Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato)
Abstract
:1. Introduction
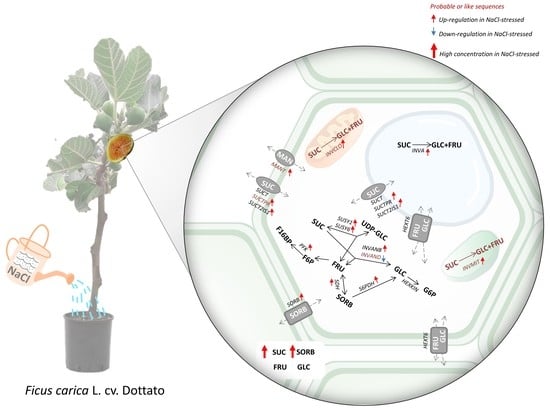
2. Results
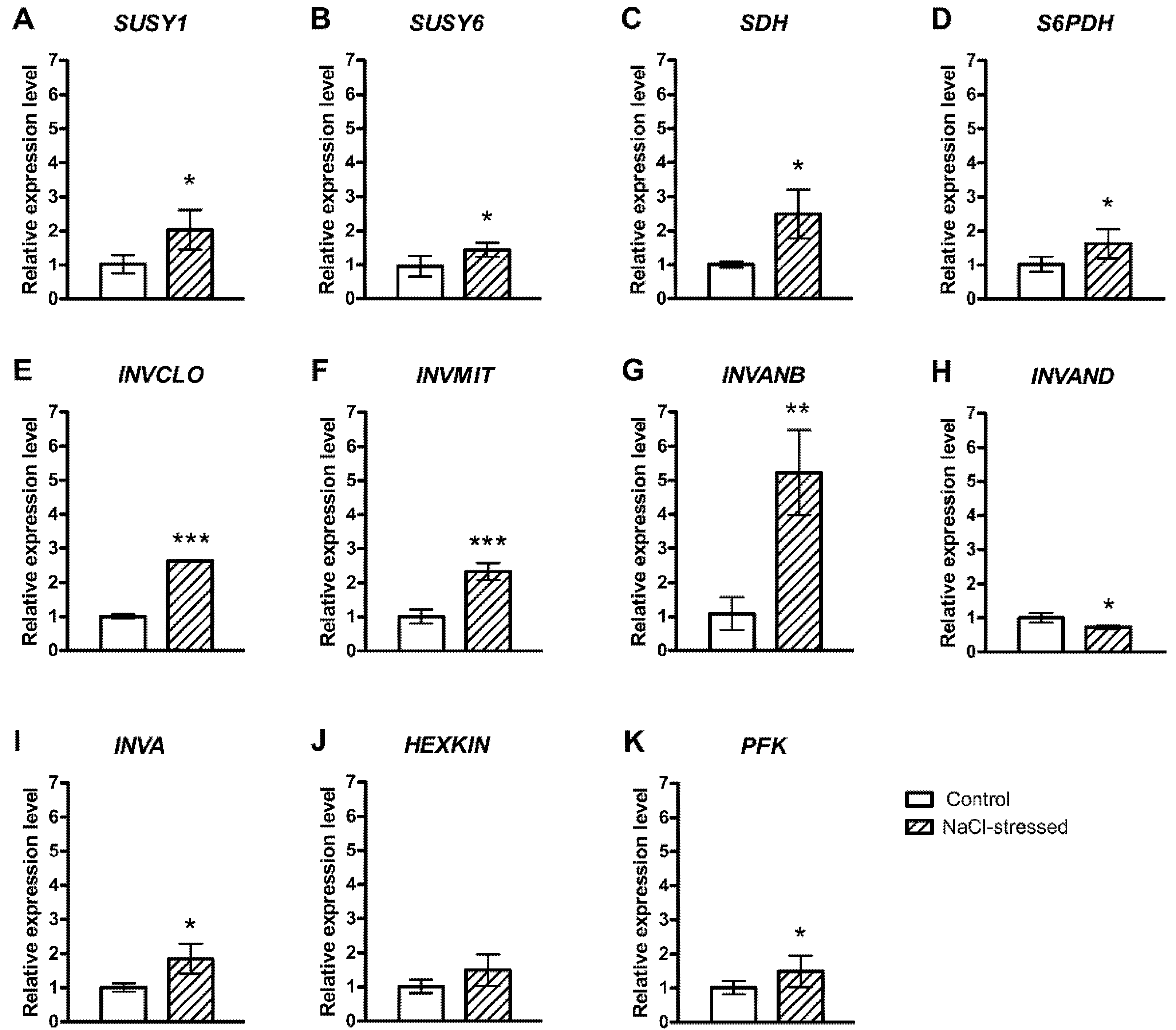
2.1. Salt-Induced Expression of Genes Involved in Soluble Carbohydrate Metabolism and Transport
2.2. Changes in Main Soluble Carbohydrates Contents in Response to Salinity
3. Discussion
4. Materials and Methods
4.1. Plant Material and Salt Treatment
4.2. Nucleic Acid Isolation and Analysis of Gene Expression
4.3. Quantitative 1H Nuclear Magnetic Resonance (NMR) for the Determination of Free Soluble Carbohydrates
4.4. Experimental Design and Statistical Analysis of Data
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAOSTAT Statistical Databases, Food and Agriculture Organization of the United Nations. Available online: http://www.fao.org/faostat/en/#home (accessed on 6 March 2021).
