Coupling Relationship of Leaf Economic and Hydraulic Traits of Alhagisparsifolia Shap. in a Hyper-Arid Desert Ecosystem
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling Sites
2.2. Measurement of Traits
2.2.1. Economic Traits
2.2.2. Hydraulic Traits
- SDada, stomata number per area on the adaxial surface of a leaf;
- SDaba, stomata number per area on the abaxial surface of a leaf;
- SLada, the length of the guard cell on the adaxial surface of a leaf;
- SLaba: the length of the guard cell on the abaxial surface of a leaf.
2.2.3. Mesophyll Structure
2.2.4. Soil Properties
2.3. Statistical Analysis
3. Results
3.1. Variation in Leaf Functional Traits
3.2. Relationships among Leaf Economic Traits, Hydraulic Traits, and Mesophyll Structure
3.3. Contributions of the Environmental Variables on Leaf Functional Traits
4. Discussion
4.1. Coupling Relationship between Leaf Economic and Hydraulic Traits Based on the Mesophyll Structure
4.2. Contributions of Environmental Variables on the Leaf Functional Traits of A. sparsifolia
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wright, I.J.; Reich, P.B.; Westoby, M.; Ackerly, D.D.; Baruch, Z.; Bongers, F.; Cavender-Bares, J.; Chapin, T.; Cornelissen, J.H.C.; Diemer, M.; et al. The worldwide leaf economics spectrum. Nature 2004, 428, 821–827. [Google Scholar] [CrossRef]
- Reich, P.B. The world-wide ‘fast-slow’ plant economics spectrum: A traits manifesto. J. Ecol. 2014, 102, 275–301. [Google Scholar] [CrossRef]
- Li, L.; McCormack, M.L.; Ma, C.; Kong, D.; Zhang, Q.; Chen, X.; Zeng, H.; Niinemets, U.; Guo, D. Leaf economics and hydraulic traits are decoupled in five species-rich tropical-subtropical forests. Ecol. Lett. 2015, 18, 899–906. [Google Scholar] [CrossRef] [PubMed]
- Yin, Q.; Wang, L.; Lei, M.; Dang, H.; Quan, J.; Tian, T.; Chai, Y.; Yue, M. The relationships between leaf economics and hydraulic traits of woody plants depend on water availability. Sci. Total Environ. 2018, 621, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Sack, L.; Cowan, P.D.; Jaikumar, N.; Holbrook, N.M. The ‘hydrology’ of leaves: Co-ordination of structure and function in temperate woody species. Plant Cell Environ. 2003, 26, 1343–1356. [Google Scholar] [CrossRef] [Green Version]
- de Boer, H.J.; Drake, P.L.; Wendt, E.; Price, C.A.; Schulze, E.D.; Turner, N.C.; Nicolle, D.; Veneklaas, E.J. Apparent Overinvestment in Leaf Venation Relaxes Leaf Morphological Constraints on Photosynthesis in Arid Habitats. Plant Physiol. 2016, 172, 2286–2299. [Google Scholar] [CrossRef] [PubMed]
- Xiong, D.; Flexas, J. Leaf economics spectrum in rice: Leaf anatomical, biochemical, and physiological trait trade-offs. J. Exp. Bot. 2018, 69, 5599–5609. [Google Scholar]
- Pathare, V.S.; Koteyeva, N.; Cousins, A.B. Increased adaxial stomatal density is associated with greater mesophyll surface area exposed to intercellular air spaces and mesophyll conductance in diverse C4 grasses. New Phytol. 2020, 225, 169–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blonder, B.; Violle, C.; Bentley, L.P.; Enquist, B.J. Venation networks and the origin of the leaf economics spectrum. EcolLett 2011, 14, 91–100. [Google Scholar] [CrossRef]
- Sack, L.; Scoffoni, C.; John, G.P.; Poorter, H.; Mason, C.M.; Mendez-Alonzo, R.; Donovan, L.A. Leaf mass per area is independent of vein length per area: Avoiding pitfalls when modelling phenotypic integration (reply to Blonder et al. 2014). J. Exp. Bot. 2014, 65, 5115–5123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, N.P.; Li, Y.; Liu, C.C.; Xu, L.; Li, M.X.; Zhang, J.H.; He, J.S.; Tang, Z.Y.; Han, X.G.; Ye, Q.; et al. Plant Trait Networks: Improved Resolution of the Dimensionality of Adaptation. Trends Ecol. Evol. 2020, 35, 908–918. [Google Scholar] [CrossRef]
- Simonin, K.A.; Limm, E.B.; Dawson, T.E. Hydraulic conductance of leaves correlates with leaf lifespan: Implications for lifetime carbon gain. New Phytol. 2012, 193, 939–947. [Google Scholar] [CrossRef]
- Blonder, B.; Violle, C.; Enquist, B.J. Assessing the causes and scales of the leaf economics spectrum using venation networks in Populus tremuloides. J. Ecol. 2013, 101, 981–989. [Google Scholar] [CrossRef]
