Genome-Wide Identification of the HMA Gene Family and Expression Analysis under Cd Stress in Barley
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials and Treatment
2.2. RNA Isolation and cDNA Synthesis
2.3. Bioinformatics Analysis of the Barley HMA Gene Family
2.3.1. Identification and Structural Analysis of Barley HMA Genes
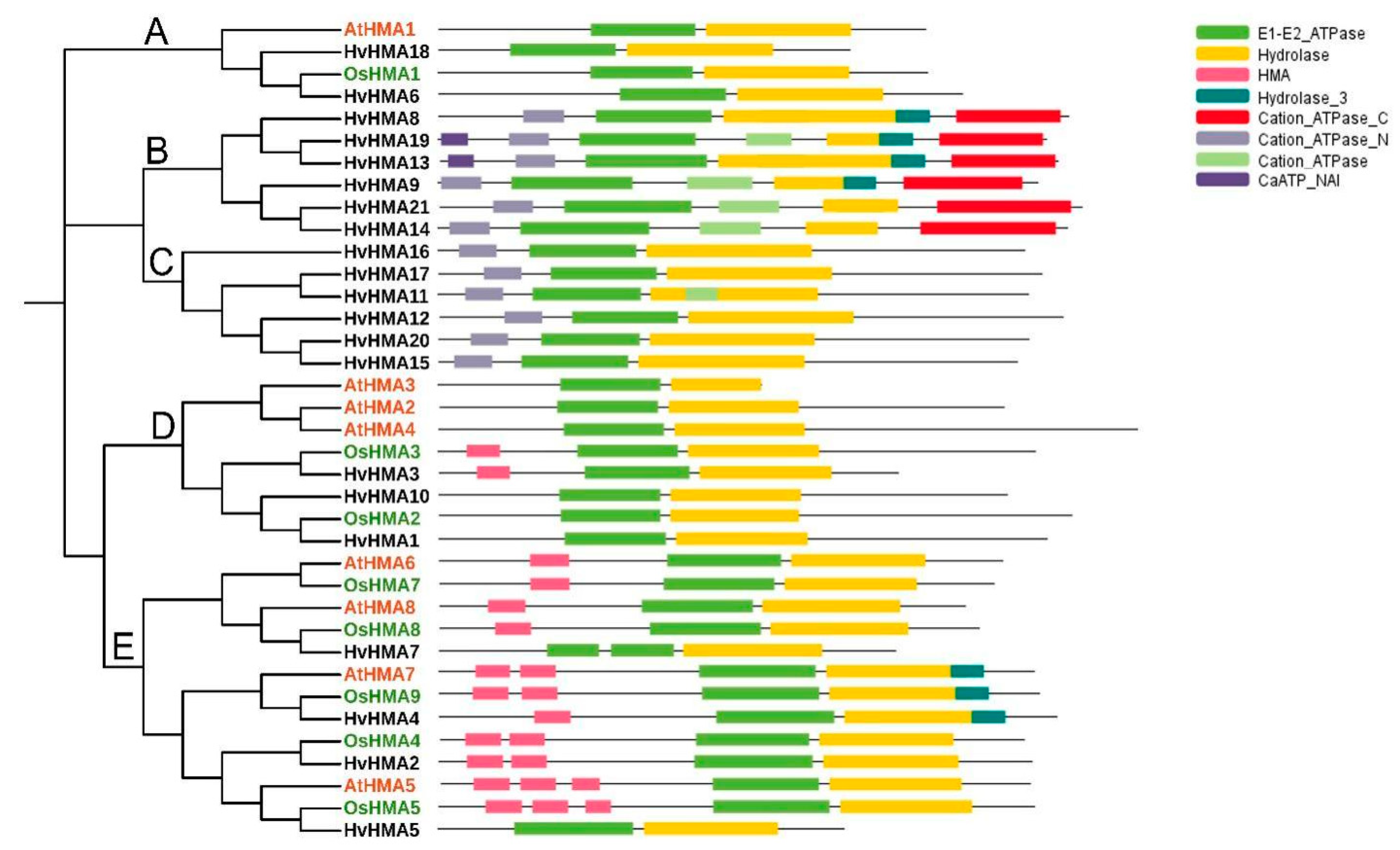
2.3.2. Phylogenetic Analysis of the Barley HMA Family
2.3.3. Expression Profiling of the Barley HMA Family and Candidate Gene Selection
2.4. Quantitative RT-PCR Analysis of Barley HMA Genes
3. Results
3.1. Identification and Molecular Characteristics of Barley HMA Proteins
3.2. Phylogenetic Analysis and Classification of Barley HMA Genes
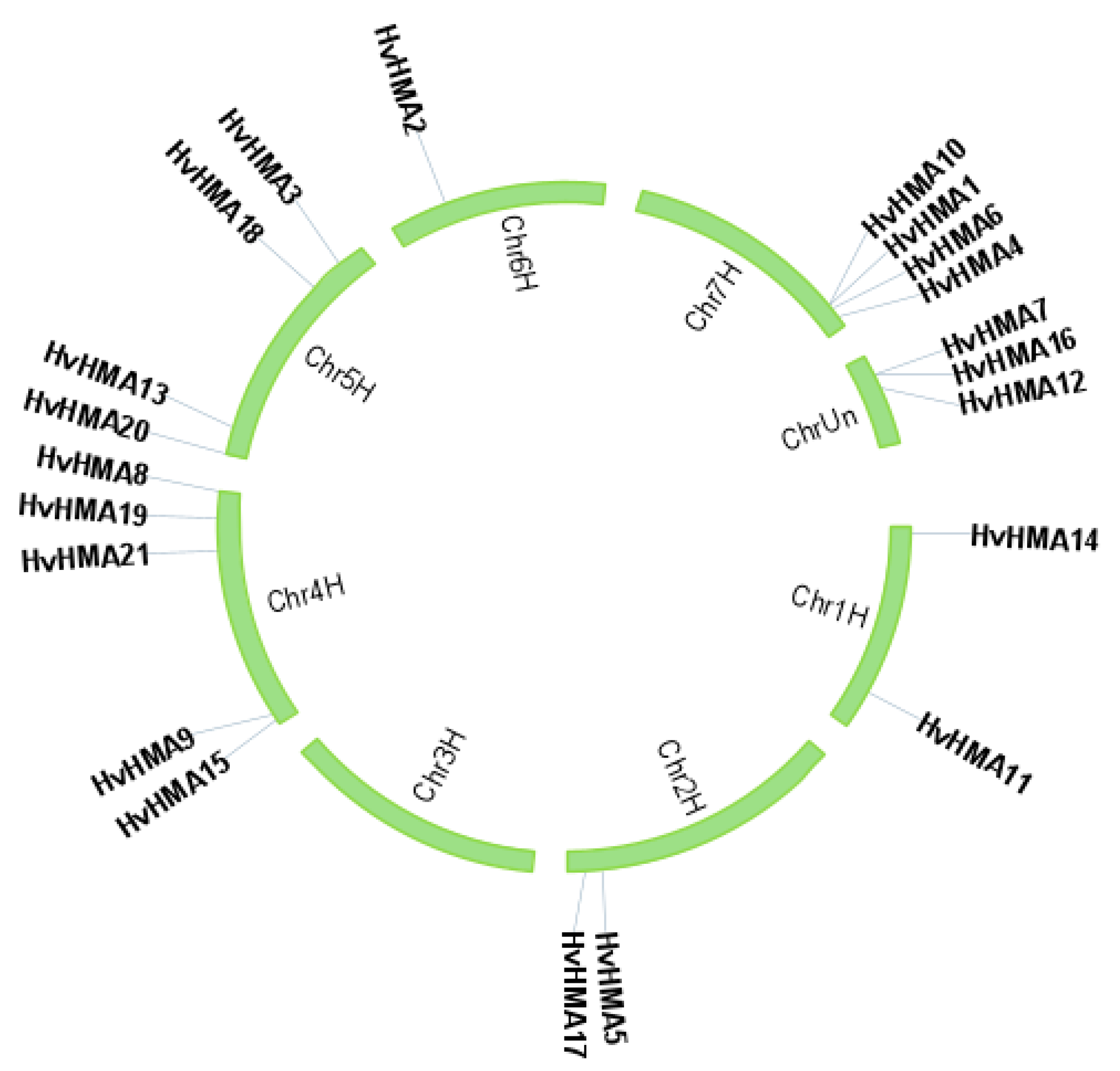
3.3. Chromosomal Location of Barley HMA Genes
3.4. Motif Composition of the Barley HMA Proteins
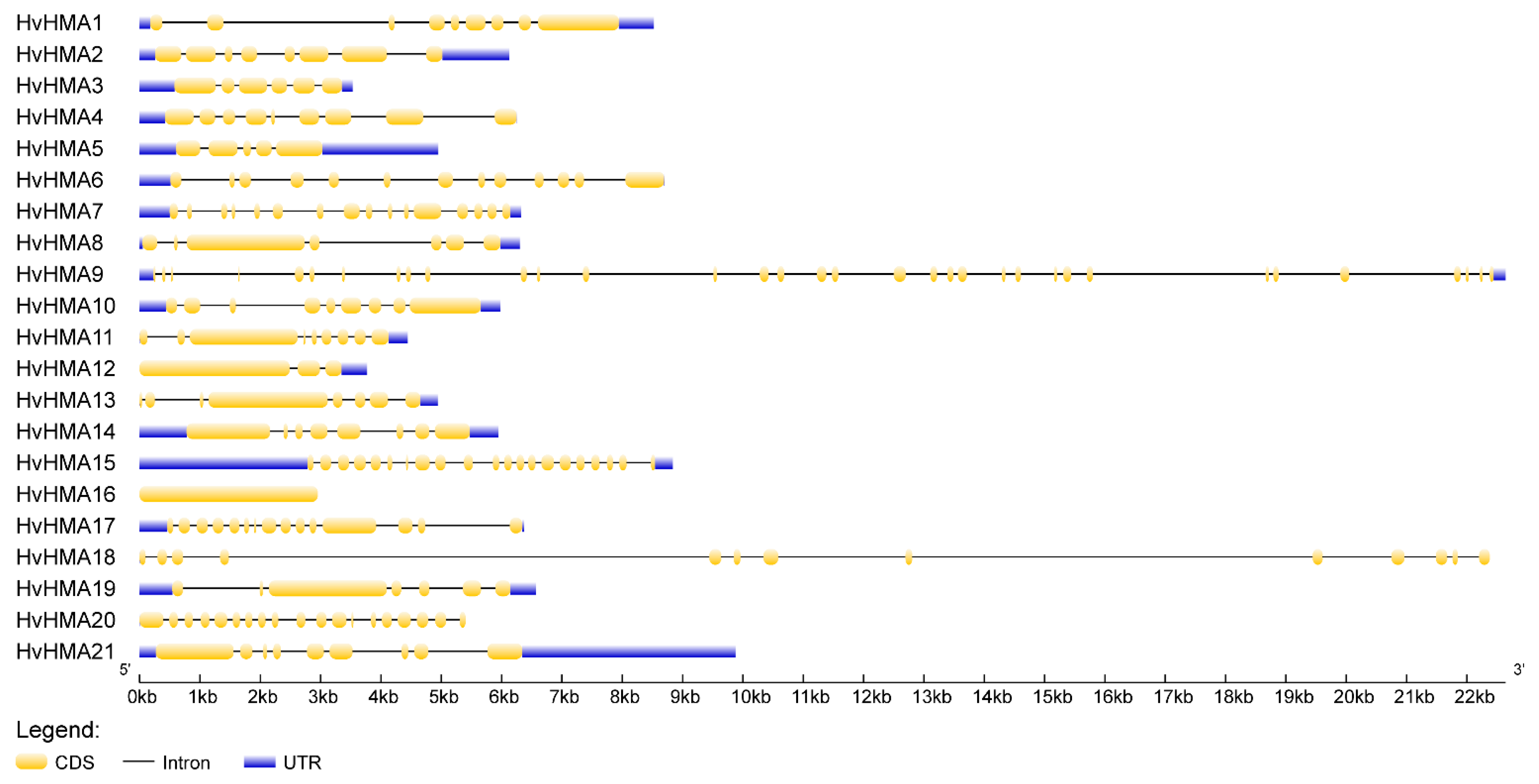
3.5. Intron-Exon Structure of Barley HMA Genes
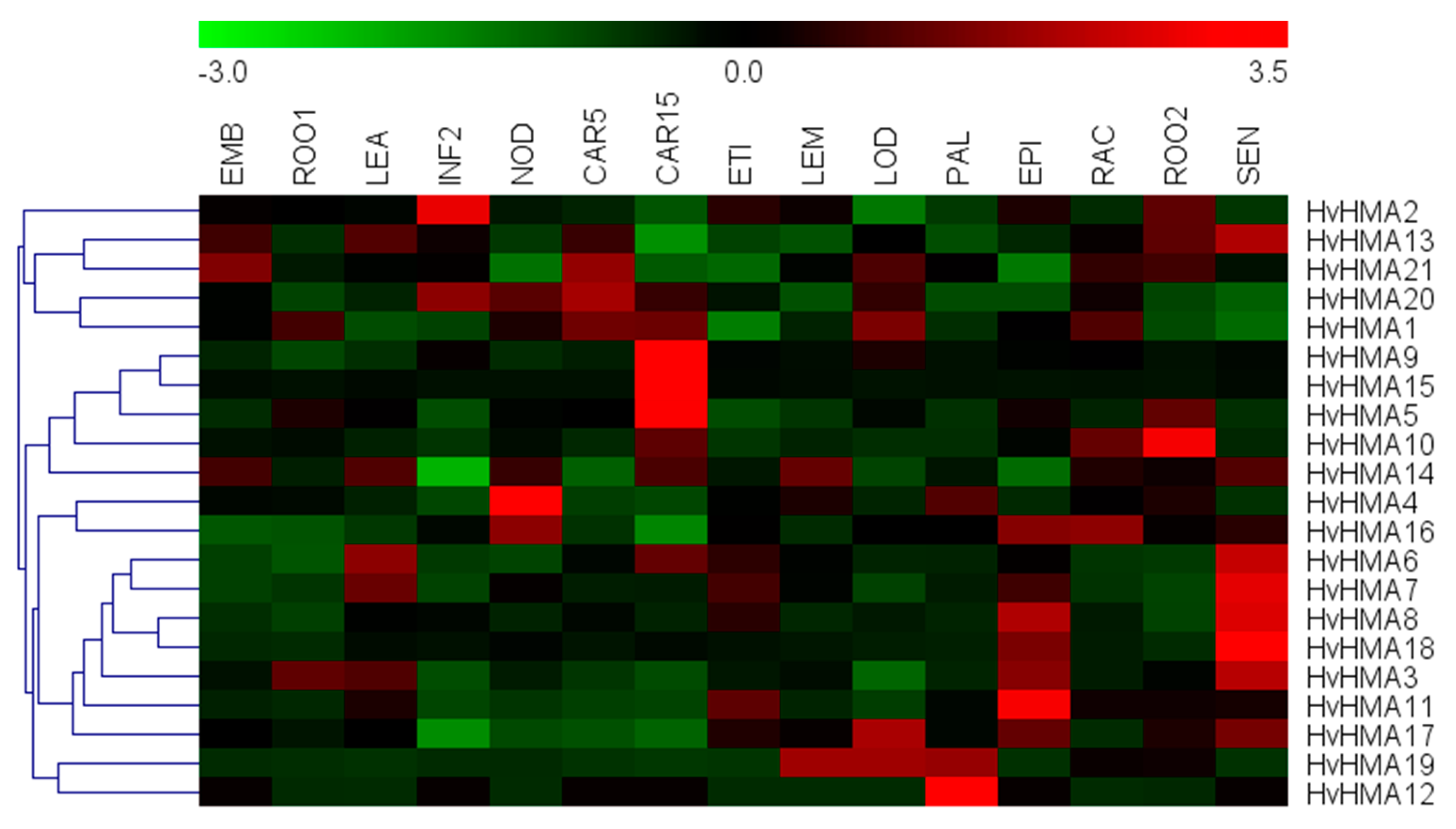
3.6. Expression Pattern Analysis of Barley HMA Genes and Target Gene Screening
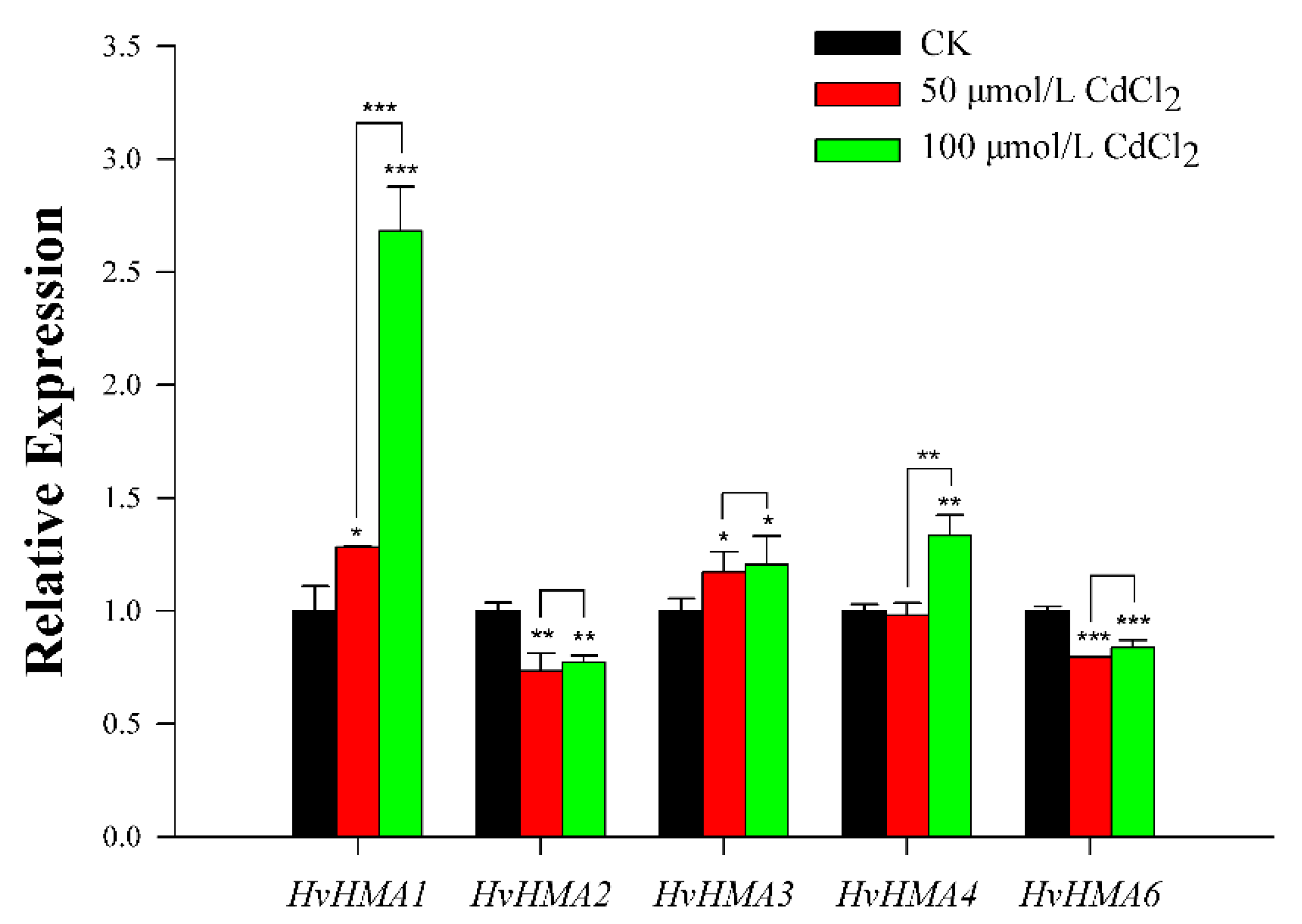
3.7. Expression Analysis of Barley HMA Genes in Response to Cd Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- DalCorso, G.; Farinati, S.; Furini, A. Regulatory networks of cadmium stress in plants. Plant. Signal. Behav. 2010, 5, 663–667. [Google Scholar] [CrossRef]
- Sanità, D.T.L.; Gabbrielli, R. Response to cadmium in higher plants. Environ. Exp. Bot 1999, 41, 105–130. [Google Scholar] [CrossRef]
- Pedas, P.; Ytting, C.K.; Fuglsang, A.T.; Jahn, T.P.; Schjoerring, J.K.; Husted, S. Manganese efficiency in barley: Identification and characterization of the metal ion transporter HvIRT1. Plant. Physiol. 2008, 148, 455–466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant. Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [Green Version]
