or as alcohols and oximes |
| 7 | 96.51 ± 1.62 | 157.0 ± 16.68 | 124.42 ± 13.22 | Rib, Gal | Gal or Glc alkylated or as alcohols and oximes |
| 8 | 82.57 ± 1.34 | 94.8 ± 9.8 | 272.17 ± 28.21 | Ara | Gal or Glc alkylated or as alcohols and oximes |
| 9 | 70.55 ± 0.65 | 114.6 ± 13.0 | 68.01 ± 7.76 | Xyl, Man, Gal | Gal or Glc alkylated or as alcohols and oximes |
| Batch Number | Total Amino Acid Content [mg g−1] | Single Amino Acids |
|---|---|---|
| 1 | 192.6 ± 0.46 | Tyr, Lys, OH-Prol, Pro, Val |
| 3 | 214.4 ± 0.52 | Tyr, Lys, OH-Prol, Phe, Pro, Val, Ile |
| 4 | 358.3 ± 0.75 | Tyr, Cys, Lys, OH-Prol, Val, Ile |
| 5 | 461.0 ± 0.68 | Tyr, Lys, OH-Prol, Phe, Pro, Val, Ile |
| 6 | 78.66 ± 0.19 | Cys, Tyr, Lys, OH-Prol, Phe, Pro, Val |
| 7 | 458.6 ± 1.54 | Tyr, OH-Prol, Lys, Arg, Val, Pro, Ala, Phe, Ile |
| 8 | 395.7 ± 0.68 | Cys, Tyr, OH-Prol, Lys, Phe, Val, Pro |
| 9 | 65.48 ± 0.13 | Tyr, Lys, OH-Prol, Cys, Ile, Pro, Val |
| Batch Number | Fatty Acids [mg g−1] | Glycerol [mg g−1] | Monosaccharides [mg g−1] | Single Monosaccharides | Monoasaccharide Derivatives |
|---|---|---|---|---|---|
| 1 a | 45.09 ± 0.31 | 313.7 ± 2.74 | 371.0 ± 3.24 | Man, Gal, Glc, GlcUA | as alcohols |
| 1 b | 184.5 ± 2.44 | 529.1 ± 7.01 | 199.0 ± 2.75 | Man, Gal, Glc | as alcohols and oximes |
| 2 | 21.81 ± 0.21 | 224.3 ± 2.53 | 448.6 ± 5.07 | Ara, Gal, Glc, GlcUA | Gal or Glc alkylated or as alcohols and oximes |
| 4 | 266.1 ± 2.55 | 304.7 ± 3.44 | 244.6 ± 2.76 | Gal, Glc | Gal or Glc alkylated or as alcohols |
| 5 a | 45.27 ± 0.31 | 267.5 ± 2.33 | 251.0 ± 2.19 | Man, Gal, Glc | as alcohols and oximes |
| 5 b | 80.56 ± 0.55 | 172.2 ± 1.51 | 255.0 ± 2.23 | Gal | as alcohols and oximes |
| 6 a | 93.43 ± 0.89 | 105.5 ± 1.19 | 50.17 ± 0.57 | Ara, Man | as alcohols and oximes |
| 6 b | 34.25 ± 0.33 | 270.6 ± 3.06 | 287.7 ± 3.25 | Ara, Man, Gal | as alcohols and oximes |
| 7 | 2.52 ± 0.02 | 20.71 ± 0.23 | 774.4 ± 8.7 | Ara, Fuc, Gul | Gal or Glc alkylated or as alcohols and oximes |
| 8 | 0.75 ± 0.01 | 106.5 ± 1.47 | 783.4 ± 10.8 | Gal, Glc, GlcUA | Gal or Glc alkylated or as alcohols and oximes |
| 9 | 8.88 ± 0.12 | 217.8 ± 3.01 | 544.4 ± 7.52 | Ara, Gal, Glc, Fuc | Gal or Glc alkylated or as oximes |
| Batch Number | Total Amino Acid Content [mg g−1] | Single Amino Acids |
|---|---|---|
| 1 a | 266.8 ± 0.58 | Arg, Ala, Tyr, Asp, OH-Prol, Pro, Gln |
| 1 b | 185.6 ± 0.44 | Ala, Tyr, Asp, Glu, OH-Prol, Pro, Gln |
| 2 | 364.2 ± 0.694 | Lys, Arg, Met, Asp, OH-Prol, Phe, Ile, Glu |
| 4 | 264.3 ± 0.59 | Lys, Arg, Met, Asp, OH-Prol, Val, Ile, Phe, Leu |
| 5 a | 368.3 ± 0.68 | Lys, Arg, Met, Asp, OH-Prol, Phe, Leu |
| 5 b | 497.7 ± 0.75 | Arg, Ala, Tyr, OH-Prol, Lys, Val, Asp, Pro, Phe, Ile, Gly |
| 6 a | 381.7 ± 0.70 | Arg, Tyr, OH-Prol, Ala, Lys, Val, Pro, Asp, Phe, Ile, Gly |
| 6 b | 457.7 ± 1.48 | Arg, Tyr, OH-Prol, Ala, Lys, Val, Pro, Asp, Phe, Ile, Gly |
| 7 | 561.9 ± 1.36 | Tyr, Arg, OH-Prol, Lys, Ala, Asp, Val, Pro, Phe, Ile, Gly |
| 8 | 273.9 ± 0.66 | Lys, Arg, Met, OH-Prol, Asp, Phe |
| 9 | 521.7 ± 3.24 | Lys, Leu, OH-Prol, Asp, Met, Phe, Thr, Ile, Pro |
| Batch Number | Fatty Acids [mg g−1] | Glycerol [mg g−1] | Monosaccharides [mg g−1] | Single Monosaccharides | Monosaccharide Derivatives | Amino Acids [mg g−1] | Single Amino Acids |