- McDowell, N.; Pockman, W.T.; Allen, C.D.; Breshears, D.D.; Cobb, N.; Kolb, T.; Plaut, J.; Sperry, J.; West, A.; Williams, D.G.; et al. Mechanisms of plant survival and mortality during drought: Why do some plants survive while others succumb to drought? New Phytol. 2008, 178, 719–739. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhou, G. Responses of leaf stomatal density to water status and its relationship with photosynthesis in a grass. J. Exp. Bot. 2008, 59, 3317–3325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.B.; Guan, Z.J.; Sun, M.; Zhang, J.J.; Cao, K.F.; Hu, H. Evolutionary association of stomatal traits with leaf vein density in Paphiopedilum, Orchidaceae. PLoS ONE 2012, 7, e40080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sack, L.; Scoffoni, C. Leaf venation: Structure, function, development, evolution, ecology and applications in the past, present and future. New Phytol. 2013, 198, 983–1000. [Google Scholar] [CrossRef] [PubMed]
- Li, F.L.; McCulloh, K.A.; Sun, S.J.; Bao, W. Linking leaf hydraulic properties, photosynthetic rates, and leaf lifespan in xerophytic species: A test of global hypotheses. Am. J. Bot. 2018, 105, 1858–1868. [Google Scholar] [CrossRef]
- Liu, C.C.; Li, Y.; Xu, L.; Chen, Z.; He, N.P. Variation in leaf morphological, stomatal, and anatomical traits and their relationships in temperate and subtropical forests. Sci. Rep. 2019, 9, 5803. [Google Scholar] [CrossRef] [Green Version]
- Martínez-Vilalta, J.; García-Forner, N. Water potential regulation, stomatalbehaviour and hydraulic transport under drought: Deconstructing the iso/anisohydric concept. Plant Cell Environ. 2016, 40, 962–976. [Google Scholar] [CrossRef] [Green Version]
- Hochberg, U.; Rockwell, F.E.; Holbrook, N.M.; Cochard, H. Iso/anisohydry: A plant-environment interaction rather than a simple hydraulic trait. Trends Plant Sci. 2018, 23, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Álvarez-Maldini, C.; Acevedo, M.; Pinto, M. Hydroscapes: A Useful Metric for Distinguishing Iso-/Anisohydric Behavior in Almond Cultivars. Plants 2021, 10, 1249. [Google Scholar] [CrossRef]
- Guo, J.S.; Hultine, K.R.; Koch, G.W.; Kropp, H.; Ogle, K. Temporal shifts in iso/anisohydry revealed from daily observations of plant water potential in a dominant desert shrub. New Phytol. 2020, 225, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Guan, K.; Li, Y.; Novick, K.A.; Feng, X.; McDowell, N.G.; Konings, A.G.; Thompson, S.E.; Kimball, J.S.; De Kauwe, M.G.; et al. Interannual variability of ecosystem iso/anisohydry is regulated by environmental dryness. New Phytol. 2020, 229, 2562–2575. [Google Scholar] [CrossRef] [PubMed]
- Harrison, S.; Spasojevic, M.J.; Li, D. Climate and plant community diversity in space and time. Proc. Natl. Acad. Sci. USA 2020, 117, 4464–4470. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, G.; Li, M.; Zhang, M.; Zhang, L.; Zhang, X.; An, L.; Xu, S. C:N:P stoichiometry and leaf traits of halophytes in an arid saline environment, northwest China. PLoS ONE 2015, 10, e0119935. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Su, Y.; Yang, R. Variation of soil organic carbon, nitrogen, and phosphorus stoichiometry and biogeographic factors across the desert ecosystem of Hexi Corridor, northwestern China. J. Soil Sediment. 2018, 19, 49–57. [Google Scholar] [CrossRef]
- Perri, S.; Katul, G.G.; Molini, A. Xylem-phloem hydraulic coupling explains multiple osmo regulatory responses to salt stress. New Phytol. 2019, 224, 644–662. [Google Scholar] [CrossRef]
- Jin, Q.H. Population chatacter of Alhagisparsifolia and plant community succession. Acta Phytoecol. Sin. 1995, 19, 255–260. (In Chinese) [Google Scholar]
- Li, X.Y.; Zhang, X.M.; Zeng, F.J.; Foetzki, A.; Thomas, F.M.; Li, X.M.; Runge, M.; He, X.Y. Water relations on Alhagisparsifolia in the southern fringe of Taklamakan Desert. Acta Bot. Sin. 2002, 44, 1219–1224. (In Chinese) [Google Scholar]
- Yang, G.; Li, Y. A study on the ecological type of Alhagi from their Anatomy. Arid Zone Res. 1992, 4, 21–25. (In Chinese) [Google Scholar]
- Drake, P.L.; de Boer, H.J.; Schymanski, S.J.; Veneklaas, E.J. Two sides to every leaf: Water and CO2 transport in hypostomatous and amphistomatous leaves. New Phytol. 2019, 222, 1179–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, C.X.; Huang, Z.S. A preliminary study of xeromorphism of some important xerophytes growing in Tungeli Desert. J. Integr. Plant Biol. 1981, 23, 278–283. (In Chinese) [Google Scholar]
- Sack, L.; Frole, K. Leaf structural diversity is related to hydraulic capacity in tropical rain forest trees. Ecology 2006, 87, 483–491. [Google Scholar] [CrossRef]