- Hall, J.L. Cellular mechanisms for heavy metal detoxification and tolerance. J. Exp. Bot. 2002, 53, 1–11. [Google Scholar] [CrossRef]
- Argüello, J.M.; Eren, E.; González-Guerrero, M. The structure and function of heavy metal transport P1B-ATPases. BioMetals 2007, 20, 233–248. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.S.; Tan, J.J.; Zhang, Y.X.; Liang, S.; Xiang, S.Q.; Wang, H.; Chai, T.Y. Isolation and characterization of a novel cadmium-regulated Yellow Stripe-Like transporter (SnYSL3) in Solanum nigrum. Plant. Cell Rep. 2017, 36, 281–296. [Google Scholar] [CrossRef] [PubMed]
- Mani, A.; Sankaranarayanan, K. In Silico Analysis of Natural Resistance-Associated Macrophage Protein (NRAMP) Family of Transporters in Rice. Protein J. 2018, 37, 237–247. [Google Scholar] [CrossRef] [PubMed]
- Mills, R.F.; Francini, A.; Ferreira da Rocha, P.S.; Baccarini, P.J.; Aylett, M.; Krijger, G.C.; Williams, L.E. The plant P1B-type ATPase AtHMA4 transports Zn and Cd and plays a role in detoxification of transition metals supplied at elevated levels. FEBS Lett. 2005, 579, 783–791. [Google Scholar] [CrossRef] [Green Version]
- Smith, A.T.; Smith, K.P.; Rosenzweig, A.C. Diversity of the metal-transporting P1B-type ATPases. J. Biol. Inorg. Chem 2014, 19, 947–960. [Google Scholar] [CrossRef] [Green Version]
- Li, D.D.; Xu, X.M.; Hu, X.Q.; Liu, Q.G.; Wang, Z.C.; Zhang, H.Z.; Wang, H.; Wei, M.; Wang, H.Z.; Liu, H.M.; et al. Genome-Wide Analysis and Heavy Metal-Induced Expression Profiling of the HMA Gene Family in Populus trichocarpa. Front. Plant. Sci. 2015, 6, 1149. [Google Scholar] [CrossRef]
- Williams, L.E.; Mills, R.F. P(1B)-ATPases--an ancient family of transition metal pumps with diverse functions in plants. Trends Plant. Sci. 2005, 10, 491–502. [Google Scholar] [CrossRef]