- Gilani, A.H.; Mehmood, M.H.; Janbaz, K.H.; Khan, A.U.; Saeed, S.A. Ethnopharmacological studies on antispasmodic and antiplatelet activities of Ficus carica. J. Ethnopharmacol. 2008, 119, 1–5. [Google Scholar] [CrossRef]
- Solomon, A.; Golubowicz, S.; Yablowicz, Z.; Grossman, S.; Bergman, M.; Gottlieb, H.E.; Altman, A.; Kerem, Z.; Flaishman, M.A. Antioxidant activities and anthocyanin content of fresh fruits of common fig (Ficus carica L.). J. Agric. Food Chem. 2006, 54, 7717–7723. [Google Scholar] [CrossRef]
- Veberic, R.; Colaric, M.; Stampar, F. Phenolic acids and flavonoids of fig fruit (Ficus carica L.) in the northern Mediterranean region. Food Chem. 2008, 106, 153–157. [Google Scholar] [CrossRef]
- Allegra, A.; Sortino, G.; Inglese, P.; Settanni, L.; Todaro, A.; Gallotta, A. The effectiveness of Opuntia ficus-indica mucilage edible coating on post-harvest maintenance of ‘Dottato’ fig (Ficus carica L.) fruit. Food Packag. Shelf Life 2017, 12, 135–141. [Google Scholar] [CrossRef]
- Allegra, A.; Gallotta, A.; Carimi, F.; Mercati, F.; Inglese, P.; Martinelli, F. Metabolic profiling and post-harvest behavior of “Dottato” fig (Ficus carica L.) fruit covered with an edible coating from O. ficus-indica. Front. Plant Sci. 2018, 9, 1321. [Google Scholar] [CrossRef]
- Golombek, S.D.; Lüdders, P. Effects of short-term salinity on leaf gas exchange of the fig (Ficus carica L.). Plant Soil 1993, 148, 21–27. [Google Scholar] [CrossRef]
- Vangelisti, A.; Zambrano, L.S.; Caruso, G.; Macheda, D.; Bernardi, R.; Usai, G.; Mascagni, F.; Giordani, T.; Gucci, R.; Cavallini, A.; et al. How an ancient, salt-tolerant fruit crop, Ficus carica L., copes with salinity: A transcriptome analysis. Sci. Rep. 2019, 9, 2561. [Google Scholar] [CrossRef] [Green Version]
- Sadder, M.T.; Alshomali, I.; Ateyyeh, A.; Musallam, A. Physiological and molecular responses for long term salinity stress in common fig (Ficus carica L.). Physiol. Mol. Biol. Plants 2021, 27, 107–117. [Google Scholar] [CrossRef]
- Caruso, G.; Gennai, C.; Ugolini, F.; Marchini, F.; Quartacci, M.F.; Gucci, R. Tolerance and physiological response of young Ficus carica L. plants irrigated with saline water. Acta Hortic. 2017, 1173, 137–141. [Google Scholar] [CrossRef]
- Tóth, G.; Adhikari, K.; Várallyay, G.; Tóth, T.; Bódis, K.; Stolbovoy, V. Update map of salt affected soils in the European Union. In Threats to Soil Quality in Europe; Office for Official Publications of the European Communities: Luxemburg, 2008; pp. 67–77. ISBN 9789279095290. [Google Scholar]
- Everard, J.D.; Gucci, R.; Kann, S.C.; Flore, J.A.; Loescher, W.H. Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol. 1994, 106, 281–292. [Google Scholar] [CrossRef] [Green Version]
- Gucci, R.; Moing, A.; Gravano, E.; Gaudillere, J. Partitioning of photosynthetic carbohydrates in leaves of salt-stressed olive plants. Aust. J. Plant Physiol. 1998, 25, 571–579. [Google Scholar] [CrossRef]
- Hoffman, G.J.; Catlin, P.B.; Mead, R.M.; Johnson, R.S.; Francois, L.E.; Goldhamer, D. Yield and foliar injury responses of mature plum trees to salinity. Irrig. Sci. 1989, 10, 215–229. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. HortScience 2006, 41, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Saied, A.S.; Keutgen, A.J.; Noga, G. The influence of NaCl salinity on growth, yield and fruit quality of strawberry cvs. “Elsanta” and “Korona”. Sci. Hortic. (Amst.) 2005, 103, 289–303. [Google Scholar] [CrossRef]