- Wang, H. An Empirical Analysis on Hydraulic Lift of A. sparsifolia and T. ramosissima at the Southern Fringe of the Taklimakan Desert. Master’s Thesis, Chinese Academy of Sciences, Beijing, China, 2014. [Google Scholar]
- Caldwell, M.M.; Richards, J.H. Hydraulic lift: Water efflux from upper roots improves effectiveness of water uptake by deep roots. Oecologia 1989, 79, 1–5. [Google Scholar] [CrossRef]
- Caldwell, M.; Dawson, T.; Richards, J. Hydraulic lift: Consequences of water efflux from the roots of plants. Oecologia 1998, 113, 151–161. [Google Scholar] [CrossRef] [PubMed]
- Domec, J.C.; Warren, J.M.; Meinzer, F.C.; Brooks, J.R.; Coulombe, R. Native root xylem embolism and stomatal closure in stands of Douglas-fir and ponderosa pine: Mitigation by hydraulic redistribution. Oecologia 2004, 141, 7–16. [Google Scholar] [CrossRef]
- Zhang, L.; Mai, M.; An, N.; Xia, Y. Effects of summer irrigation on morphological characteristics, community ecological structure and natural regeneration of A. sparsifolia. Arid Zone Res. 1995, 12, 34–40. (In Chinese) [Google Scholar]
- Wang, J.; Ding, J.; Yu, D.; Teng, D.; He, B.; Chen, X.; Ge, X.; Zhang, Z.; Wang, Y.; Yang, X.; et al. Machine learning-based detection of soil salinity in an arid desert region, Northwest China: A comparison between Landsat-8 OLI and Sentinel-2 MSI. Sci. Total Environ. 2020, 707, 136092. [Google Scholar] [CrossRef]
- Munns, R. Comparative physiology of salt and water stress. Plant Cell Environ. 2002, 25, 239–250. [Google Scholar] [CrossRef]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Saf. 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Perri, S.; Entekhabi, D.; Molini, A. Plant osmoregulation as an emergent water-saving adaptation. Water Resour. Res. 2018, 54, 2781–2798. [Google Scholar] [CrossRef]
- Perri, S.; Suweis, S.; Holmes, A.; Marpu, P.R.; Entekhabi, D.; Molini, A. River basin salinization as a form of aridity. Proc. Natl. Acad. Sci. USA 2020, 117, 17635–17642. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.; Chu, L.; Lu, H.; Qi, W.; Chen, X.; Liu, J.; Kuang, S.; Tang, B.; Wong, V. Towards sustainable agriculture for the salt-affected soil. Land Degrad. Dev. 2019, 30, 574–579. [Google Scholar] [CrossRef]
- Zhao, K.; Song, J.; Feng, G.; Zhao, M.; Liu, J. Species, types, distribution, and economic potential of halophytes in China. Plant Soil. 2010, 342, 495–509. [Google Scholar] [CrossRef]
- Luo, H.; Zeng, F.; Zhang, B.; Liu, B.; Zhao, S.; Gao, H. Ion distribution, absorption and translocation characteristics of Alhagisparsifolia in adaptation to saline habitat. Chin. J. App. Ecol. 2016, 27, 3514–3520. (In Chinese) [Google Scholar]
- Zhao, S.; Zeng, F.; Zhang, B.; Liu, B.; Gao, H.; Luo, H. Effect of salt stress on leaf traits of Alhagisparsifolia. Pratacult. Sci. 2016, 33, 1770–1778. (In Chinese) [Google Scholar]
- Arndt, S.K.; Kahmen, A.; Arampatsis, C.; Popp, M.; Adams, M. Nitrogen fixation and metabolism by groundwater-dependent perennial plants in a hyperarid desert. Oecologia 2004, 141, 385–394. [Google Scholar] [CrossRef]
- Li, M.M.; Petrie, M.D.; Tariq, A.; Zeng, F. Response of nodulation, nitrogen fixation to salt stress in a desert legume Alhagi sparsifolia. Environ. Exp. Bot. 2021, 183, 104348. [Google Scholar] [CrossRef]
- Fick, S.E.; Hijmans, R.J. WorldClim 2: New 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 2017, 37, 4302–4315. [Google Scholar] [CrossRef]
- Gomes, A.L.; Revermann, R.; Meller, P.; Goncalves, F.M.P.; Aidar, M.P.M.; Lages, F.; Finckh, M. Functional traits and symbiotic associations of geoxyles and trees explain the dominance of detarioid legumes in miombo ecosystems. New Phytol. 2021, 230, 510–520. [Google Scholar] [CrossRef] [PubMed]
- Franks, P.J.; Drake, P.L.; Beerling, D.J. Plasticity in maximum stomatal conductance constrained by negative correlation between stomatal size and density: An analysis using Eucalyptus globulus. Plant Cell Environ. 2009, 32, 1737–1748. [Google Scholar] [CrossRef]
- Bao, S.D. Agro-Chemical Analysis of Soil; China Agricultural Press: Beijing, China, 2000. [Google Scholar]
- Malacarne, R.L. Canonical Correlation Analysis. Math. J. 2014. [Google Scholar] [CrossRef] [Green Version]
- The SPSSAU Project. SPSSAU (Version 20.0). 2020. Available online: https://www.spssau.com (accessed on 15 July 2020).