- Zhiguo, E.; Li, T.T.; Chen, C.; Wang, L. Genome-wide survey and expression analysis of P1B-ATPases in rice, Maize and Sorghum. Rice Sci. 2018, 25, 208–217. [Google Scholar] [CrossRef]
- Fang, X.L.; Wang, L.; Deng, X.J.; Wang, P.; Ma, Q.B.; Nian, H.; Wang, Y.X.; Yang, C.Y. Genome-wide characterization of soybean P1B-ATPases gene family provides functional implications in cadmium responses. BMC Genom. 2016, 17, 376. [Google Scholar] [CrossRef] [Green Version]
- Wong, C.K.E.; Cobbett, C.S. HMA P-type ATPases are the major mechanism for root-to-shoot Cd translocation in Arabidopsis thaliana. New Phytol. 2009, 181, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Milner, M.J.; Yamaji, N.; Yokosho, K.; Koyama, E.; Clemencia Zambrano, M.; Kaskie, M.; Ebbs, S.; Kochian, L.V.; Ma, J.F. Elevated expression of TcHMA3 plays a key role in the extreme Cd tolerance in a Cd-hyperaccumulating ecotype of Thlaspi caerulescens. Plant. J. 2011, 66, 852–862. [Google Scholar] [CrossRef]
- Miyadate, H.; Adachi, S.; Hiraizumi, A.; Tezuka, K.; Nakazawa, N.; Kawamoto, T.; Katou, K.; Kodama, I.; Sakurai, K.; Takahashi, H.; et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. New Phytol. 2011, 189, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.S.; Guo, M.; Si, E.J.; Yao, L.R.; Wang, J.C.; Li, B.C.; Yang, K.; Meng, Y.X.; Ma, X.L.; Wang, H.J. Response of barley to toxin stress of barley stripe bacteria. J. Wheat Crop. 2021, 3, 1–7. [Google Scholar]
- Tong, T.; Fang, Y.X.; Zhang, Z.L.; Zheng, J.J.; Lu, X.L.; Zhang, X.Q.; Xue, D.W. Genome-wide identification, phylogenetic and expression analysis of SBP-box gene family in barley (Hordeum vulgare L.). Plant. Growth Regul. Int. J. Plant. Growth Dev. 2020, 90, 1–13. [Google Scholar] [CrossRef]