- Saito, T.; Matsukura, C.; Ban, Y.; Shoji, K.; Sugiyama, M.; Fukuda, N.; Nishimura, S. Salinity stress affects assimilate metabolism at the gene-expression level during fruit development and improves fruit quality in tomato (Solanum lycopersicum L.). J. Jpn. Soc. Hortic. Sci. 2008, 77, 61–68. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.G.; Kobayashi, Y.; Sanuki, A.; Kondo, S.; Fukuda, N.; Ezura, H.; Sugaya, S.; Matsukura, C. Salinity induces carbohydrate accumulation and sugar-regulated starch biosynthetic genes in tomato (Solanum lycopersicum L. cv. ’Micro-Tom’) fruits in an ABA-and osmotic stress-independent manner. J. Exp. Bot. 2010, 61, 563–574. [Google Scholar] [CrossRef]
- Vemmos, S.N.; Petri, E.; Stournaras, V. Seasonal changes in photosynthetic activity and carbohydrate content in leaves and fruit of three fig cultivars (Ficus carica L.). Sci. Hortic. (Amst.) 2013, 160, 198–207. [Google Scholar] [CrossRef]
- Brown, P.H.; Hu, H. Phloem mobility of boron is species dependent: Evidence for phloem mobility in sorbitol-rich species. Ann. Bot. 1996, 77, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Usai, G.; Mascagni, F.; Giordani, T.; Vangelisti, A.; Bosi, E.; Zuccolo, A.; Ceccarelli, M.; King, R.; Hassani-Pak, K.; Zambrano, L.S.; et al. Epigenetic patterns within the haplotype phased fig (Ficus carica L.) genome. Plant J. 2020, 102, 600–614. [Google Scholar] [CrossRef] [Green Version]
- Usai, G.; Vangelisti, A.; Simoni, S.; Giordani, T.; Natali, L.; Cavallini, A.; Mascagni, F. DNA modification patterns within the transposable elements of the fig (Ficus carica L.) genome. Plants 2021, 10, 451. [Google Scholar] [CrossRef]
- Vangelisti, A.; Simoni, S.; Usai, G.; Ventimiglia, M.; Natali, L.; Cavallini, A.; Mascagni, F.; Giordani, T. LTR-retrotransposon dynamics in common fig (Ficus carica L.) genome. BMC Plant Biol. 2021, 21, 221. [Google Scholar] [CrossRef]
- Mori, K.; Shirasawa, K.; Nogata, H.; Hirata, C.; Tashiro, K.; Habu, T.; Kim, S.; Himeno, S.; Kuhara, S.; Ikegami, H. Identification of RAN1 orthologue associated with sex determination through whole genome sequencing analysis in fig (Ficus carica L.). Sci. Rep. 2017, 7, 41124. [Google Scholar] [CrossRef] [Green Version]
- Usai, G.; Vangelisti, A.; Solorzano-Zambrano, L.; Mascagni, F.; Giordani, T.; Cavallini, A.; Natali, L. Transcriptome comparison between two fig (Ficus carica L.) cultivars. Agrochimica 2017, 61, 340–354. [Google Scholar] [CrossRef]
- Fattorini, C.; Bernardi, R.; Quartacci, M.F.; Mascgni, F.; Caruso, G.; Cavallini, A.; Gucci, R.; Natali, L. Expression of genes involved in metabolism and transport of soluble carbohydrates during fruit ripening in two cultivars of Ficus carica L. Agrochimica 2021, 65, 117–136. [Google Scholar] [CrossRef]
- Ikegami, H.; Habu, T.; Mori, K.; Nogata, H.; Hirata, C.; Hirashima, K.; Tashiro, K.; Kuhara, S. De novo sequencing and comparative analysis of expressed sequence tags from gynodioecious fig (Ficus carica L.) fruits: Caprifig and common fig. Tree Genet. Genomes 2013, 9, 1075–1088. [Google Scholar] [CrossRef]