- Heilmeier, H. Functional traits explaining plant responses to past and future climate changes. Flora 2019, 254, 1–11. [Google Scholar] [CrossRef]
- Michelaki, C.; Fyllas, N.M.; Galanidis, A.; Aloupi, M.; Evangelou, E.; Arianoutsou, M.; Dimitrakopoulos, P.G. An integrated phenotypic trait-network in thermo-Mediterranean vegetation describing alternative, coexisting resource-use strategies. Sci. Total Environ. 2019, 672, 583–592. [Google Scholar] [CrossRef]
- Hetherington, A.M.; Woodward, F.I. The role of stomata in sensing and driving environmental change. Nature 2003, 424, 901–908. [Google Scholar] [CrossRef]
- Franks, P.J.; Farquhar, G.D. A relationship between humidity response, growth form and photosynthetic operating point in C-3 plants. Plant Cell Environ. 1999, 22, 1337–1349. [Google Scholar] [CrossRef]
- Deng, X.; Li, X.; Zhang, X.; Ye, W. A study of the gas exchange characteristics of four desert plants. Acta Phytoecol. Sin. 2002, 26, 605–612. (In Chinese) [Google Scholar]
- Buckley, T.N. The contributions of apoplastic, symplastic and gas phase pathways for water transport outside the bundle sheath in leaves. Plant Cell Environ. 2015, 38, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Flexas, J.; Barbour, M.M.; Brendel, O.; Cabrera, H.M.; Carriqui, M.; Diaz-Espejo, A.; Douthe, C.; Dreyerc, E.; Ferrio, J.P.; Gago, J.; et al. Mesophyll diffusion conductance to CO2: An unappreciated central player in photosynthesis. Plant Sci. 2012, 193–194, 70–84. [Google Scholar] [CrossRef]
- Ren, T.; Weraduwage, S.M.; Sharkey, T.D. Prospects for enhancing leaf photosynthetic capacity by manipulating mesophyll cell morphology. J Exp. Bot. 2019, 70, 1153–1165. [Google Scholar] [CrossRef]
- Parkhurst, D.F. Adaptive significance of stomatal occurrence on one or both surfaces of leaves. J. Ecol. 1978, 66, 367–383. [Google Scholar] [CrossRef]
- Parkhurst, D.F.; Mott, K.A. Intercellular diffusion limits to CO2 uptake in leaves. Plant Physiol. 1990, 94, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Richardson, F.; Brodribb, T.J.; Jordan, G.J. Amphistomatic leaf surfaces independently regulate gas exchange in response to variations in evaporative demand. Tree Physiol. 2017, 37, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Mott, K.A. Leaf hydraulic conductivity and stomatal responses to humidity in amphistomatous leaves. Plant Cell Environ. 2007, 30, 1444–1449. [Google Scholar] [CrossRef]