- Zheng, J.J.; Zhang, Z.L.; Tong, T.; Fang, Y.X.; Zhang, X.; Niu, C.Y.; Li, J.; Wu, Y.H.; Xue, D.W.; Zhang, X.Q. Genome-Wide Identification of WRKY Gene Family and Expression Analysis under Abiotic Stress in Barley. Agronomy 2021, 11, 521. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Tong, T.; Fang, Y.X.; Zheng, J.J.; Zhang, X.; Niu, C.Y.; Li, J.; Zhang, X.Q.; Xue, D.W. Genome-Wide Identification of Barley ABC Genes and Their Expression in Response to Abiotic Stress Treatment. Plants 2020, 9, 1281. [Google Scholar] [CrossRef]
- Zhang, L.T.; Wang, S.Y.; Chen, Y.Y.; Dong, M.Y.; Fang, Y.X.; Zhang, X.; Tong, T.; Zhang, Z.L.; Zheng, J.J.; Xue, D.W.; et al. Genome-wide identification of the F-box gene family and expression analysis under drought and salt stress in barley. Phyton-Int. J. Exp. Bot. 2020, 89, 229–251. [Google Scholar]
- Zhang, X.; Zhang, L.T.; Chen, Y.Y.; Wang, S.Y.; Fang, Y.X.; Zhang, X.Q.; Wu, Y.H.; Xue, D.W. Genome-wide identification of the SOD gene family and expression analysis under drought and salt stress in barley. Plant. Growth Regul. 2021, 94, 49–60. [Google Scholar] [CrossRef]
- Mikkelsen, M.D.; Pedas, P.; Schiller, M.; Vincze, E.; Mills, R.F.; Borg, S.; Møller, A.; Schjoerring, J.K.; Williams, L.E.; Baekgaard, L.; et al. Barley HvHMA1 is a heavy metal pump involved in mobilizing organellar Zn and Cu and plays a role in metal loading into grains. PLoS ONE 2012, 7, e49027. [Google Scholar] [CrossRef]
- Kintlová, M.; Vrána, J.; Hobza, R.; Blavet, N.; Hudzieczek, V. Transcriptome Response to Cadmium Exposure in Barley (Hordeum vulgare L.). Front. Plant. Sci. 2021, 12, 629089. [Google Scholar] [CrossRef]