- Desnoues, E.; Gibon, Y.; Baldazzi, V.; Signoret, V.; Génard, M.; Quilot-Turion, B. Profiling sugar metabolism during fruit development in a peach progeny with different fructose-to-glucose ratios. BMC Plant Biol. 2014, 14, 12–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaki, S.; Moriguchi, T. Seasonal fluctuation of sorbitol-related enzymes and invertase activities accompanying maturation of Japanese pear (Pyrus serotina Rehder var. culta Rehder) fruit. J. Jpn. Soc. Hortic. Sci. 1989, 57, 602–607. [Google Scholar] [CrossRef] [Green Version]
- Smirnoff, N.; Cumbes, Q.J. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Williamson, J.D.; Stoop, J.M.H.; Massel, M.O.; Conkling, M.A.; Pharr, D.M. Sequence analysis of a mannitol dehydrogenase cDNA from plants reveals a function for the pathogenesis-related protein ELI3. Proc. Natl. Acad. Sci. USA 1995, 92, 7148–7152. [Google Scholar] [CrossRef] [Green Version]
- Jennings, D.B.; Ehrenshaft, M.; Mason Pharr, D.; Williamson, J.D. Roles for mannitol and mannitol dehydrogenase in active oxygen-mediated plant defense. Proc. Natl. Acad. Sci. USA 1998, 95, 15129–15133. [Google Scholar] [CrossRef] [Green Version]
- Conde, C.; Silva, P.; Agasse, A.; Lemoine, R.; Delrot, S.; Tavares, R.; Gerós, H. Utilization and transport of mannitol in Olea europaea and implications for salt stress tolerance. Plant Cell Physiol. 2007, 48, 42–53. [Google Scholar] [CrossRef] [Green Version]
- Gupta, B.; Huang, B. Mechanism of Salinity Tolerance in Plants: Physiological, Biochemical, and Molecular Characterization. Int. J. Genom. 2014, 2014, 701596. [Google Scholar] [CrossRef]
- Conde, C.; Delrot, S.; Gerós, H. Physiological, biochemical and molecular changes occurring during olive development and ripening. J. Plant Physiol. 2008, 165, 1545–1562. [Google Scholar] [CrossRef]
- Lu, S.; Li, T.; Jiang, J. Effects of salinity on sucrose metabolism during tomato fruit development. Afr. J. Biotechnol. 2010, 9, 842–849. [Google Scholar]
- Saito, T.; Fukuda, N.; Matsukura, C.; Nishimura, S. Effects of salinity on distribution of photosynthates and carbohydrate metabolism in tomato grown using nutrient film technique. J. Jpn. Soc. Hortic. Sci. 2009, 78, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Lü, H.; Li, J.; Huang, Y.; Zhang, M.; Zhang, S.; Wu, J. Genome-wide identification, expression and functional analysis of the phosphofructokinase gene family in Chinese white pear (Pyrus bretschneideri). Gene 2019, 702, 133–142. [Google Scholar] [CrossRef] [PubMed]
- Mustroph, A.; Stock, J.; Hess, N.; Aldous, S.; Dreilich, A.; Grimm, B. Characterization of the phosphofructokinase gene family in rice and its expression under oxygen deficiency stress. Front. Plant Sci. 2013, 4, 125. [Google Scholar] [CrossRef] [Green Version]
- Moles, T.M.; de Brito Francisco, R.; Mariotti, L.; Pompeiano, A.; Lupini, A.; Incrocci, L.; Carmassi, G.; Scartazza, A.; Pistelli, L.; Guglielminetti, L.; et al. Salinity in autumn-winter season and fruit quality of tomato landraces. Front. Plant Sci. 2019, 10, 1078. [Google Scholar] [CrossRef]
- Gao, Z.; Sagi, M.; Lips, S.H. Carbohydrate metabolism in leaves and assimilate partitioning in fruits of tomato (Lycopersicon esculentum L.) as affected by salinity. Plant Sci. 1998, 135, 149–159. [Google Scholar] [CrossRef]