- Mott, K.A.; Peak, D. Effects of the mesophyll on stomatal responses in amphistomatous leaves. Plant Cell Environ. 2018, 41, 2835–2843. [Google Scholar] [CrossRef]
- Mott, K.A.; O’Leary, J.W. Stomatal Behavior and CO2 Exchange Characteristics in Amphistomatous Leaves. Plant Physiol. 1984, 74, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynth. Res. 2017, 131, 333–350. [Google Scholar] [CrossRef]
- Serbin, S.P.; Wu, J.; Ely, K.S.; Kruger, E.L.; Townsend, P.A.; Meng, R.; Wolfe, B.T.; Chlus, A.; Wang, Z.H.; Rogers, A. From the Arctic to the tropics: Multibiome prediction of leaf mass per area using leaf reflectance. New Phytol. 2019, 224, 1557–1568. [Google Scholar] [CrossRef]
- Niinemets, U.; Diaz-Espejo, A.; Flexas, J.; Galmes, J.; Warren, C.R. Role of mesophyll diffusion conductance in constraining potential photosynthetic productivity in the field. J. Exp. Bot. 2009, 60, 2249–2270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lambers, H.; Poorter, H. Inherent variation in growth-rate between higher plants-a search for physiological cause and ecological consequences. Adv. Ecol. Res. 1992, 23, 187–261. [Google Scholar]
- Brodribb, T.J.; Feild, T.S.; Sack, L. Viewing leaf structure and evolution from a hydraulic perspective. Funct. Plant Biol. 2010, 37, 488–498. [Google Scholar] [CrossRef]
- Waite, M.; Sack, L. How does moss photosynthesis relate to leaf and canopy structure? Trait relationships for 10 Hawaiian species of contrasting light habitats. New Phytol. 2010, 185, 156–172. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Peng, Q.; Li, K.; Gong, Y.; Liu, Y.; Han, W. Patterns of nitrogen and phosphorus stoichiometry among leaf, stem and root of desert plants and responses to climate and soil factors in Xinjiang, China. Catena 2021, 199, 105100. [Google Scholar] [CrossRef]