- Zhang, X.Q.; Tong, T.; Tian, B.; Fang, Y.X.; Pan, J.J.; Zheng, J.J.; Xue, D.W. Physiological, Biochemical and Molecular Responses of Barley Seedlings to Aluminum Stress. Phyton-Int. J. Exp. Bot. 2019, 88, 253–260. [Google Scholar] [CrossRef]
- Wheeler, T.J.; Eddy, S.R. nhmmer: DNA homology search with profile HMMs. Bioinformatics 2013, 29, 2487–2489. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Niu, B.; Gao, Y.; Fu, L.; Li, W. CD-HIT Suite: A web server for clustering and comparing biological sequences. Bioinformatics 2010, 26, 680–682. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART 7: Recent updates to the protein domain annotation resource. Nucleic Acids Res. 2012, 40, D302–D305. [Google Scholar] [CrossRef]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. WoLF PSORT: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, T.L.; Boden, M.; Buske, F.A.; Frith, M.; Grant, C.E.; Clementi, L.; Ren, J.; Li, W.W.; Noble, W.S. MEME SUITE: Tools for motif discovery and searching. Nucleic Acids Res. 2009, 37, W202–W208. [Google Scholar] [CrossRef]
- Hu, B.; Jin, J.; Guo, A.Y.; Zhang, H.; Luo, J.; Gao, G. GSDS 2.0: An upgraded gene feature visualization server. Bioinformatics 2015, 31, 1296–1297. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, 256–259. [Google Scholar] [CrossRef] [Green Version]
- Imran, M.; Shafiq, S.; Farooq, M.A.; Naeem, M.K.; Widemann, E.; Bakhsh, A.; Jensen, K.B.; Wang, R.R. Comparative Genome-wide Analysis and Expression Profiling of Histone Acetyltransferase (HAT) Gene Family in Response to Hormonal Applications, Metal and Abiotic Stresses in Cotton. Int. J. Mol. Sci. 2019, 20, 5311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt Method. Methods (San Diego. Calif.) 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Jain, M.; Khurana, P.; Tyagi, A.K.; Khurana, J.P. Genome-wide analysis of intronless genes in rice and Arabidopsis. Funct. Integr. Genom. 2008, 8, 69–78. [Google Scholar] [CrossRef]