- Ersoy, N. Changes in sugar contents of fig fruit (Ficus carica L. cv. Bursa Siyahı) during development. SDÜ Ziraat Fakültesi Derg. 2007, 2, 22–26. [Google Scholar]
- Slatnar, A.; Klancar, U.; Stampar, F.; Veberic, R. Effect of drying of figs (Ficus carica L.) on the contents of sugars, organic acids, and phenolic compounds. J. Agric. Food Chem. 2011, 59, 11696–11702. [Google Scholar] [CrossRef] [PubMed]
- Sedaghat, S.; Rahemi, M. Enzyme activity regarding sugar and organic acid changes during developmental stages in rainfed fig (Ficus carica L.cv Sabz). Int. J. Fruit Sci. 2018, 18, 14–28. [Google Scholar] [CrossRef]
- Lo Bianco, R.; Rieger, M.; Sung, S.J.S. Effect of drought on sorbitol and sucrose metabolism in sinks and sources of peach. Physiol. Plant. 2000, 108, 71–78. [Google Scholar] [CrossRef]
- Li, M.; Li, P.; Ma, F.; Dandekar, A.M.; Cheng, L. Sugar metabolism and accumulation in the fruit of transgenic apple trees with decreased sorbitol synthesis. Hortic. Res. 2018, 5. [Google Scholar] [CrossRef] [Green Version]
- Marei, N.; Crane, J.C. Growth and respiratory response of fig (Ficus carica L. cv. Mission) fruits to ethylene. Plant Physiol. 1971, 48, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Choi, Y.H.; Verpoorte, R. NMR-based metabolomic analysis of plants. Nat. Protoc. 2010, 5, 536–549. [Google Scholar] [CrossRef]


| Soluble Carbohydrates | Control | 100 mM NaCl-Stressed | p-Value |
|---|---|---|---|
| D-Fructose | 242.68 ± 7.73 | 235.87 ± 3.64 | 0.24 |
| D-Glucose | 240.92 ± 15.71 | 219.30 ± 5.29 | 0.09 |
| Sucrose | 27.9 ± 0.3 | 34.31 ± 3.51 | 0.03 |
| D-Sorbitol | 0.99 ± 0.23 | 1.48 ± 0.15 | 0.04 |
| Total | 511.68 ± 23.04 | 490.97 ± 2.14 | 0.18 |
| Ratios | |||
| Glucose/Fructose | 99.22 ± 3.76 | 92.99 ± 3.04 | 0.09 |
| Sucrose/Fructose | 11.5 ± 0.25 | 14.55 ± 1.55 | 0.03 |
| Sucrose/Glucose | 11.61 ± 0.64 | 15.68 ± 1.95 | 0.03 |
| Sucrose/Glucose + Fructose | 5.78 ± 0.22 | 7.55 ± 0.87 | 0.03 |
| Sorbitol/Sucrose | 3.55 ± 0.82 | 4.35 ± 0.69 | 0.26 |
| Sorbitol/Glucose | 0.42 ± 0.12 | 0.68 ± 0.08 | 0.03 |
| Sorbitol/Fructose | 0.41 ± 0.10 | 0.63 ± 0.06 | 0.03 |
| Sorbitol/Glucose + Fructose | 0.21 ± 0.05 | 0.33 ± 0.03 | 0.03 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascellani, A.; Natali, L.; Cavallini, A.; Mascagni, F.; Caruso, G.; Gucci, R.; Havlik, J.; Bernardi, R. Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato). Plants 2021, 10, 1861. https://doi.org/10.3390/plants10091861
Mascellani A, Natali L, Cavallini A, Mascagni F, Caruso G, Gucci R, Havlik J, Bernardi R. Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato). Plants. 2021; 10(9):1861. https://doi.org/10.3390/plants10091861
Chicago/Turabian StyleMascellani, Anna, Lucia Natali, Andrea Cavallini, Flavia Mascagni, Giovanni Caruso, Riccardo Gucci, Jaroslav Havlik, and Rodolfo Bernardi. 2021. "Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato)" Plants 10, no. 9: 1861. https://doi.org/10.3390/plants10091861
APA StyleMascellani, A., Natali, L., Cavallini, A., Mascagni, F., Caruso, G., Gucci, R., Havlik, J., & Bernardi, R. (2021). Moderate Salinity Stress Affects Expression of Main Sugar Metabolism and Transport Genes and Soluble Carbohydrate Content in Ripe Fig Fruits (Ficus carica L. cv. Dottato). Plants, 10(9), 1861. https://doi.org/10.3390/plants10091861