- He, M.; Zhang, K.; Tan, H.; Hu, R.; Su, J.; Wang, J.; Huang, L.; Zhang, Y.; Li, X. Nutrient levels within leaves, stems, and roots of the xeric species Reaumuria soongorica in relation to geographical, climatic, and soil conditions. Ecol. Evol. 2015, 5, 1494–1503. [Google Scholar] [CrossRef]
- Laughlin, D.C. Applying trait-based models to achieve functional targets for theory-driven ecological restoration. Ecol. Lett. 2014, 17, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Ferrarini, A.; Dai, J.H.; Bai, Y.; Alatalo, J.M. Redefining the climate niche of plant species: A novel approach for realistic predictions of species distribution under climate change. Sci. Total Environ. 2019, 671, 1086–1093. [Google Scholar] [CrossRef]
- Guo, Y.; Jiang, M.; Liu, Q.; Xie, Z.; Tang, Z. Climate and vegetation together control the vertical distribution of soil carbon, nitrogen and phosphorus in shrublands in China. Plant Soil. 2020, 456, 15–26. [Google Scholar] [CrossRef]
- Felle, H.H.; Hanstein, S.; Steinmeyer, R.; Hedrich, R. Dynamics of ionic activities in the apoplast of the sub-stomatal cavity of intact Viciafaba leaves during stomatal closure evoked by ABA and darkness. Plant J. 2000, 24, 297–304. [Google Scholar] [CrossRef]
- Nunes, T.D.G.; Zhang, D.; Raissig, M.T. Form, development and function of grass stomata. Plant J. 2020, 101, 780–799. [Google Scholar] [CrossRef]
- Poorter, H.; Niinemets, Ü.; Poorter, L.; Wright, I.J.; Villar, R. Causes and consequences of variation in leaf mass per area (LMA): A meta-analysis. New Phytol. 2009, 182, 565–588. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Sun, J.; Zarif, N.; Khan, K.; Jamil, M.A.; Yang, L.; Clothier, B.; Rewald, B. Effects of Increased N Deposition on Leaf Functional Traits of Four Contrasting Tree Species in Northeast China. Plants 2020, 9, 1231. [Google Scholar] [CrossRef]
- Shields, L.M. Leaf xeromorphy as related to physiological and structural influences. Bot. Rev. 1950, 16, 399–447. [Google Scholar] [CrossRef]
- Longstreth, D.J.; Nobel, P.S. Salinity effects on leaf anatomy. Consequences for photosynthesis. Plant Physiol. 1979, 63, 700–703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlowski, T.T. Responses of woody plants to flooding and salinity. Tree Physiol. 1997, 1, 1–29. [Google Scholar] [CrossRef]