- Catania, F.; Lynch, M. Where do introns come from? PLoS Biol. 2008, 6, e283. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Zhang, Y.; Long, M. Extensive structural renovation of retrogenes in the evolution of the Populus genome. Plant. Physiol. 2009, 151, 1943–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Long, M.; Betrán, E.; Thornton, K.; Wang, W. The origin of new genes: Glimpses from the young and old. Nat. Rev. Genet. 2003, 4, 865–875. [Google Scholar] [CrossRef]
- Takahashi, R.; Bashir, K.; Ishimaru, Y.; Nishizawa, N.K.; Nakanishi, H. The role of heavy-metal ATPases, HMAs, in zinc and cadmium transport in rice. Plant. Signal. Behav. 2012, 7, 1605–1607. [Google Scholar] [CrossRef]
- Nocito, F.F.; Lancilli, C.; Dendena, B.; Lucchini, G.; Sacchi, G.A. Cadmium retention in rice roots is influenced by cadmium availability, chelation and translocation. Plant. Cell Environ. 2011, 34, 994–1008. [Google Scholar] [CrossRef]
- Satoh-Nagasawa, N.; Mori, M.; Nakazawa, N.; Kawamoto, T.; Nagato, Y.; Sakurai, K.; Takahashi, H.; Watanabe, A.; Akagi, H. Mutations in rice (Oryza sativa) heavy metal ATPase 2 (OsHMA2) restrict the translocation of zinc and cadmium. Plant. Cell Physiol. 2012, 53, 213–224. [Google Scholar] [CrossRef]
- Takahashi, R.; Ishimaru, Y.; Shimo, H.; Ogo, Y.; Senoura, T.; Nishizawa, N.K.; Nakanishi, H. The OsHMA2 transporter is involved in root-to-shoot translocation of Zn and Cd in rice. Plant. Cell Environ. 2012, 35, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, Y.Y.; Lee, Y.; An, G. Rice P1B-type heavy-metal ATPase, OsHMA9, is a metal efflux protein. Plant. Physiol. 2007, 145, 831–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, R.F.; Peaston, K.A.; Runions, J.; Williams, L.E. HvHMA2, a P(1B)-ATPase from barley, is highly conserved among cereals and functions in Zn and Cd transport. PLoS ONE 2012, 7, e42640. [Google Scholar] [CrossRef] [Green Version]