|---|---|---|---|---|---|---|---|
| 5 | 49.1 ± 0.77 | 78.7 ± 1.68 | 588.2 ± 12.6 | Ara, Man, Gal, Glc | as alcohols and oximes | 461.5 ± 1.54 | Lys, Arg, Met, Asp, OH-Prol, Phe, Leu |
| 6 | 60.1 ± 1.01 | 119.1 ± 2.48 | 230.6 ± 4.81 | Rib, Man, Gal, Glc | as alcohols and oximes | 392.1 ± 0.86 | Arg, Ala, Tyr, OH-Prol, Lys, Val, Asp, Pro, Phe, Ile, Gly |
| 7 | 52.4 ± 0.82 | 89.6 ± 1.86 | 316.6 ± 6.77 | Man, Gal | alkylated sugars only | 368.7 ± 0.71 | Tyr, Arg, OH-Prol, Lys, Ala, Asp, Val, Pro, Phe, Ile, Gly |
| 5 * | 366.6 ± 5.83 | 6.9 ± 0.45 | 14.62 ± 0.31 | Man, Gal | alkylated sugars only | 18.2 ± 0.03 | Tyr, Lys, Arg |
| 6 * | 374.5 ± 5.95 | 9.1 ± 0.61 | 16.6 ± 0.35 | Man, Gal | alkylated sugars only | 11.8 ± 0.02 | Tyr, Lys, Arg |
| 7 * | 435.0 ± 6.91 | 7.2 ± 0.53 | 9.2 ± 0.21 | Man, Gal | alkylated sugars only | 13.6 ± 0.02 | Tyr, Lys, Arg |
| Batch No. | Fraction | Solvent | Concentration [g L−1] | Immobilized Daphnids 1 | Immobilized Enchytraeids 2 | Immobilized Collembola 3 | Bacterial Enzyme Activity 4 |
|---|---|---|---|---|---|---|---|
| 1 | EPS-P | Medium | 1 a | 0.10 | 0.06 ± 0.13 | 0.30 ± 0.20 | no effect |
| 3 | 1.0 | 1.0 ± 0 | 0.50 ± 0.23 | no effect | |||
| 5 | 0 | 1.0 ± 0 | 0 | no effect | |||
| 7 | 0 | 1.0 ± 0 | 0 | no effect | |||
| 9 | 0 | 1.0 ± 0 | 0 | n.r. | |||
| 1 | medium-P | Medium | 1 | 0 | 0.31 ± 0.13 | 0 | no effect |
| 6 | 0 | 0 | 0 | n.r. | |||
| 7 | 0 | 0 | 0 | n.r. | |||
| 8 | 0 | 0 | 0 | n.r. | |||
| 1 | DMSO | 0.1 b | 0 | 0.06 ± 0.13 | 0.16 ± 0.17 | no effect | |
| 6 | 0 | 0 | 0.03 ± 0.09 | no effect | |||
| 7 | 0 | 0 | 0.22 ± 0.36 | no effect | |||
| 8 | 0 | 0 | 0.06 ± 0.12 | no effect | |||
| 3 | cells-P | Medium | 1 | 0 | 1.0 ± 0 | 0 | n.r. |
| 5 | 0 | 0.06 ± 0.13 | 0 | no effect | |||
| 6 | 0 | 1.0 ± 0 | 0 | no effect | |||
| 3 | DMSO | 0.1 b | 0 | 0 | 0.19 ± 0.22 | no effect | |
| 5 | 0 | 0 | 0.25 ± 0.38 | no effect | |||
| 6 | 0 | 0.06 ± 0.13 | 0.15 ± 0.24 | no effect |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gavalás-Olea, A.; Siol, A.; Sakka, Y.; Köser, J.; Nentwig, N.; Hauser, T.; Filser, J.; Thöming, J.; Lang, I. Potential of the Red Alga Dixoniella grisea for the Production of Additives for Lubricants. Plants 2021, 10, 1836. https://doi.org/10.3390/plants10091836
Gavalás-Olea A, Siol A, Sakka Y, Köser J, Nentwig N, Hauser T, Filser J, Thöming J, Lang I. Potential of the Red Alga Dixoniella grisea for the Production of Additives for Lubricants. Plants. 2021; 10(9):1836. https://doi.org/10.3390/plants10091836
Chicago/Turabian StyleGavalás-Olea, Antonio, Antje Siol, Yvonne Sakka, Jan Köser, Nina Nentwig, Thomas Hauser, Juliane Filser, Jorg Thöming, and Imke Lang. 2021. "Potential of the Red Alga Dixoniella grisea for the Production of Additives for Lubricants" Plants 10, no. 9: 1836. https://doi.org/10.3390/plants10091836
APA StyleGavalás-Olea, A., Siol, A., Sakka, Y., Köser, J., Nentwig, N., Hauser, T., Filser, J., Thöming, J., & Lang, I. (2021). Potential of the Red Alga Dixoniella grisea for the Production of Additives for Lubricants. Plants, 10(9), 1836. https://doi.org/10.3390/plants10091836