- Keiser, A.D.; Smith, M.; Bell, S.; Hofmockel, K.S. Peatland microbial community response to altered climate tempered by nutrient availability. Soil Boil Biochem. 2019, 137, 107561. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Xiong, K.; Yu, Y.; Min, X. Changes of leaf functional traits in karst rocky desertification ecological environment and the driving factors. Glob. Ecol. Conserv. 2020, 24, e01381. [Google Scholar] [CrossRef]
- Kamaluddin, M.; Zwiazek, J.J. Effects of root medium pH on water transport in paper birch (Betula papyrifera) seedlings in relation to root temperature and abscisic acid treatments. Tree Physiol. 2004, 24, 1173–1180. [Google Scholar] [CrossRef] [Green Version]
- Siemens, J.A.; Zwiazek, J.J. Hebeloma crustuliniforme modifies root hydraulic responses of trembling aspen (Populus tremuloides) seedlings to changes in external pH. Plant Soil. 2011, 345, 247–256. [Google Scholar] [CrossRef]
- Seligmana, N.G.; Sinclair, T.R. Global environment change and simulated forage quality of wheat II. Water and nitrogen stress. Field Crop Res. 1995, 40, 29–37. [Google Scholar] [CrossRef]
- Wright, I.J.; Reich, P.B.; Westoby, M. Strategy shifts in leaf physiology, structure and nutrient content between species of high- and low-rainfall and high- and low-nutrient habitats. Funct. Ecol. 2001, 15, 423–434. [Google Scholar] [CrossRef] [Green Version]
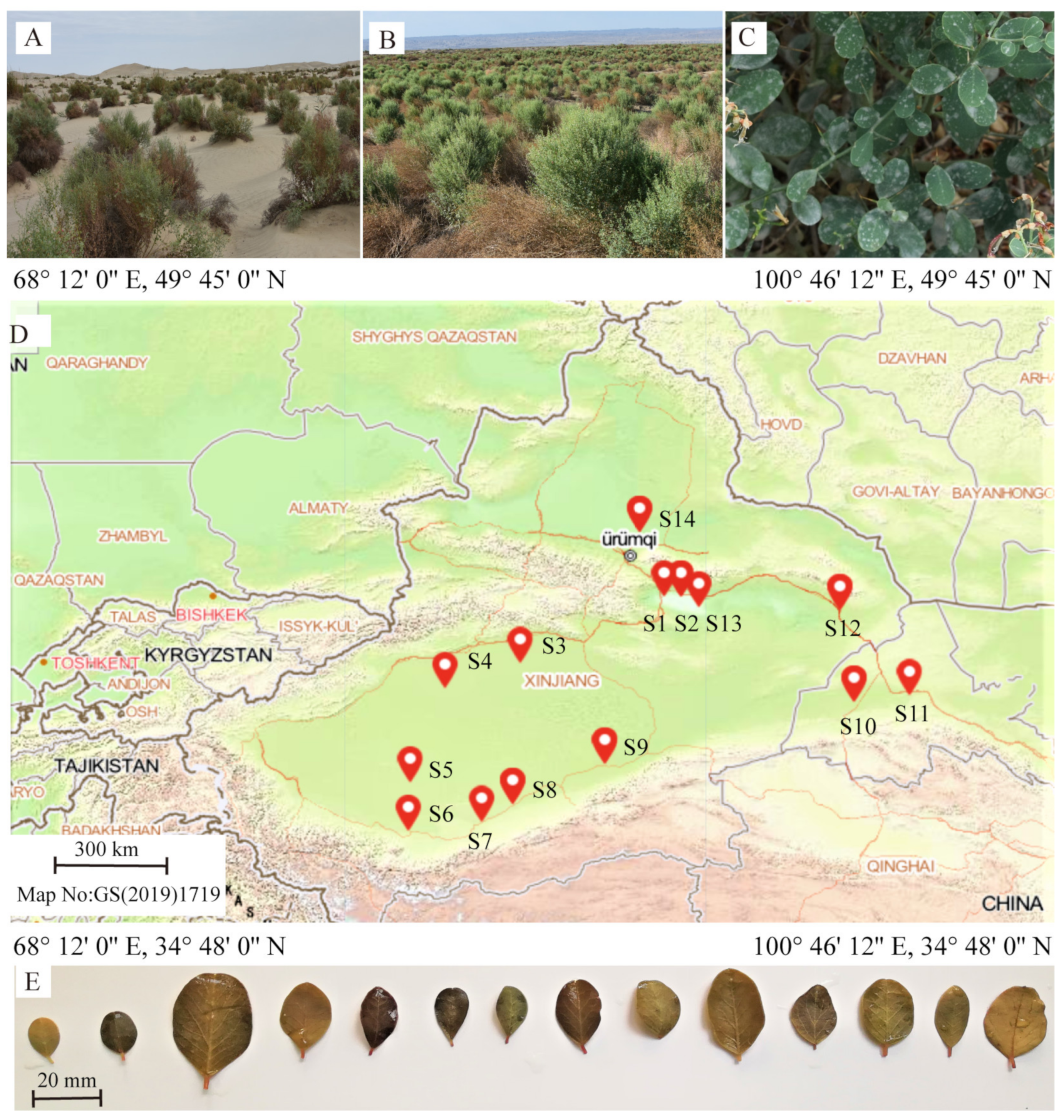
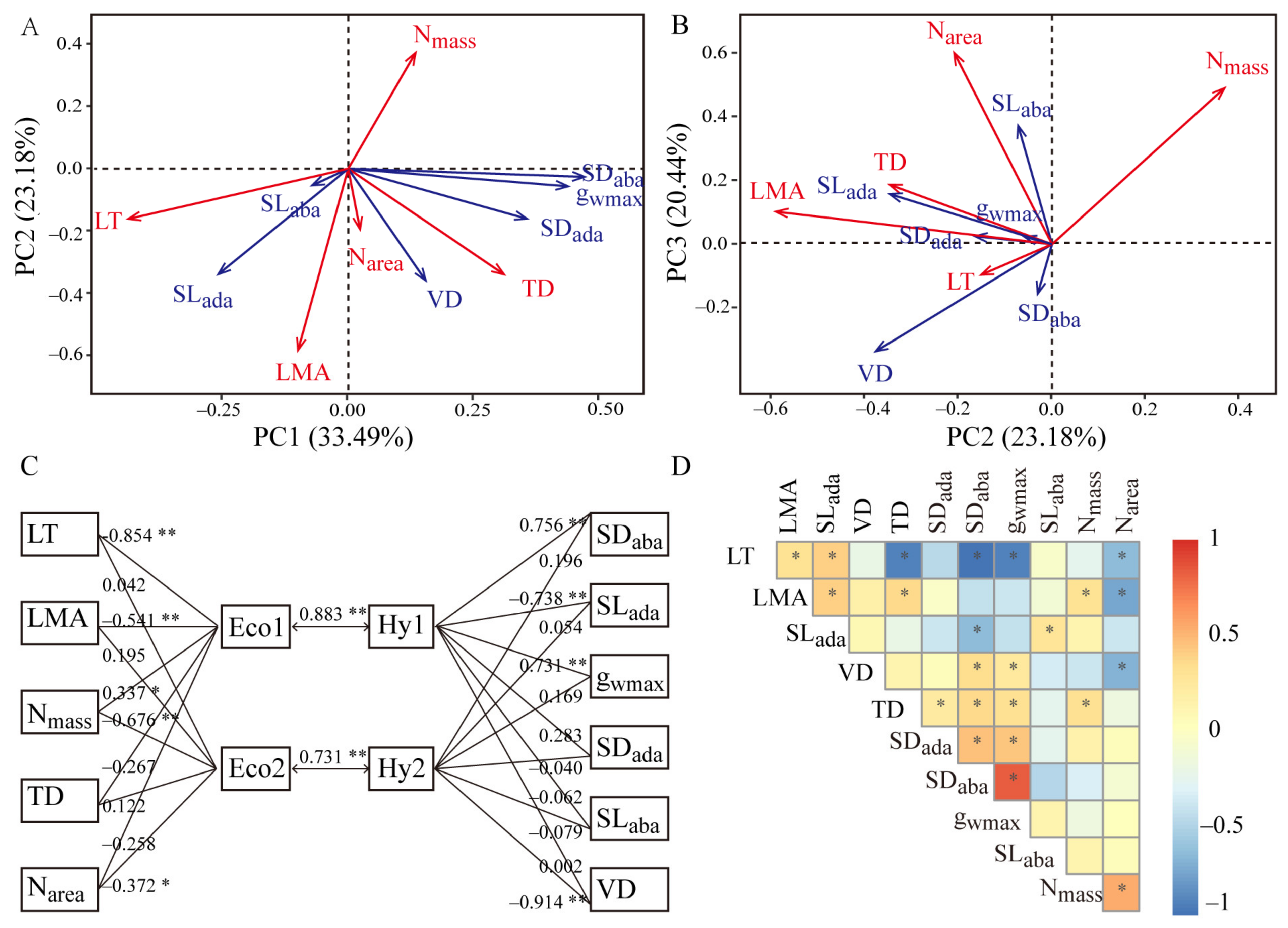

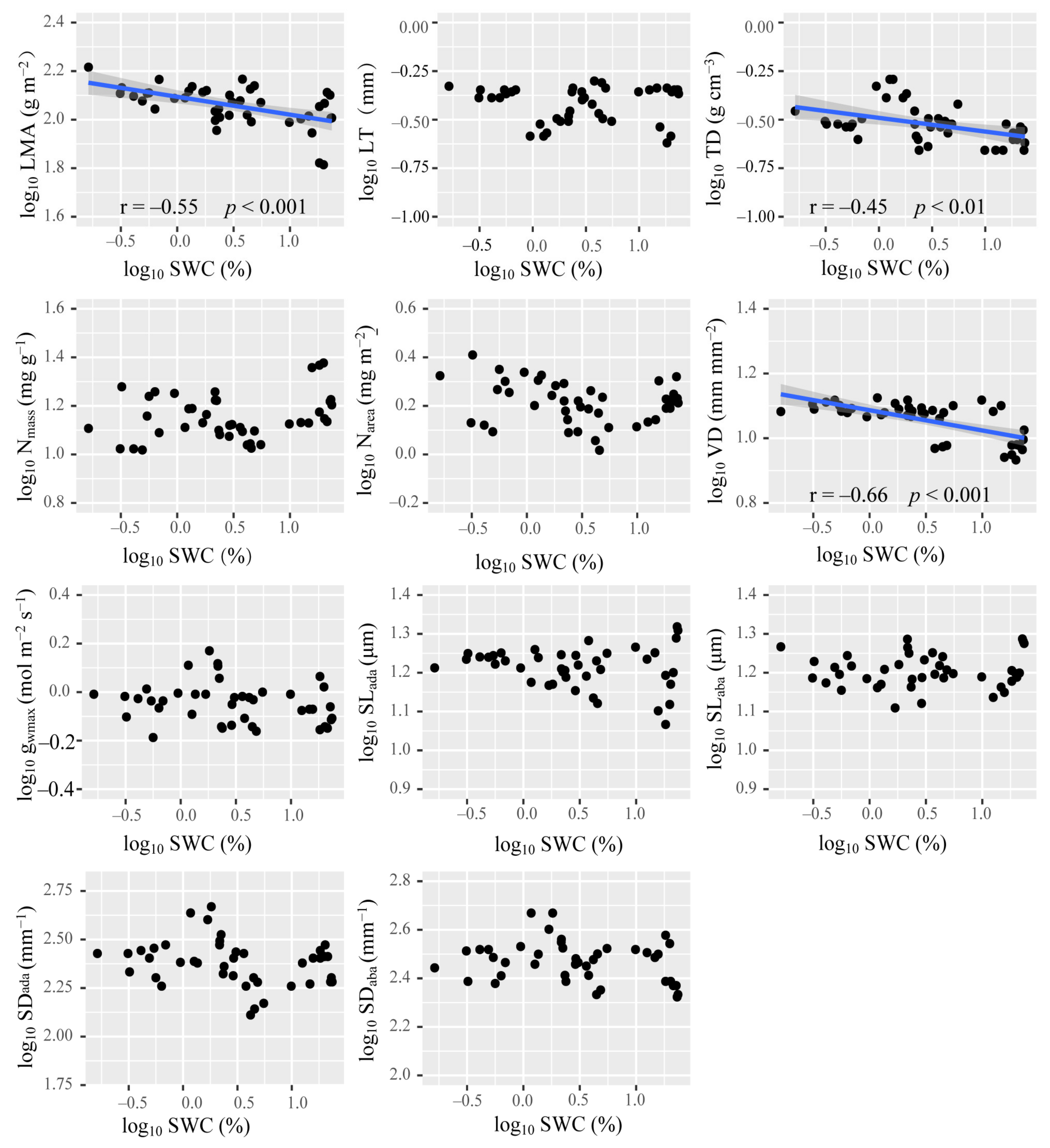

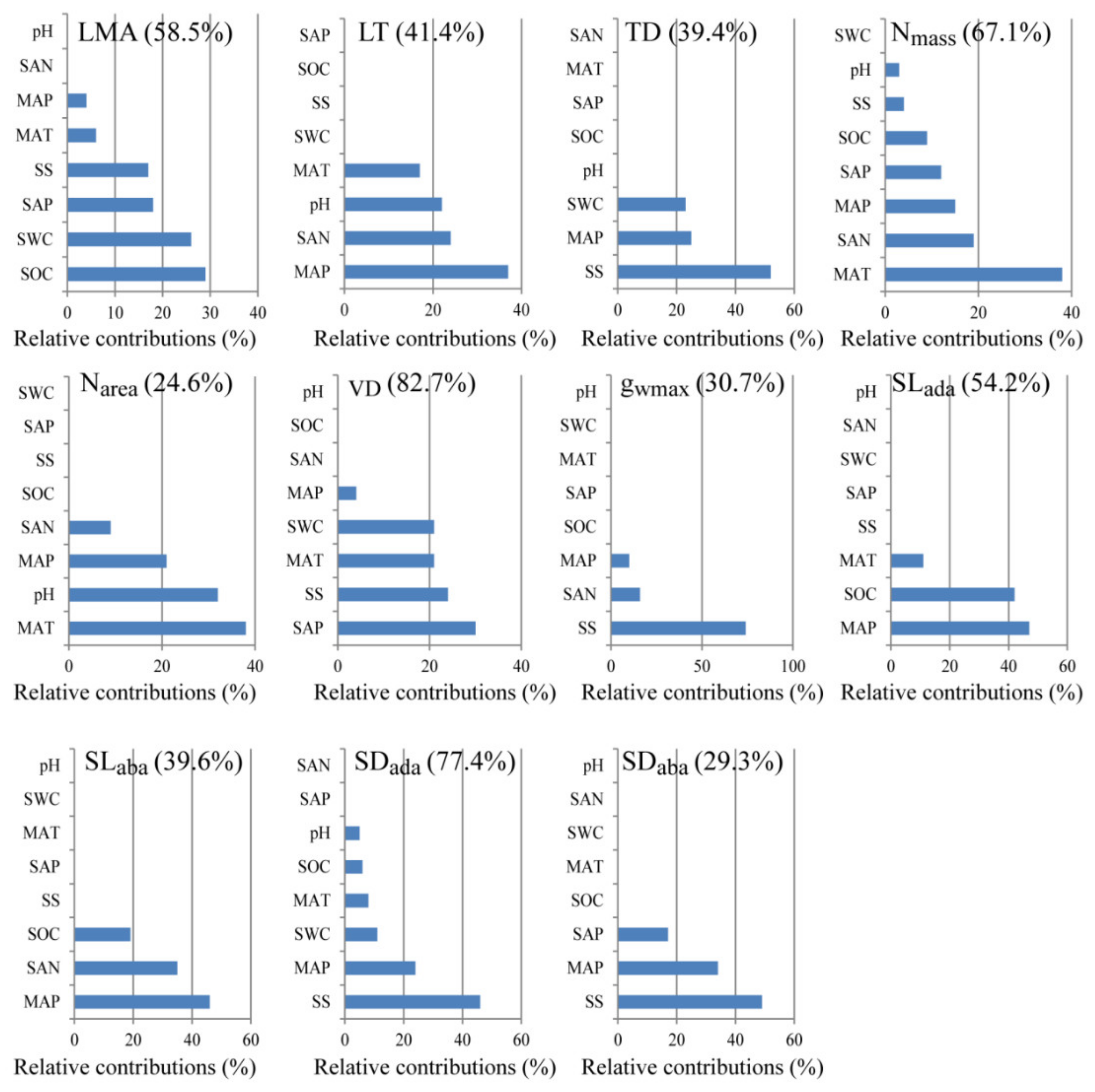

| Traits Name | Abbre | Unit | Mean ± SE | CV (%) | |
|---|---|---|---|---|---|
| Economic traits | Leaf dry mass per area | LMA | g m−2 | 116.4 ± 3.1 | 17.16 |
| Leaf thickness | LT | mm | 0.39 ± 0.01 | 19.35 | |
| Leaf tissue density | TD | g cm−3 | 0.31 ± 0.01 | 23.83 | |
| Leaf nitrogen concentration per mass | Nmass | mg g−1 | 14.60 ± 0.52 | 23.13 | |
| Leaf nitrogen concentration per area | Narea | g m−2 | 1.67 ± 0.05 | 20.23 | |
| Hydraulic traits | Vein density | VD | mm mm−2 | 11.49 ± 0.22 | 12.46 |
| Maximum stomatal conductance to water vapor | gwmax | mol m−2 s−1 | 0.92 ± 0.03 | 20.37 | |