- Lei, G.J.; Fujii, K.M.; Wu, D.Z.; Hisano, H.S.D.; Deng, F.L.; Yamaji, N.; Sato, K.; Zhao, F.J.; Ma, J.F. Breeding for low cadmium barley by introgression of a Sukkula-like transposable element. Nat. Food 2020, 1, 489–499. [Google Scholar] [CrossRef]

| Gene Name | Gene ID | Forward Primer Sequence (5′-3′) | Reverse Primer Sequence (5′-3′) |
|---|---|---|---|
| HvHMA1 | HORVU7Hr1G097240.1 | TGGCGAAGAAATGCTGTGCT | AACCGCCTGTTGATACATTCTC |
| HvHMA2 | HORVU6Hr1G033380.2 | TGGAGGTGTCATTTCAGAAGTGG | CAACACCATCAACTGGGACCTT |
| HvHMA3 | HORVU5Hr1G094430.8 | ACATCGCCGTGAGGACAAGT | GCGTCTTGGACTTGCTCTGC |
| HvHMA4 | HORVU7Hr1G108890.1 | TCAGCCTAAGTCACAGAAGACATTG | CCTGGACGATTTCATCCTTGC |
| HvHMA6 | HORVU7Hr1G100160.2 | GCTAAGGCATCTATCGGTTCC | ATGCAGAACACTTTACTGCCTCT |
| HvActin | HORVU1Hr1G002840 | TGGATCGGAGGGTCCATCCT | GCACTTCCTGTGGACGATCGCTG |
| Gene Name | Protein Number | ORF (aa) | MW (Da) | PI | Subcellular Localization | Hydrophilicity Index |
|---|---|---|---|---|---|---|
| HvHMA1 | A0A287XH51 | 1009 | 108464.81 | 6.68 | Plasma membrane | −0.090 |
| HvHMA2 | A0A287TV87 | 987 | 106112.96 | 5.58 | Plasma membrane | 0.171 |
| HvHMA3 | A0A287SBM8 | 765 | 80511.64 | 5.80 | Plasma membrane | 0.422 |
| HvHMA4 | A0A287XS00 | 1023 | 102819.64 | 5.27 | Plasma membrane | 0.268 |
| HvHMA5 | A0A287J245 | 672 | 73112.29 | 6.19 | Plasma membrane | 0.277 |
| HvHMA6 | A0A287XKH5 | 871 | 92584.78 | 7.59 | Plasma membrane | 0.106 |
| HvHMA7 | M0X9Y2 | 761 | 80246.05 | 5.36 | Plasma membrane | 0.223 |
| HvHMA8 | A0A287NAD1 | 1050 | 113188.47 | 5.00 | Plasma membrane | 0.193 |