| Stomatal length on the adaxial surface of the leaf | SLada | μm | 16.46 ± 0.30 | 11.97 | |
| Stomatal length on the abaxial surface of the leaf | SLaba | μm | 16.08 ± 0.25 | 10.12 | |
| Stomatal density on the adaxial surface of the leaf | SDada | mm−1 | 248.4 ± 10.86 | 28.34 | |
| Stomatal density on the abaxial surface of the leaf | SDaba | mm−1 | 300.2 ± 9.30 | 20.05 |
| LMA | LT | TD | Nmass | Narea | VD | gwmax | SLada | SLaba | SDada | SDaba | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| UPT | 0.37 | 0.86 | −0.51 | −0.28 | 0.03 | −0.05 | −0.47 | 0.51 | 0.24 | −0.12 | −0.60 |
| LPT | 0.31 | 0.70 | −0.40 | −0.55 | −0.30 | 0.07 | −0.37 | 0.13 | −0.18 | −0.03 | −0.27 |
| PT | 0.39 | 0.76 | −0.42 | −0.47 | −0.14 | 0.09 | −0.38 | 0.21 | −0.07 | -0.05 | −0.34 |
| ST | 0.23 | 0.63 | −0.40 | −0.21 | −0.01 | −0.24 | −0.51 | 0.24 | 0.17 | −0.36 | −0.61 |
| PT/ST | 0.06 | −0.07 | 0.09 | −0.13 | −0.09 | 0.31 | 0.24 | −0.09 | −0.22 | 0.33 | 0.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.; Tariq, A.; Zhang, B.; Lv, G.; Zeng, F.; Graciano, C.; Santos, M.; Zhang, Z.; Wang, P.; Mu, S. Coupling Relationship of Leaf Economic and Hydraulic Traits of Alhagisparsifolia Shap. in a Hyper-Arid Desert Ecosystem. Plants 2021, 10, 1867. https://doi.org/10.3390/plants10091867
Yin H, Tariq A, Zhang B, Lv G, Zeng F, Graciano C, Santos M, Zhang Z, Wang P, Mu S. Coupling Relationship of Leaf Economic and Hydraulic Traits of Alhagisparsifolia Shap. in a Hyper-Arid Desert Ecosystem. Plants. 2021; 10(9):1867. https://doi.org/10.3390/plants10091867
Chicago/Turabian StyleYin, Hui, Akash Tariq, Bo Zhang, Guanghui Lv, Fanjiang Zeng, Corina Graciano, Mauro Santos, Zhihao Zhang, Peng Wang, and Shuyong Mu. 2021. "Coupling Relationship of Leaf Economic and Hydraulic Traits of Alhagisparsifolia Shap. in a Hyper-Arid Desert Ecosystem" Plants 10, no. 9: 1867. https://doi.org/10.3390/plants10091867
APA StyleYin, H., Tariq, A., Zhang, B., Lv, G., Zeng, F., Graciano, C., Santos, M., Zhang, Z., Wang, P., & Mu, S. (2021). Coupling Relationship of Leaf Economic and Hydraulic Traits of Alhagisparsifolia Shap. in a Hyper-Arid Desert Ecosystem. Plants, 10(9), 1867. https://doi.org/10.3390/plants10091867