| HvHMA9 | A0A287N6A1 | 1000 | 109664.92 | 5.72 | Endoplasmic reticulum | 0.171 |
| HvHMA10 | M0WLW4 | 946 | 100370.69 | 7.04 | Plasma membrane | −0.016 |
| HvHMA11 | A0A287FY78 | 981 | 107543.64 | 6.42 | Plasma membrane | 0.083 |
| HvHMA12 | A0A287E4C3 | 1039 | 114071.38 | 6.95 | Plasma membrane | −0.042 |
| HvHMA13 | A0A287QI47 | 1033 | 111820.68 | 6.07 | Plasma membrane | 0.183 |
| HvHMA14 | A0A287EJS4 | 1049 | 113618.24 | 5.49 | Endoplasmic reticulum | 0.140 |
| HvHMA15 | M0WME5 | 962 | 105942.79 | 6.15 | Plasma membrane | 0.094 |
| HvHMA16 | A0A287DYP4 | 983 | 107457.12 | 6.10 | Plasma membrane | 0.063 |
| HvHMA17 | A0A287JG07 | 1010 | 106141.11 | 7.82 | Plasma membrane | 0.074 |
| HvHMA18 | A0A287RKR1 | 690 | 73747.50 | 6.73 | Plasma membrane | 0.219 |
| HvHMA19 | M0WL52 | 1020 | 110187.74 | 6.21 | Endoplasmic reticulum | 0.220 |
| HvHMA20 | A0A287Q5Y4 | 994 | 109774.70 | 7.45 | Plasma membrane | 0.142 |
| HvHMA21 | A0A287P785 | 1083 | 118116.93 | 5.49 | Plasma membrane | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yang, Q.; Zhang, X.; Zhang, X.; Yu, T.; Wu, Y.; Fang, Y.; Xue, D. Genome-Wide Identification of the HMA Gene Family and Expression Analysis under Cd Stress in Barley. Plants 2021, 10, 1849. https://doi.org/10.3390/plants10091849
Zhang C, Yang Q, Zhang X, Zhang X, Yu T, Wu Y, Fang Y, Xue D. Genome-Wide Identification of the HMA Gene Family and Expression Analysis under Cd Stress in Barley. Plants. 2021; 10(9):1849. https://doi.org/10.3390/plants10091849
Chicago/Turabian StyleZhang, Chiran, Qianhui Yang, Xiaoqin Zhang, Xian Zhang, Tongyuan Yu, Yuhuan Wu, Yunxia Fang, and Dawei Xue. 2021. "Genome-Wide Identification of the HMA Gene Family and Expression Analysis under Cd Stress in Barley" Plants 10, no. 9: 1849. https://doi.org/10.3390/plants10091849






